Abstract
Initiating prehospital resuscitation with plasma in patients with trauma-associated hemorrhagic shock will result in more rapid and durable clot formation and, thus, the need for fewer packed cell infusions, less frequent use of cryoprecipitate, and more ventilator-free hospital days compared with those of patients randomized to standard crystalloid field resuscitation.
BLOOD’S PREEMINENCE
Hemorrhage is the most preventable cause of trauma-related fatalities.1 The uniqueness of blood’s oxygen-carrying red cells in combination with procoagulant factors and oncotic properties became more apparent in the interim between World Wars I and II. In World War II, resuscitation with reconstituted, freeze-dried plasma (FDP) during evacuation was an effective in-transit strategy, but required blood availability at a field hospital to achieve its full potential. Physicians serving in Korea and Vietnam had ready access to blood, but triple isotope studies of Shires et al2 in 1961 defined a third-space fluid loss that prompted an American Vietnam military hospital policy of infusing a ratio of 3 L of crystalloid for each unit of blood transfused.
Casualties reaching combat hospitals have had a progressively better chance of survival in successive wars, which rebounded in Vietnam (Fig 1).3 The comfortable explanation was that unsalvageable patients who would have died in the field in previous wars were now reaching a care center. A plausible alternative is that potentially salvageable patients were more likely to bleed from dilutive coagulopathy, despite frequent use of fresh-drawn whole blood.
Fig 1.
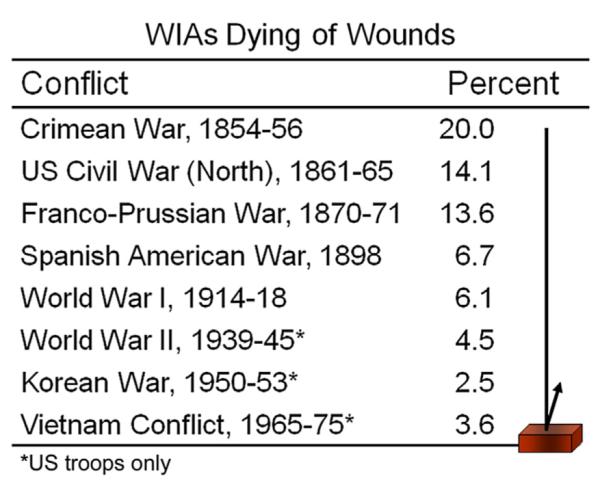
Troops wounded in action (WIA) who do not survive after reaching a medical treatment facility are categorized as dying of wounds (DOW), as opposed to having been killed in action (KIA).3 (Color version of figure is available online.)
Multiple studies now support restricted crystalloid resuscitation, and Ley et al4 identified ≥1.5 L as being associated with increased mortality among 3000 trauma patients. Brohi et al’s5 often quoted 24.4% incidence of trauma-associated, acute coagulopathy on admission to the emergency department at a median of 73 minutes (interquartile range, 57–75) after injury involved patients who had all received <1,500 mL of prehospital crystalloid. This report reignited interest in proscribed transfusion ratios, but with a shift in emphasis from crystalloid to FFP for preemptively addressing trauma-associated, acute coagulopathy. Giving FFP soon after injury is paramount, because the survival benefit is primarily in the first 24 hours.1 Yet, ratios of FFP to packed RBC are based typically on 24-hour treatment intervals and, therefore, are not truly preemptive. They also are often not achieved because of clinical exigencies and fall behind as less effective “catch-up therapy.” For example, the statistical model of Holcomb et al,6 derived from their multicenter, retrospective study indicates specifying a 1:1 ratio would be needed to ensure an actual delivery ratio of 1:2.
PLASMA, NATURE’S “ORGANIC” PROCOAGULANT COLLOID THEN AND NOW
Plasma, unlike blood, can be preserved by freezing at −18°C (fresh frozen plasma [FFP]) or FDP by spraying in vacuum (lyophilization or cryodessication). Because freeze or spray drying removes CO2 and water, FDP should be reconstituted with a weak acid. FDP has a room temperature shelf-life measured in years and can be used for 24 hours after reconstitution, which takes <5 minutes. World War II FDP was derived from pooled plasma, which increased the probability of transmission of viral hepatitis at a time when contemporary donor screening was not sufficient to make even single-donor plasma 100% safe, particularly when donors were paid. The emergence of the acquired immunodeficiency syndrome in the 1980s and subsequent identification of the human immunodeficiency virus ensured the apparent permanent retirement of FDP. Nucleic acid testing has brought it back by ensuring that fresh drawn pooled plasma is virtually virus free before its preservation.
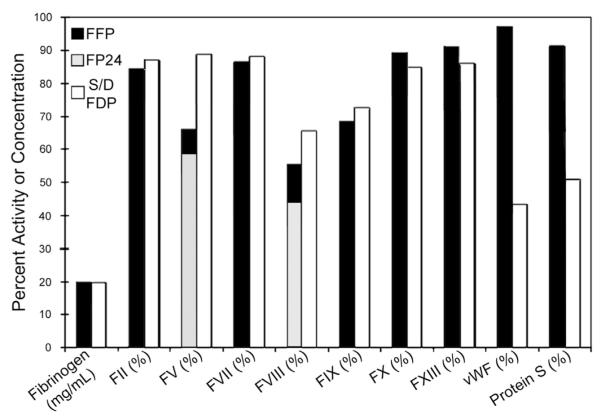
FFP designates plasma that has been frozen within 6–8 hours of collection and is typically single donor plasma. FFP has been largely supplanted by FP24, which can be frozen within 24 hours of collection, allowing for large mobile blood drives. Both FFP and FP24 must be stored at −18°C, have a storage limit of 12 months (7 years at −65°C), can take 15 minutes to thaw, and once thawed, should be used within 24 hours. Freeze or spray dried (lyophilized) plasma (FDP) has been continuously available in South Africa and France since 1998 as a solvent-detergent-treated, FDP (S/DFDP). This preparation has gone through multiple processing iterations, mainly in Europe, and is now available from ≥3 European manufacturers.7 FFP, FP24, and S/DFDP all contain substantially less fibrinogen than fresh plasma (Fig 2).8 FP24 differs from FFP by having insufficient factor VIII and is contraindicated for treating hemophiliac bleeding. S/DFDP is relatively deficient in von Willebrand factor, protein S, α1-antitrypsin, and α2-antiplasmin. Diminished protein S was blamed for occasional thromboembolic events, and paradoxically increased bleeding was ascribed to antitrypsin and antiplasmin deficiencies when multiple units of American S/DFDP had been given to patients with severe liver disease, causing its sole manufacturer to cease production in 2002. Neither aberrant event has been observed in patients undergoing liver transplants who have received current European S/DFDP iterations, which have a slightly greater level of plasmin inhibitor.7
Fig 2.
Percent retained coagulation factor activity in thawed FFP (freshly drawn plasma frozen within 8 hours); in FP24 for factors V and VIII, where freezing within 24 hours differs from FFP; and in room temperature–stored, reconstituted solvent-detergent treated freeze dried plasma (S/DFDP). vWF, von Willebrand factor.8
In the United States, male-only plasma donors have decreased the risk of plasma-induced, transfusion-related acute lung injury (TRALI), but TRALI is still the leading cause of transfusion-related deaths in the United States. In Europe, solvent-detergent treatment of relatively large pools of FDP has eliminated completely the risk of TRALI from plasma (0 incidences after 106 infusions) by diluting and neutralizing leukocyte antibodies and washing out activated lipids.7 The treatment removes >5 logs of abnormal prion protein and most enveloped viruses, including HIV, hepatitis C, and West Nile. Pooling types A, B, and AB plasma in ratios approximating their relative presence in the population and excluding type O donors also allows A and B antigens to neutralize naturally occurring anti-A and anti-B hemagglutinins as well as possible anti-idiotypic antibodies, thereby constituting a universal, pathogen-reduced plasma for all blood types that also minimizes allergic reactions.9
The German and French armies are using S/DFDP in Afghanistan and have used it effectively in treating several US Special Forces casualties. The US Department of Defense would like to have it, but the US Food and Drug Administration is skeptical and maintains a high bar for approval of any new FDP format. Consequently, European manufacturers and the former American manufacturer are reluctant to undertake the expensive studies that might allow them to enter or reenter the US market.
PLASMA PROTEOMICS
The protein concentration of plasma is approximately 65 g/L. Albumin, transferrin, and immunoglobulins comprise the majority (up to 80%) of protein. The next most abundant 50 proteins include (1) additional transport and apolipoproteins for storage, delivery, and clearance of lipids, iron, and hydrophobic hormone carriers; (2) several protease inhibitors, including members of the serpin family, which inhibit classic serum proteases, coagulation, and fibrinolytic factors, and many matrix metalloproteases; (3) coagulation factors; (4) acute phase components; and (5) enzymes responsible for the bioconversion of small molecules.
A proteomic analysis of female versus male plasma revealed female plasma to have approximately twice the concentrations of anti-proteases, α2-macroglobulin, and α1-antitrypsin, which oppose enzymatic clot degradation and males to have almost 15-fold more transgelin-2.10 The latter “gels” actin at a ratio of 1:6 preventing the formation of actin filaments that, if formed in the circulation, get trapped in the pulmonary capillaries.
We and others have observed significant differences in these more abundant plasma components, in various animal models of trauma research as well as in clinical samples. Qian et al11 identified 110 of 313 proteins that exhibited a significant change in abundance in the plasma of 15 severe burn patients compared with a pool of healthy controls. The most striking findings were that the abundant protease inhibitors α-1-antichymotrypsin, α-1-antitrypsin–related protein, plasma protease C1 inhibitor, and β-2-micro-globulin all increased with injury, whereas antithrombin III, α-2 antiplasmin, and inter–α-trypsin inhibitor heavy chains-H1 and -H2 were decreased uniformly.11 The protective mode of action of plasma does not necessarily reflect the beneficial effect of a specific component, but rather the balance of components and their ability to “buffer” a wide range of shock-generated abnormalities. Such abnormalities after shock states include endothelial permeability, inappropriate coagulation, and other protease–protease inhibitor imbalances. Advances in proteomic techniques make it possible to monitor hundreds to thousands of proteins in a targeted way so that we can begin to understand the role of a fluid as dynamic and complex as plasma in the setting of trauma-associated hemorrhagic shock.12
PROPOSAL
It is time to leverage our greater understanding of critical care and the superior safety profile of European universal donor S/DFDP to test the effectiveness of its preemptive field use with a randomized, prospective controlled trial versus standard prehospital crystalloid resuscitation. This study can be done at any busy, level 1 trauma center where severely injured patients are encountered commonly; such patients predictably require whole blood transfusion based on their on-site blood pressure and pulse rates and heuristic observation of such factors as penetrating torso wounds and an unstable pelvis.1
Ambulances could carry S/DFDP and ascorbic acid solution units for its reconstitution that takes <5 minutes. Trained EMTs could determine eligibility and do the randomization on site. Further management would be in accord with ATLS and local protocols, monitoring coagulation with sequential thromboelastography and coagulation panels. The primary endpoints would be usage of whole blood and components, with ventilator-free days and hospital mortality as secondary endpoints. US Food and Drug Administration regulations for patients with life-threatening medical conditions where prospective informed consent is not possible can be found in 21 CFR §50.24, which includes descriptions of requisite community consultation and public disclosure that must occur before beginning patient enrollment.
Obtaining US Food and Drug Administration approval for this specific use will require cooperation from a European manufacturer and will not be an easy task. Developing evidence of the superiority of S/DFDP over crystalloid for field resuscitation will not add substantially to the excellent safety profile of the European universal donor S/DFDP. But it could be the lynch pin to engage manufacturer interest and segue into a unique US Department of Defense–European-manufacturer-partnered IND (Investigational New Drug) application, pitting S/DFDP against FP24 US standard of care for generalized use.
Acknowledgments
Funded in part by NIH grants: P50-GM049222 and T32-GM008315.
REFERENCES
- 1.Burman S, Cotton BA. Trauma patients at risk for massive transfusion: the role of scoring systems and the impact of early identification on patient outcomes. Expert Rev Hematol. 2012;5:211–8. doi: 10.1586/ehm.11.85. [DOI] [PubMed] [Google Scholar]
- 2.Shires T, Williams J, Brown F. Acute change in extracellular fluids associated with major surgical procedures. Ann Surg. 1961;154:803–10. doi: 10.1097/00000658-196111000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garfield RM, Neugut AI. Epidemiologic analysis of warfare. A historical review. JAMA. 1991;266:688–92. [PubMed] [Google Scholar]
- 4.Ley EJ, Clond MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, et al. Emergency department crystalloid resuscitation of 1.5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011;70:398–400. doi: 10.1097/TA.0b013e318208f99b. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 7.Hellstern P, Solheim BG. The use of solvent/detergent treatment in pathogen reduction of plasma. Transfus Med Hemother. 2011;38:65–70. doi: 10.1159/000323552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theusinger OM, Baulig W, Seifert B, Emmert MY, Spahn DR, Asmis LM. Relative concentrations of haemostatic factors and cytokines in solvent/detergent-treated and fresh-frozen plasma. Br J Anaesth. 2011;106:505–11. doi: 10.1093/bja/aer003. [DOI] [PubMed] [Google Scholar]
- 9.Solheim BG, Chetty R, Flesland O. Indications for use and cost-effectiveness of pathogen-reduced ABO-universal plasma. Curr Opin Hematol. 2008;15:612–7. doi: 10.1097/MOH.0b013e32831366d3. [DOI] [PubMed] [Google Scholar]
- 10.Silliman CC, Dzieciatkowska M, Moore EE, Kelher MR, Banerjee A, Liang X, et al. Proteomic analyses of human plasma: Venus versus Mars. Transfusion. 2012;52:417–24. doi: 10.1111/j.1537-2995.2011.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian WJ, Petritis BO, Kaushal A, Finnerty CC, Jeschke MG, Monroe ME, et al. Plasma proteome response to severe burn injury revealed by 18O-labeled “universal” reference-based quantitative proteomics. J Proteome Res. 2010;9:4779–89. doi: 10.1021/pr1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omenn GS, Baker MS, Aebersold R. Recent Workshops of the HUPO Human Plasma Proteome Project (HPPP): a bridge with the HUPO CardioVascular Initiative and the emergence of SRM targeted proteomics. Proteomics. 2011;11:3439–43. doi: 10.1002/pmic.201100382. [DOI] [PubMed] [Google Scholar]