Abstract
BACKGROUND
Coagulopathy in traumatic brain injury (CTBI) is a well-established phenomenon, but its mechanism is poorly understood. Various studies implicate protein C activation related to the global insult of hemorrhagic shock or brain tissue factor release with resultant platelet dysfunction and depletion of coagulation factors. We hypothesized that the platelet dysfunction of CTBI is a distinct phenomenon from the coagulopathy following hemorrhagic shock.
METHODS
We used thrombelastography with platelet mapping as a measure of platelet function, assessing the degree of inhibition of the adenosine diphosphate (ADP) and arachidonic acid (AA) receptor pathways. First, we studied the early effect of TBI on platelet inhibition by performing thrombelastography with platelet mapping on rats. We then conducted an analysis of admission blood samples from trauma patients with isolated head injury (n = 70). Patients in shock or on clopidogrel or aspirin were excluded.
RESULTS
In rats, ADP receptor inhibition at 15 minutes after injury was 77.6% ± 6.7% versus 39.0% ± 5.3% for controls (p < 0.0001). Humans with severe TBI (Glasgow Coma Scale [GCS] score ≤ 8) showed an increase in ADP receptor inhibition at 93.1% (interquartile range [IQR], 44.8–98.3%; n = 29) compared with 56.5% (IQR, 35–79.1%; n = 41) in milder TBI and 15.5% (IQR, 13.2–29.1%) in controls (p = 0.0014 and p < 0.0001, respectively). No patient had significant hypotension or acidosis. Parallel trends were noted in AA receptor inhibition.
CONCLUSION
Platelet ADP and AA receptor inhibition is a prominent early feature of CTBI in humans and rats and is linked to the severity of brain injury in patients with isolated head trauma. This phenomenon is observed in the absence of hemorrhagic shock or multisystem injury. Thus, TBI alone is shown to be sufficient to induce a profound platelet dysfunction. (J Trauma Acute Care Surg. 2014;76: 1169–1176.
Keywords: Traumatic brain injury, coagulopathy, platelet, thrombelastography, ADP receptor
Trauma-induced coagulopathy affects approximately one third of severely injured trauma patients and is likely multifactorial in its etiology.1 The coagulopathy of traumatic brain injury (CTBI) is a recognized component of trauma-induced coagulopathy, but its mechanism is poorly understood. Some studies indicate that CTBI stems from maladaptive protein C activation and hyper-fibrinolysis related to the global insult of hemorrhagic shock and tissue injury.2 Conversely, other data implicate tissue factor (TF) release from the injured brain, with resultant platelet dysfunction and depletion of coagulation factors.3,4
Several studies have also shown a link between the severity of TBI and platelet dysfunction.3-5 Recently, platelet dysfunction was described as the earliest manifestation of CTBI in the setting of multisystem trauma.4,5 The severity of early platelet dysfunction in multisystem trauma has a positive correlation with the overall degree of injury as measured by the Injury Severity Score (ISS), hypoperfusion as measured by the base deficit (BD), and mortality.3-8 However, efforts to prove a mechanistic link between brain injury and platelet inhibition have been confounded by the frequent concomitant presence of global hypoperfusion and acidosis (either from ischemia or respiratory insufficiency), which could account for the observed platelet defect.3-10
We hypothesized that the platelet dysfunction of CTBI is, in fact, an intrinsic effect of brain injury and is a distinct phenomenon from the coagulopathy induced by hemorrhagic shock and general tissue injury in trauma. Thus, (1) the platelet dysfunction of CTBI should be immediately observable in TBI patients, independent of global ischemia and acidosis, and (2) the severity of platelet dysfunction will correlatewith the severity of TBI.5,6 We first developed a rat model for TBI, to test the hypothesis that early platelet inhibition occurs in isolated brain injury. We then observed our human TBI patients prospectively, to explore the relationship between the severity of TBI and platelet dysfunction (as measured by thrombelastography with platelet mapping [TEG/PM]) in the subset of TBI patients with isolated TBI and without evidence of shock or acidosis.
PATIENTS AND METHODS
Rat TBI Model
Twenty-five male Sprague-Dawley rats, weighing 245 g to 285 g, were anesthetized with isoflurane and subjected to blunt TBI, administered via a rubber-tipped metal impactor applied to the exposed skull, at a speed of 6 m/s.11 Upon awakening, the rats displayed behavioral markers of TBI, such as imbalance, posturing, and spasticity. Blood was collected from the inferior vena cava at 15 minutes after injury and analyzed immediately with the TEG/PM assay. Blood was also taken for TEG/PM from 20 uninjured controls. This methodology was approved by the University of Notre Dame Institutional Animal Care and Use Committee.
Human TBI Patients
This study was part of an ongoing prospective observational study conducted at Denver Health Medical Center, Denver, Colorado, and Memorial Hospital of South Bend, Indiana, aimed at characterizing patterns of acute coagulopathy in trauma.8 The subset evaluation from the Memorial Hospital focused on the assessment of platelet function in TBI using TEG/PM. Field or emergency department (ED) admission blood was collected on consecutive trauma patients between December 2010 and June 2013. Only patients with isolated TBI were included for this study (n = 70). We defined isolated TBI as an head Abbreviated Injury Scale (AIS) score of 3 or greater and other AIS score of 2 or lower, without evidence of circulatory shock (systolic blood pressure [SBP] < 90 mm Hg) or acidosis (venous BD ≥ 8).4,6-9,11 Average elapsed time from injury to blood sample collection was approximately 30 minutes.11 Patients younger than 15 years; intoxicated or sedated patients; those receiving blood components before admission or with a significant (≥3 hour) delay between injury and admission; those on anticoagulants, clopidogrel, aspirin, or nonsteroidal anti-inflammatory drugs; and those deemed moribund in the field and not expected to survive beyond the ED were excluded.2,5,8,12
Data Collection
Admission blood samples were analyzed by TEG/PM at the point of care by the ED perfusionist.
Conventional coagulation tests (CCTs) (i.e., partial thromboplastin time [PTT], prothrombin time/international normalized ratio [INR], and platelet count) and serum chemistries were performed by the hospital core laboratory. BD was calculated from venous serum bicarbonate using the standard Henderson-Hasselbalch formula. With the use of a BD derived by this methodology, a deficit of 8 mEq/L corresponds to a deficit of 6 mEq/L derived from an arterial blood gas, the usual threshold for prediction of poor outcomes in trauma.6,8,13,14 Demographic and clinical data collected included age, sex, injury mechanism, SBP, Glasgow Coma Scale (GCS) score, ISS and all-cause 30-day mortality. The admission GCS score was used to stratify TBI into severe (GCS score ≤ 8) versus mild-to-moderate (GCS score ≤ 8) categories, based on the standard of clinical practice of prophylactically intubating TBI patients with a GCS score of 8 or lower.8 Data collection and storage processes were in compliance with Health Insurance Portability and Accountability Act regulations and were approved by both institutional review boards. Informed consent was obtained from volunteers. Waivers of informed consent were obtained for trauma patients under protocols approved by the respective institutional review boards.
Healthy Controls
Tenhealthy volunteers older than18 yearswere recruited at the Memorial Hospital of South Bend, Indiana, for use as controls. Sixty percent of the controls were male, and the mean age was 42 ± 5.1 years. These patients underwent TEG/PM and CCTs. Pregnant volunteers; those with genetic bleeding disorders; those taking oral contraceptives, antiplatelet, or anti-coagulant agents; and those with a history of trauma were excluded. These controls are part of a larger prospective study regarding platelet function during trauma.5,8,15
TEG and TEG/PM
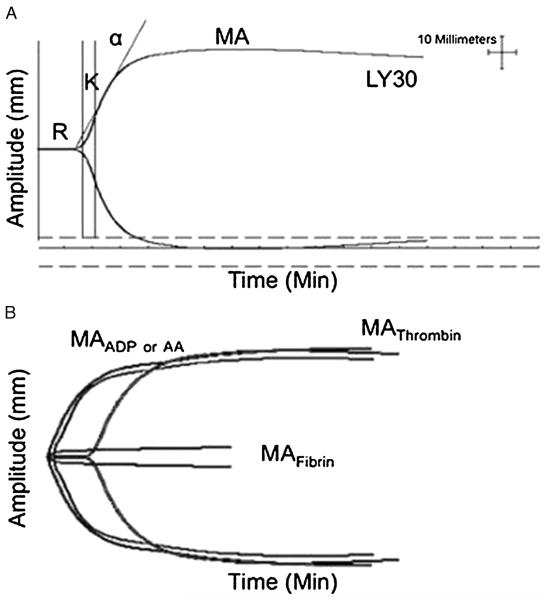
In both humans and rats, platelet function was analyzed using the TEG/PM assay (Haemonetics, Braintree, MA).16 Whole blood was collected in two tubes, one containing citrate (3.2%) and the other containing heparin (17 U/mL). TEG/PM assays were performed within 30 minutes of sample collection, in accordance with the manufacturer’s instructions. TEG/PM values are reported in terms of percent inhibition of the platelet response to stimulation by either adenosine diphosphate (ADP) or arachidonic acid (AA).5,8,16 This parameter is a calculated value obtained by comparing the viscoelastic strength of the thrombus,in termsofmaximum amplitude (MA), ofthree separate TEG tracings as follows: (1) a kaolin/Ca2+ activated whole-blood sample collected in citrate, representing the patient’s maximum hemostatic activity (MAThrombin); (2) a fibrin-only clot formed by an activator solution composed of reptilase and factor XIIIa in heparinized blood (MAFibrin); and (3) a platelet-containing clot, stimulated with either 2-mM ADP (MAADP) or 1-mM AA (MAAA) as platelet agonists, again in heparinized blood. The percentage of platelet inhibition in response to either the ADP or AA agonist was calculated using these three MA values in the following equation (Fig. 1):16
Figure 1.
Overview of TEG and TEG/PM. A, Standard TEG parameters: enzymatic time to clot formation (R), clotting time until 20-mm amplitude is achieved (K), measurement of clot kinetics (α angle), maximum clot strength reported as MA in millimeters, and clot lysis 30 minutes after MA (LY30). B, TEG/PM. The amplitude (millimeters), depicted on the y-axis, in response to platelet agonists MAADP or MAAA is compared with the whole-blood clot strength when clotting is initiated via the usual thrombin pathway (MAThrombin) minus the fibrin contribution (MAFibrin). This normal control tracing shows ADP- and AA-stimulated thrombus formation similar to the whole-blood clot strength (MAADP or MAAA near MAThrombin) with an ADP inhibition of 8% and AA inhibition of 1%. Figure 1 is modified (with permission) from Neurocrit Care 2013;18:201–208.
Statistical Analysis
All statistics and plots were generated with Prism version 5 software (GraphPad Software, La Jolla, CA). Normality was determined by a D’Agostino-Pearson omnibus test. Non-Gaussian distributed data sets were expressed as median and interquartile range (IQR), and the two-tailed, nonparametric Mann-Whitney U-test was used for comparisons. Box and whisker plots were filtered to 5th to 95th percentile, with outliers and mean values denoted.
RESULTS
Rats
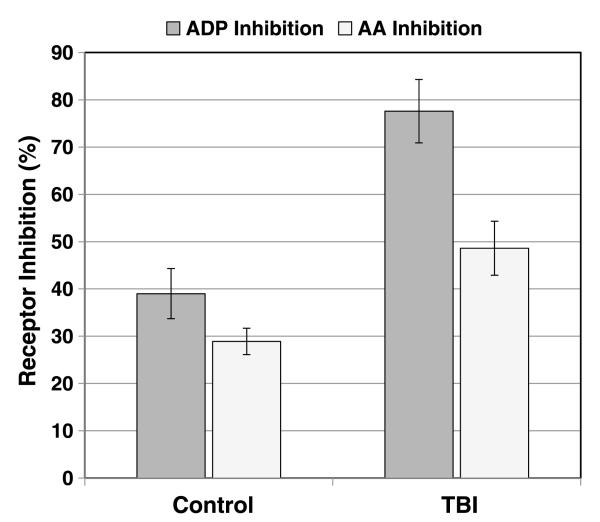
In our rat model of isolatedsevere blunt TBI, ADP receptor inhibition at 15 minutes after injury was 77.6% ± 6.7% versus 39.0% ± 5.3% for uninjured controls (p < 0.0001, n = 20–25 per group).
Similarly, AA receptor inhibition was 48.6 ± 5.7 versus 28.9 ± 2.8 for controls at the same time point (p = 0.0005) (Fig. 2).
Figure 2.
Platelet inhibition in rats following severe isolated blunt TBI. ADP receptor inhibition at 15 minutes after injury was 77.6% ± 6.7% (n = 25) versus 39.0% ± 5.3% for uninjured controls (n = 20) (p < 0.0001). A parallel trend of lesser magnitude was noted for AA receptor inhibition in the TBI group at 48.6% ± 5.7% versus 28.9 ± 2.8 for uninjured controls (p = 0.0005).
Human Subjects
All 70 patients enrolled with isolated TBI had imaging-proven intracranial bleeding, diffuse axonal injury, or brain parenchymal disruption from a penetrating injury. Subdural hematoma was the most frequent radiographic finding. Seventy-six percent of the enrollees were male, and the median age was 45.9 ± 2.5 years. Eighty-seven percent of the enrollees had a blunt mechanism, including 19 ground level falls, 17 motor vehicle collisions, 11 motorcycle collisions, 7 elevated falls, and 7 blunt assaults. The remainder was composed of three gunshot wounds and six other penetrating cases. Median ISS was 26 (IQR, 17–29), with a head AIS score of 4 (IQR, 3–5). Subdividing this group into patients with severe TBI (GCS score ≤ 8) and those with mild-to-moderate TBI (GCS score ≤ 8), ISS was 27 (IQR, 18–30) and head AIS score was 4.5 (IQR, 3–5) in the severe TBI group compared with an ISS of 25 (IQR, 17–26) and head AIS score of 4 (3.75–5) in the mild-to-moderate group. Overall, the mean GCS score was 9.7 ± 0.6. The median GCS score in the severe TBI subgroup was 4 (IQR, 3–6) versus 14 (IQR, 11.8–15) for the mild-to-moderate subgroup. There was no difference in age or sex between GCS subgroups. Fifteen patients died of their injuries.
Human TEG/PM
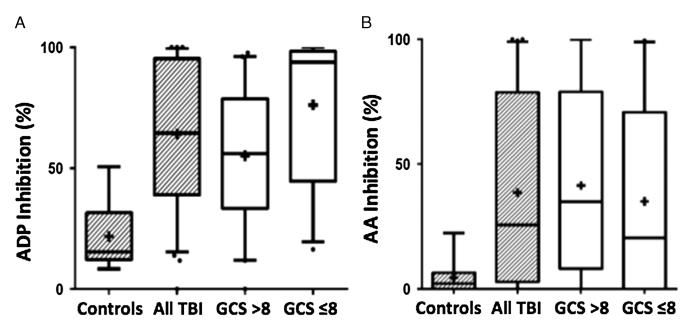
In TBI patients, the median inhibition of platelet function with respect to stimulation by the ADP pathway was 64.5% (IQR, 39.3-95.1%), compared with 15.5% (IQR, 13.2–29.1%) in the healthy controls (nonparametric Mann-Whitney U-test, p < 0.0001). When stratified based on severity of TBI, the severe (GCS score ≤ 8) cohort showed a median ADP inhibition of 93.1% (IQR, 44.8–98.3%, n = 29) compared with 56.5% (IQR, 35–79.1%, n = 41) in the mild-to-moderate (GCS score ≤ 8) cohort (p = 0.0014). With respect to platelet function stimulated via the AA pathway, the cohort of all TBI patients displayed 25.6% (IQR, 3.1–76.7%) inhibition compared with 2.2% (IQR, 0.0–5.8%) in the controls (p = 0.0027). Stratifying by severity of brain injury did not reveal significant differences with respect to AA pathway inhibition between the severe and mild-to-moderate cohorts (14.4% [IQR, 0–62.2%] vs. 40.4% [IQR, 12.9–78.9%]) (Fig. 3 and Table 1).
Figure 3.
Box and whisker plots of ADP and AA receptor inhibition in control versus TBI cohorts. A, ADP receptor inhibtion of all TBI is 64.5% (IQR, 39.3–95.1%, n = 70) compared with 15.5% (IQR, 13.2–29.1%, n = 10) (Mann-Whitney U-test p < 0.0001) in the healthy controls. ADP inhibition of severe TBI cohort, GCS score of 8 or lower, was 93.1% (IQR, 44.8–98.3%, n = 29) and 56.5% (IQR, 35–79.1%, n = 41) (p = 0.0006) in the cohort with GCS score greater than 8. Mean values denoted by “+.” B, AA receptor inhibition of all TBI is 25.6% (IQR, 3.1–76.7%, n = 70) compared with 2.2% (IQR, 0.0–5.8%, n = 10) (p = 0.0027) in the healthy controls. AA inhibition of severe TBI, GCS score of 8 or lower, was 14.4% (IQR, 0–62.2%, n = 29) and 40.4% (IQR, 12.9–78.9%, n = 41) (p = 0.3460) in the cohort with a GCS score greater than 8. Mean values denoted by “+.”
TABLE 1.
Comparison of Platelet Function, Physiologic Markers of Perfusion, and CCTs Between TBI Patients and Controls
| N | % ADP Inhibition, Median (IQR) |
% AA Inhibition, Median (IQR) |
BD, mean ± SEM |
SBP, Median (IQR) |
Platelet Count, Mean ± SEM |
INR Median (IQR) |
PTT, Median (IQR) |
RValue Median (IQR) |
MA Median (IQR) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | 10 | 15.5 (13.2–29.1) | 2.2 (0–5.8) | NA | NA | 229 ± 28 | 0.81 (0.74–0.98) | 33.0 (29.9–36.6) | 7.5 (6.15–8.2) | 63.3 (60.8–65) |
| All TBI | 70 | 64.5 (39.3–95.1) | 25.6 (3.1–76.7) | −0.6 ± 0.36 | 132 (119.5–150) | 204 ± 8.9 | 1.1 (1–1.2) | 29.3 (26.6–32.4) | 5.75 (4.7–6.7) | 61.5 (55.8–65.4) |
| GCS score > 8 | 41 | 56.5 (35–79.1) | 40.4 (12.9–78.9) | −1.1 ±0.47 | 140 (122–152) | 181 ± 11.1 | 1(1–1.2) | 29.2 (27.7–32) | 6.2 (5–7.4) | 61.6 (57.6–64.4) |
| GCS score ≤ 8 | 29 | 93.1 (44.8–98.3) | 14.4 (0–62.2) | 0.10 ± 0.56 | 128 (119–147) | 236 ± 12.7 | 1.1 (1–1.2) | 29.4 (25.4–32.6) | 5.4 (4.2–6) | 61.1 (55.2–66.2) |
Patients with any degree of TBI (defined as head AIS score >3) have a marked inhibition of platelet stimulation by both the ADP and AA pathways, compared with the uninjured controls. Moreover, the degree of ADP pathway inhibition is significantly higher in the subgroup of TBI patients with a GCS score of 8 or lower, the standard clinical cutoff for GCS at which patients are considered to have a “severe” TBI and are prophylactically intubated. Conversely, there is no correlation between BD, SBP, INR, PTT, platelet count, TEG R-time, or MA with the severity of TBI as measured by GCS. Although the INR shows a slight prolongation in the group of all TBI, compared with controls, both cohorts are within the reference range. Reference ranges: BD, −2 mEq/L to 2 mEq/L; platelet count, 150 × 103/μL to 400 × 103/μL; INR, 0.8 to 1.2; PTT, 23 seconds to 37 seconds; TEG R-time, 4 minutes to 8 minutes; TEG MA, 54 mm to 72 mm.
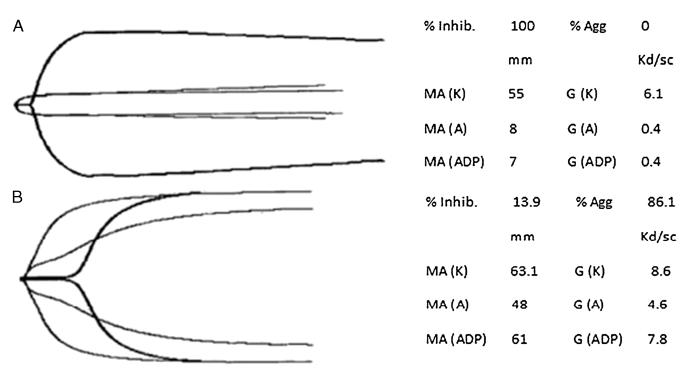
Representative TEG/PM traces of a severe TBI patient (GCS score, 3) and a healthy volunteer are shown in Figure 4.
Figure 4.
Representative TEG/PM tracings of a severe TBI patient versus an uninjured control. A, Severe TBI. In this patient with a GCS score of 3, ADP receptor inhibition is 100%, as evidenced by the lack of increase in clot strength when platelets are stimulated by ADP, compared with a fibrin-only clot. B, Uninjured control. ADP receptor inhibition of a healthy volunteer with a GCS score of 15. The ADP receptor inhibition is very low at 13.9%, denoting a normal platelet contribution to clot strength when stimulated by exogenous ADP.
Human Physiologic Data
No patients had significant acidosis or hypotension. The mean based deficit (BD) in the cohort of all TBI patients was −0.6 ± 0.36 mEq/L. There was a slight difference in BD between the severe (GCS score ≤ 8) and mild-to-moderate (GCS score ≤ 8) cohorts (0.10 ± 0.56 vs. −1.1 ± 0.47 mEq/L, respectively) but with both values centered within the reference range and with the worst BD (being 5) occurring in a mildly injured patient with GCS score of 13. Similarly, SBP was normal (≥90 mm Hg) in all subjects, with a mean of 137 ± 3 mm Hg. There was no significant difference in SBP between cohorts with GCS score greater than 8 and those with GCS score of 8 or lower.
CCTs and Kaolin TEG
In addition to TEG/PM, CCTs were also performed on TBI patients and controls. There was no difference in platelet count between the cohort of all TBI patients and healthy controls (204 ± 8.9 × 103/μL vs. 229 ± 28 × 103/μL). Whenstratifying by TBI severity, platelet count was somewhat lower in the mild-to-moderate cohort (181 ± 11.1 × 103/μL) than in the severe TBI cohort (236 ± 12.7 × 103/μL), but both were within normal limits. There was a slight prolongation of INR in the cohort of all TBI patients (1.1; IQR, 1.0–1.2) compared with controls (0.8; IQR, 0.7–0.9), but these values were still within the reference range, and there was no difference between severe and mild-to-moderate TBI subcohorts. Conversely, PTT was slightly short-ened in the TBI cohort compared with the control (30.0 ± 0.7 seconds vs. 33.0 ± 1.6 seconds), but this was not statistically significant, and again, no difference was observed between the severe and mild-to-moderate TBI subcohorts. Kaolin TEG R-time (clot kinetics) and MA (clot strength) were within the reference range for all cohorts.
DISCUSSION
In this prospective study of coagulopathy in isolated TBI, patients with any degree of TBI (defined as head AIS score ≥ 3) have a marked inhibition of platelet stimulation by both the ADP and AA pathways, compared with uninjured controls. Moreover, the degree of ADP pathway inhibition is significantly higher in the more severe subgroup of TBI patients with a GCS score of 8 or lower (the standard clinical cutoff for GCS score at which patients are considered to have a “severe” TBI and are prophylactically intubated) compared with those with mild-to-moderate TBI. Conversely, there is no clinically significant derangement of BD, SBP, INR, PTT, or platelet count in this cohort of patients with isolated head injury. These findings tend to confirm our hypothesis that TBI alone is sufficient to produce a marked impairment of platelet function, and this is further supported by a parallel observation in our rat model of isolated TBI.
Cohen et al.2 cite tissue hypoperfusion-mediated activation of the aPC pathway with downstream enhancement of fibrinolysis and other anticoagulant pathways as a central component necessary for the development of the CTBI. However, Lustenberger et al.6 have challenged the concept of tissue hypoperfusion-mediated coagulopathy by demonstrating that 60.4% of their patient group had coagulopathy, as defined by CCT parameters (INR ≤ 1.2, PTT ≤ 36, and platelet count < 100,000/μL), in the absence of significant BD. In the present study, none of our TBI patients had evidence of global hypoperfusion, as manifested by a significant BD or hypotension. This finding supports the hypothesis that platelet dysfunction in TBI is a distinct phenomenon from the platelet dysfunction observed in multisystem trauma and hemorrhagic shock.
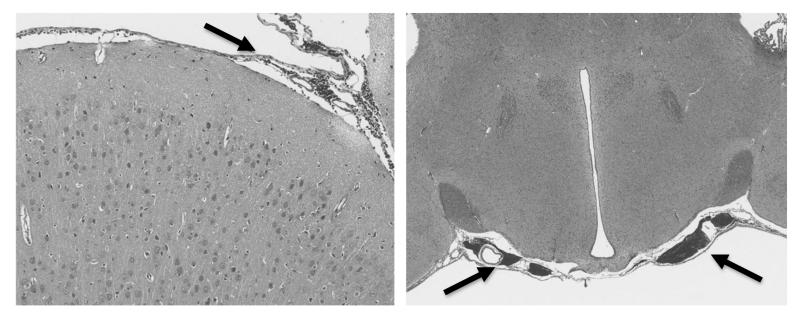
As the platelet dysfunction observed in this study stems from an isolated TBI and occurs almost immediately after injury (as demonstrated in both rats and humans), it is conceivable that the phenomenon is caused by a substance liberated from the brain parenchyma secondary to mechanical disruption of the blood-brain barrier (BBB). Histopathologic demonstration of sub-arachnoid hemorrhage in our rat model (as shown in Fig. 5) and the consistent finding of extraaxial hemorrhage in our human patients confirm disruption of the BBB in our TBI subjects.
Figure 5.
Representative histopathologic sections of post-TBI rat brains (hematoxylin and eosin, original magnification 40×). In the slides taken 30 minutes after injury, subarachnoid hemorrhage was a consistent finding (arrows), confirming disruption of the BBB in our TBI model.
One candidate for this hypothetical substance released by BBB disruption is TF. Brain TF is qualitatively different from that found in most other tissues inasmuch as it is isolated by the BBB and is unexposed to soluble clotting factors and largely unsaturated by factor VIIa. Therefore, liberation of this “free” TF into the circulation, where factor VIIawould normally exist in excess, provokes TF binding to VIIa on a massive scale, which would result in the stimulation of thrombin production in the “initiation” phase of coagulation as described by Hoffman and Monroe.17 It is conceivable that the early result of this flood of TF-generated thrombin after TBI is a “platelet exhaustion syndrome,” wherein large numbers of circulating platelets exist in an activated but refractory state.18 These platelets are incapable of stimulation and cannot form a stable thrombus via the usual pathways.3,5,8,9,12,17,19 Furthermore, hyperstimulated platelets may shed microparticles with disruption of the membrane skeleton that may contribute further to platelet dysfunction and to an overall consumptive coagulopathy.3,11,12,19-21
More immediately, the finding of an early and severe platelet dysfunction in isolated TBI has profound implications for the therapeutic management of patients with these injuries. While one limitation of the present study was an inability to evaluate the effect of the observed platelet dysfunction on intraoperative bleeding (as most patients who underwent a neurosurgical procedure first received a platelet transfusion), excessive bleeding has been noted in cardiology and cardiothoracic surgery patientswith ADP inhibitionin excess of 60%.22 The levels of ADP receptor dysfunction we observe in TBI are well in excess of this value and are found to be coupled to significant AA receptor inhibition as well, presenting a clinical scenario similar to that of a patient taking a combination of clopidogrel and nonsteroidal anti-inflammatory drugs. Thus, this finding on TEG/PM supports early platelet transfusion in the context of an intracranial hemorrhage at risk for expansion.
It is important to note that the vast majority of these patients not only had normal platelet counts and other CCTs but also had relatively normal kaolin TEG parameters (R, angle, and MA) (Table 1). Thus, in the setting of a largely isolated platelet dysfunction, hemostatic agents such as rVIIa and FFP should be given with extreme caution to avoid thrombosis of the microcirculation in early TBI.4,23-25 Moreover, we and others have previously shown that platelet dysfunction is a much more sensitive indicator of coagulopathy than the CCTs and these CCTs have a low and variable sensitivity for predicting clinical bleeding in trauma and varying cutoffs for definition of CTBI.3-9,26 Therefore, rather than giving empiric therapy, TBI patients should receive hemostatic agents and platelet transfusions guided by the best possible indicators of whole-blood hemostatic competence, including TEG/PM or other direct metrics of qualitative platelet function and not merely their quantitative abundance. Even if such tests are not readily available, our findings suggest maintaining a high index of suspicion for platelet dysfunction in isolated TBI patients and a low threshold for platelet transfusion if evidence of bleeding exists.23-25,27
ACKNOWLEDGMENT
Hanuma Swetha Chita, Joseph Cappanari, Megan Maloney, and Rachel Kurcz provided invaluable assistance in the development of our database.
DISCLOSURE
This study received research support from the Haemonetics Corporation, Niles, Illinois. The animal portion of this study was funded in part by NIH(NHLBI) grant #HL019982.
Biographies
DISCUSSION
Dr. Mitchell Jay Cohen (San Francisco, California): It is my great pleasure and honor to act as a discussant for this excellent science performed by Dr. Chapman and the very productive and excellent groups from Denver and Notre Dame.
In this study the authors address the incidence of platelet dysfunction after traumatic brain injury in rats and humans. In their study they report ADP and arachodonic acid receptor inhibition in rats and ADP receptor inhibition in humans. This study represents excellent translational work examining the important topic of platelet dysfunction after injury.
Initial evidence that platelet dysfunction may occur after trauma manifests in data that platelet transfusion was protective in both polytrauma and TBI despite normal platelet counts. Our group in San Francisco and others subsequently reported using platelet aggregometry that there is a profound platelet dysfunction in approximately 50% of patients, despite normal platelet counts.
Here the Denver/Notre Dame groups extend and expand these analyses in translational work on TBI in both rats and humans. Parenthetically, this work represents translational animal and human investigation which, in my opinion, is desperately needed as we all work to unravel the mechanisms of coagulopathy after injury. I do have a few questions:
First, in the controlled cortical impact rat model was there sectioning and histology of the brain injury? Was there quantification of the bleeding and volume of brain injury in this model?
Second, in terms of human subjects, what was the degree of confirmed brain injury? As you know, there is great variability and sometimes coding error in the AIS scores for head. I see you quantify what kinds of injuries the patients suffered but was there any volumetric or biomarker severity grading? Were there different types of injuries associated with differing platelet receptor deficits of severities, depending on the type of traumatic brain injury?
Third, importantly, how do you reconcile the fact that there is platelet inhibition but otherwise completely normal coagulation by both INR and TEG?
Fourth, in the very well-written manuscript, you state the that the “finding supports the hypothesis that platelet dysfunction in TBI is a distinct phenomenon from the platelet dysfunction observed in multisystem trauma and hemorrhagic shock.” I will admit that after several re-reads I don’t understand this.
Our group and, for that matter, your group have presented data on activated protein C coagulopathy in other mechanisms which suggest an aggregate that shock and injury are not necessary to cause coagulopathy. Matthew Kutcher’s platelet dysfunction data presented at the AAST does not necessarily need both shock and injury. And this is in keeping with your data so please explain how isolated TBI occurs by some different effect or mechanism.
Lastly, while I very much appreciate your mechanistic thinking and speculation behind the reasons for platelet dysfunction, I note that there are several other hypotheses regarding platelet dysfunction, including involving microparticle shedding, endothelial interactions and direct platelet inhibition.
I’m curious, what will you do or what have you done to quantify this? And have you actually looked at tissue factor to prove your hypothesis of platelet exhaustion?
Overall, I congratulate the authors on an excellent work in a very important, interesting field. And I look forward to years of additional collaborative work from this group.
Thank you very much for the podium.
Dr. Michael P. Chapman (Denver, Colorado): Thank you, Dr. Cohen. You’ve obviously made a very thorough read of this and those are very insightful points.
I’m not going to answer them in order, though. I’m going to address your most complicated ones first and, in the interest of time, I will try to get to them all.
Perhaps your most important point is that it’s unclear how we support the hypothesis that the platelet dysfunction that we observe is a distinct phenomenon from the accepted coagulopathy of trauma that your group and our group has been working on understanding for many years.
The previous work of both you and others in animals seems to indicate that classical trauma-induced coagulopathy is dependent, to some degree, on two hits: tissue injury and ischemia; although, certainly there are cases where we have observed this with tissue injury alone.
Kutcher’s paper explicitly implicates shock, though, noting that base deficits of nearly seven was the average in his cohort that displayed marked platelet inhibition. None of the patients in our study had any evidence of shock whatsoever, let alone profound enough and persistent enough shock to render the patient globally acidotic and ischemic.
Additionally, the trauma-induced coagulopathy that we see in most of our patients, both sort of anecdotally and in our more rigorous studies, is typically a poly-coagulopathy with disorders of the enzymatic and cellular components of coagulation and some component of fibrinolysis frequently thrown in as well.
As the phenomenon that we describe here is very consistently isolated platelet dysfunction and occurs without the ischemia that we think of as the sine qua non of classic TIC, I would tend to say that this is pretty much by definition mechanistally different, at least at the initiation phase.
That said, I think we are increasingly coming to the understanding that TIC is, as I alluded to, a polymorphic entity that is comprised of several components governed, to some extent, by the patient’s phenotype, the types of injured vascular beds, the type of denuded endothelium that’s involved, and many other factors that we are probably yet to understand, which is all the more reason why I think it’s valuable to study TIC in a reductionist manner whenever possible, look at isolated organ system injury.
This is hard to do in people; it’s something that a lot of people do in animals. This isolated TBI is one opportunity to look at that in isolation, which leads into your question, of course, about mechanism. Really, these two go hand-in-hand.
And we absolutely did indulge in a bit of speculation in the discussion section of our paper. Not so much from a desire to wax lyrical, but because we felt that it was necessary to at least present some sort of plausible mechanism for our finding and a direction for others and ourselves to pursue.
Certainly the vast amount of free tissue factor in the brain is one hypothesis. The unique endothelial environment of the brain and particularly its disruption and the disruption of the blood brain barrier, the exposure of platelets to a novel environment that they don’t usually interact with, all these things may well be the case.
I think testing these involves, as you said, looking for biomarkers to really prove a good dose response and better developed animal models, particularly in probably some larger animals like swine, which gets into your other two questions about how we quantitate this in people. So, a more granular analysis of radiographic abnormalities is in the works.
Every patient in this study had some sort of radiographic abnormality, be that intracranial blood or diffuse exonal injury. And doing volumetric studies and things like that with it is a future step that we absolutely think is critical. We agree with you with regard to that.
And the same would go for the rats. We have the brains collected. We have not, indeed, done volumetric analysis on that tissue. Again, I think we may focus our efforts on larger animal studies at this point.
Your other very important question, it’s that it is a little odd that we see this isolated platelet dysfunction with no other coagulopathy. I think on its face it is a little surprising: perfectly normal TEGS, perfectly normal coags and bad platelets. But I think it’s careful patient selection that may be responsible for that.
We truly have patients with nothing else wrong with them other than a blow to the head. And this platelet injury, this platelet dysfunction, would normally go unrecognized without the use of things like aggregometry or platelet mapping.
What the specific bad humor is that is coming out of your brain is speculative and that’s where we are heading next.
Thanks.
Footnotes
This paper was presented at the 72nd annual meeting of the American Association for the Surgery of Trauma, September 18–21, 2013, in San Francisco, California.
AUTHORSHIP
M.W., M.P.C., S.T., M.V.W., R.Y., and E.E.M. designed this study. M.P.C., M.W., B.F., and E.E.M. prepared the manuscript and figures. Data were analyzed and interpreted by M.P.C., M.W., S.T., E.E.M., and B.F. E.E., B.F., and P.D. collected human subject data. F.J.C., V.P., and D.L.D. performed the rat TBI experiments.
REFERENCES
- 1.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma. 2007;63:1254–1261. doi: 10.1097/TA.0b013e318156ee4c. discussion 1261-1262. [DOI] [PubMed] [Google Scholar]
- 3.Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion. 2013;53:28S–37S. doi: 10.1111/trf.12033. [DOI] [PubMed] [Google Scholar]
- 4.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma. 2013;73:13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, Moore EE, Wohlauer MV, Howard J, Ploplis V, et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18(2):201–208. doi: 10.1007/s12028-012-9745-6. [DOI] [PubMed] [Google Scholar]
- 6.Lustenberger T, Talving P, Kobayashi L, Barmparas G, Inaba K, Lam L, Branco BC, Demetriades D. Early coagulopathy after isolated severe traumatic brain injury: relationship with hypoperfusion challenged. J Trauma. 2010;69:1410–1414. doi: 10.1097/TA.0b013e3181cdae81. [DOI] [PubMed] [Google Scholar]
- 7.Lustenberger T, Talving P, Kobayashi L, Inaba K, Lam L, Plurad D, Demetriades D. Time course of coagulopathy in isolated severe traumatic brain injury. Injury. 2010;41(9):924–928. doi: 10.1016/j.injury.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214(5):739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan L, Demetriades D. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. 2009;66(1):55–61. doi: 10.1097/TA.0b013e318190c3c0. discussion 61-62. [DOI] [PubMed] [Google Scholar]
- 10.Sillesen M1, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam AM, Hwabejire J, Lu J, Duggan M, Velmahos G, et al. Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J Trauma. 2013;74:1252–1259. doi: 10.1097/TA.0b013e31828c7a6b. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Katayana Y, Kawamata T, Aoyama N, Mori T. Hemodynamic depression and microthrombosis in the peripheral areas of cortical contusion in the rat: role of platelet activating factor. Acta Neurochir Suppl. 1997;70:102–105. doi: 10.1007/978-3-7091-6837-0_32. [DOI] [PubMed] [Google Scholar]
- 12.Ostrowski SR, Sorensen AM, Larsen CF, Johansson PI. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study. Scand J Trauma Resusc Emerg Med. 2011;19:64. doi: 10.1186/1757-7241-19-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FitzSullivan E, Salim A, Demetriades D, Asensio J, Martin M. Serum bicarbonate may replace arterial base deficit in the trauma intensive care unit. Am J Surg. 2005;190:941–946. doi: 10.1016/j.amjsurg.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Eachempati SR, Reed RL, II, Barie PS. Serum bicarbonate concentration correlates with arterial base deficit in critically ill patients. Surg Infect (Larchmt) 2003;4(2):193–197. doi: 10.1089/109629603766956988. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thrombelastography. Semin Thromb Hemost. 2010;36:723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boschen L, Wiinberg B, Kjelgaard-Hansen M, Steinbruchel DA, Johansson PI. Evaluation of the TEG platelet mapping assay in blood donors. Thromb J. 2007;5:3. doi: 10.1186/1477-9560-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman M, Monroe DM. Tissue factor in brain is not saturated with factor VIIa: implications for factor VIIa: dosing in intracerebral hemorrhage. Stroke. 2009;40:2882–2884. doi: 10.1161/STROKEAHA.109.555433. [DOI] [PubMed] [Google Scholar]
- 18.Pareti FI, Capitanio A, Mannucci L, Ponticelli C, Mannucci PM. Acquired dysfunction due to the circulation of “exhausted” platelets. Am J Med. 1980;69:235–240. doi: 10.1016/0002-9343(80)90383-6. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51:639–647. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Morel N, Moel O, Petit L, Hugel B, Cochard JF, Freyssinet JM, Sztark F, Dabadie P. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J Trauma. 2008;64:698–704. doi: 10.1097/TA.0b013e31816493ad. [DOI] [PubMed] [Google Scholar]
- 21.Johansson PI, Sorensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, Ostrowski SR. High sCD40L levels early after trauma are associated with enhanced shock, sympathoadrenal activation, tissue and endothelial damage, coagulopathy and mortality. J Thromb Haemost. 2012;10:207–216. doi: 10.1111/j.1538-7836.2011.04589.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Bracey AW, Radovancevic R, Cooper JR, Collard CD, Vaughn WK, Nussmeier NA. Clopidogrel and bleeding in patients undergoing elective coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;128:425–431. doi: 10.1016/j.jtcvs.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70:1334–1345. doi: 10.1227/NEU.0b013e31824d179b. [DOI] [PubMed] [Google Scholar]
- 24.Stein S, Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70:1345. doi: 10.1227/NEU.0b013e31824d179b. Comment in. [DOI] [PubMed] [Google Scholar]
- 25.Anglin CO, Spence JS, Warner MA, Paliotta C, Harper C, Moore C, Sarode R, Madden C, Diaz-Arrastia R. Effects of platelet and plasma transfusion on outcome in traumatic brain injury patients with moderate bleeding diatheses. J Neurosurg. 2013;118:676–686. doi: 10.3171/2012.11.JNS12622. [DOI] [PubMed] [Google Scholar]
- 26.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 27.Brasel KJ, Vercruysse G, Spinella PC, Wade CE, Blackbourne LH, Borgman MA, Zarzabal LA, Du F, Perkins JG, Maegele M, et al. The association of blood component use ratios with the survival of massively transfused trauma patients with and without severe brain injury. J Trauma. 2011;71(2 Suppl 3):S343–S352. doi: 10.1097/TA.0b013e318227ef2d. [DOI] [PubMed] [Google Scholar]