Abstract
Background
The N-methyl-D-aspartate receptor antagonist ketamine has rapid antidepressant effects in major depression. Psychotomimetic symptoms, dissociation and hemodynamic changes are known side effects of ketamine, but it is unclear if these side effects relate to its antidepressant efficacy.
Methods
Data from 108 treatment-resistant inpatients meeting criteria for major depressive disorder and bipolar disorder who received a single subanesthetic ketamine infusion were analyzed. Pearson correlations were performed to examine potential associations between rapid changes in dissociation and psychotomimesis with the Clinician-Administered Dissociative States Scale (CADSS) and Brief Psychiatric Rating Scale (BPRS), respectively, manic symptoms with Young Mania Rating Scale (YMRS), and vital sign changes, with percent change in the 17-item Hamilton Depression Rating scale (HDRS) at 40 and 230 min and Days 1 and 7.
Results
Pearson correlations showed significant association between increased CADSS score at 40 min and percent improvement with ketamine in HDRS at 230 min (r= −0.35, p=0.007) and Day 7 (r=−0.41, p=0.01). Changes in YMRS or BPRS Positive Symptom score at 40 min were not significantly correlated with percent HDRS improvement at any time point with ketamine. Changes in systolic blood pressure, diastolic blood pressure, and pulse were also not significantly related to HDRS change.
Limitations
Secondary data analysis, combined diagnostic groups, potential unblinding.
Conclusions
Among the examined mediators of ketamine’s antidepressant response, only dissociative side effects predicted a more robust and sustained antidepressant. Prospective, mechanistic investigations are critically needed to understand why intra-infusion dissociation correlates with a more robust antidepressant efficacy of ketamine.
Keywords: Blood pressure, Psychotomimetic, Predictors, Major depressive disorder, Bipolar depression, Ketamine
1. Introduction
A single subanesthetic dose of ketamine has been shown to reduce depressive symptoms within hours in major depression (aan het Rot et al., 2010; Berman et al., 2000; Mathew et al., 2010; Messer et al., 2010; Murrough et al., 2013; Valentine et al., 2011; Zarate et al., 2006) and bipolar disorder (Diazgranados et al., 2010; Zarate et al., 2012) patients. This effect is sustained for approximately 1–2 weeks (Ibrahim et al., 2012). While results are encouraging, most patients experience transient dissociation and psychotomimetic side effects and hemodynamic changes (e.g., increases in blood pressure) that limit its clinical use (Green and Johnson, 1990).
Other noncompetitive (Zarate et al., 2013) and more specific NMDA receptor antagonists (Ibrahim et al., 2012; Preskorn et al., 2008) have antidepressant efficacy in major depression and are relatively devoid of psychotomimetic and dissociative side effects. The effects of these other antagonists, however, are not as robust as ketamine, which may reflect their decreased binding/affinity for the NMDA receptor complex (Aan Het Rot et al., 2012). Thus, it is unclear if the psychotomimetic sequelae, dissociative experiences, and/or hyperdynamic vital sign changes associated with a sub-anesthetic dose of ketamine are necessary to achieve antidepressant effects. Therefore, the objective of this analysis was to determine whether increased sympathomimetic and hypoglutamatergic effects were related to ketamine’s antidepressant efficacy. We hypothesized that increased sympathomimetic and hypoglutamatergic (psychotomimetic and dissociative) effects would correlate with changes in depression on ketamine.
2. Methods
We analyzed data from 108 treatment-resistant depression patients (MDD=74; BD=34) in a current major depressive episode without psychotic features, diagnosed according to the Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Version (First et al., 2002) (see Diazgranados et al., 2010; Ibrahim et al., 2012; Zarate et al., 2012, 2006 for study details). For two studies, ketamine was administered double-blind (Diazgranados et al., 2010; Zarate et al., 2012, 2006) while in the third study, ketamine was delivered open-label (MDD, n=42) (Ibrahim et al., 2012). The inpatient studies were conducted at the National Institutes of Mental Health Clinical Research Center in Bethesda, MD, USA. All participants provided written informed consent as approved by the NIH Combined Neuroscience Institutional Review Board. Subjects had at least a moderate severity episode of major depression, as measured as ≥18 on the 21-item Hamilton Depression Rating Scale (HDRS) or ≥20 on the Montgomery-Asberg Depression Rating Scale (MADRS) for at least four weeks at screening and the start of each infusion, and had a history of at least one failed antidepressant drug trial in a current or past depressive episode. Exclusion criteria included a DSM-IV diagnosis of a lifetime psychotic spectrum disorder, drug or alcohol dependence or abuse within the past three months and serious, unstable medical illness.
Patients were psychotropic medication-free for at least two weeks prior to the first infusion (five weeks for fluoxetine), with the exception of BD patients who were maintained on therapeutic levels of either lithium (0.6–1.2 mEq/L) or valproate (50–125 μg/mL).
2.1. Ketamine administration
Subjects received a single subanesthetic dose (0.5 mg/kg) of ketamine by intravenous infusion over 40 min. Ratings of depression, hypo/mania, psychotomimetic, and dissociative symptoms were measured using the HDRS (Hamilton, 1960, 1980), Young Mania Rating Scale (YMRS) (Young et al., 1978), Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962), and Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998). Baseline and intra-infusion blood pressure and pulse were measured every 5 min for the first 40 min after starting the infusion with the subject reclining using a Philips Suresigns VS3 vital signs monitor. Psychiatric ratings were collected before and at 40, 80, 110, and 230 min after the start of infusion, and at various time points post-infusion.
2.2. Data analysis
Linear mixed models with restricted maximum likelihood estimation and compound symmetry covariance structures were used to examine changes in blood pressure and pulse over the course of the first 40 min and clinical ratings over the fisrst 230 min of the ketamine crossover studies. The phase-specific baseline was a covariate where a drug and time interaction was included in the model with their main effects. Bonferroni adjusted post hoc tests were used to examine drug differences at individual time points.
Using data from all ketamine treated subjects, Pearson correlations were calculated to examine the relationship between absolute changes in CADSS, BPRS total and positive symptoms, YMRS, and vital signs from baseline to 40 min and percent changes in HDRS at 230 min, Days 1 and 7 post-infusion. Significance was evaluated at p<0.05, two-tailed.
3. Results
All patients received a single ketamine infusion. Patients randomized to receive riluzole as an add-on treatment (Ibrahim et al., 2012) were excluded from analyses at Days 1 and 7.
The sample had moderate-to-severe depression in the current episode lasting an average of 55.7 (SD=97.2) months (Table 1). The sample was 85% Caucasian (n=92) with half females (n=54) and 21% (n=21) current smokers.
Table 1.
Demographic and clinical features of 108 treatment-resistant patients with major depression receiving a single subanesthetic dose (0.5 mg/kg for 40 min) as a rapidly-acting antidepressant
| Mean | SD | |
|---|---|---|
| Age | 47.2 | 12.0 |
| BMI | 30.5 | 6.9 |
| Age of onset | 20.2 | 11.3 |
| Length of current episode (months) | 55.7 | 97.2 |
| Length of illness (years) | 27.0 | 12.9 |
| Number of previous MDEs | 26.2 | 37.6 |
| Clinical ratings | ||
| HDRS (17 items) | 21.2 | 4.4 |
| BPRS total | 36.8 | 5.8 |
| BPRS positive symptoms | 9.8 | 1.5 |
| CADSS | 3.9 | 6.5 |
| YMRS | 4.9 | 2.6 |
| Percent change in HDRS (17 items) | ||
| 230 min | −39.6 | 25.3 |
| Day 1 | −35.9 | 30.7 |
| Day 7 | −23.4 | 29.7 |
| n | % | |
|---|---|---|
| Diagnosis (bipolar) | 34 | 32 |
| Male | 54 | 50 |
| Race (caucasian) | 92 | 85 |
| Smoking (current) | 21 | 21 |
Abbreviations: BPRS=Brief Psychiatric Rating Scale; BMI=body mass index; CADSS= Clinician-Administered Dissociative State Scale; HDRS=Hamilton Depression Rating Scale; MDE=major depressive episode; YMRS=Young Mania Rating Scale.
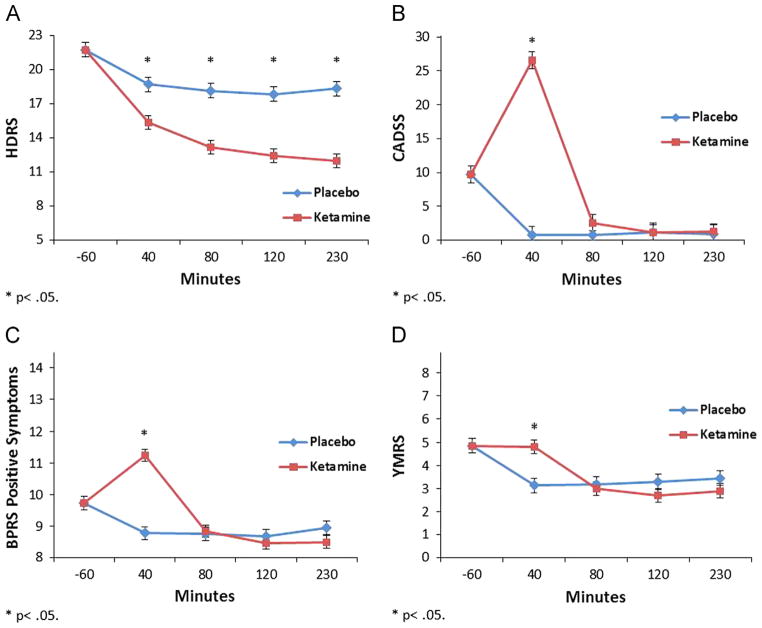
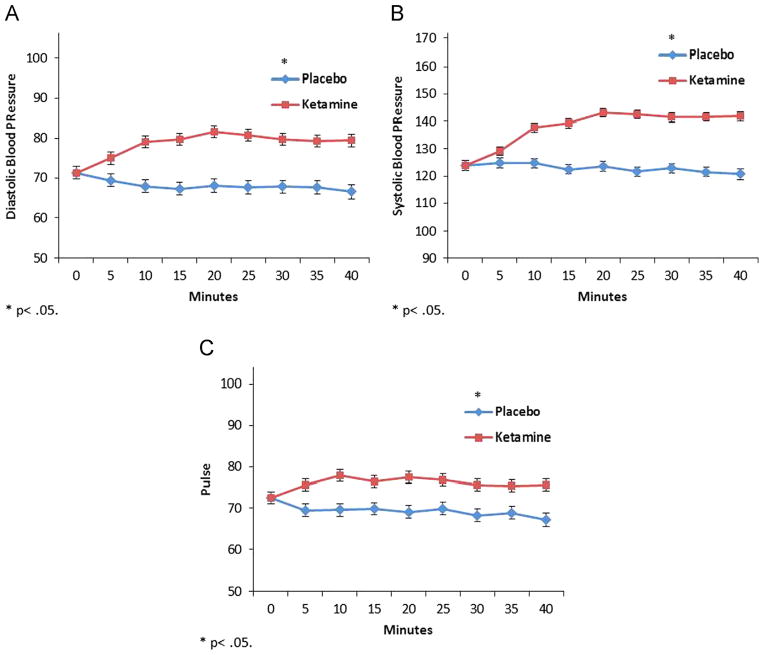
A linear mixed model showed a significant drug by time interaction indicating HDRS ratings were significantly lower on ketamine than placebo from 40 to 230 min (F=3.14, df=3,333, p=0.026) (Fig. 1A). CADSS, YMRS, and BPRS positive symptoms were significantly increased on ketamine at 40 min only (CADSS: F=55.48, df=3,209, p<0.001; YMRS: F=7.69, df=3,332, p<0.001; BPRS Positive: F=27.38, df=3,322, p<0.001) (Fig. 1B–D). Similar models demonstrated significantly higher diastolic (F=264.95, df=1,640, p<0.001) and systolic (F=429.80, df=1,652, p<0.001) blood pressure and pulse (F=175.22, df=1,609, p<0.001) on ketamine versus placebo from 5 to 40 min post-infusion (Fig. 2).
Fig. 1.
Infusion day (up to 230 min post-infusion) baseline-corrected clinical ratings from 108 treatment-resistant patients with major depression who received a single subanesthetic dose (0.5 mg/kg for 40 min) for treatment-resistant major depression. A. 17-item Hamilton Depression Rating Scale (HDRS), B. Clinician-Administered Dissociative Scale (CADSS), C. Brief Psychiatric Rating Scale (BPRS) Positive Symptoms Subscale, and D. Young Mania Rating Scale (YMRS); *=p<0.05.
Fig. 2.
Intra-ketamine infusion (every 5 min) baseline-corrected vital sign recordings from 108 treatment-resistant patients with major depression who received a single subanesthetic dose (0.5 mg/kg for 40 min) for treatment-resistant major depression. A. Diastolic Blood Pressure, B. Systolic Blood Pressure, and C. Pulse; *=p<0.05.
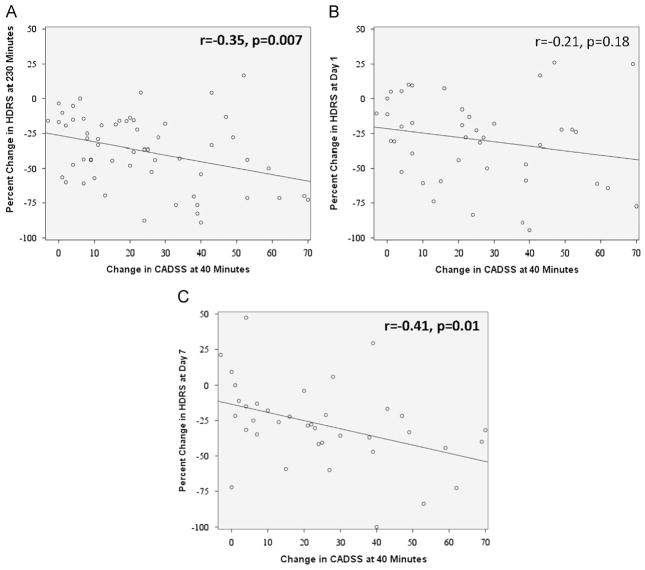
Correlations were significant between increased CADSS at 40 min and percent improvement in HDRS at 230 min (r= −0.35, p=0.007) and Day 7 (r= −0.41, p=0.01), but not Day 1 (r= −0.21, p=0.18 Fig. 3). Changes in YMRS, BPRS total, or BPRS positive symptoms at 40 min were not significantly related to HDRS percent change at any point (Table 2). Changes in systolic and diastolic blood pressure and pulse were not significantly related to depression changes.
Fig. 3.
Pearson Correlations with Clinician-Administered Dissociative States Scale (CADSS) at 40 min Post-Ketamine Infusion with 17-item Hamilton Depressing Rating Scale (HDRS) scores at A. 230 min Post-Ketamine Infusion; B. Day 1 Post-Ketamine Infusion; and C. Day 7 Post-Ketamine Infusion. The strength of the correlation is presented with significance level set at p<0.05 (bolded).
Table 2.
Pearson correlations of change in rating scales and levels with percent change in HDRS (from baseline).
| 230 min
|
Day 1
|
Day 7
|
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| CADSS | −.35 | .007 | −.21 | .18 | −.41 | .01 |
| BPRS total | .21 | .08 | .16 | .19 | −.01 | .94 |
| BPRS positive symptoms | −.15 | .22 | −.13 | .36 | −.21 | .15 |
| YMRS | −.19 | .13 | −.11 | .44 | −.10 | .51 |
| Systolic blood pressure | −.21 | .08 | −.01 | .95 | −.14 | .36 |
| Diastolic blood pressure | −.10 | .44 | .00 | 1.00 | −.13 | .39 |
| Pulse | −.01 | .93 | .08 | .59 | −.06 | .68 |
| Ketamine level | .04 | .81 | .18 | .29 | .16 | .36 |
| Norketamine level | .05 | .76 | .03 | .84 | −.12 | .50 |
Abbreviations: BPRS=Brief Psychiatric Rating Scale; CADSS=Clinician-Administered Dissociative State Scale; HDRS=Hamilton Depression Rating Scale; YMRS=Young Mania Rating Scale.
Next, at 40 min, change in CADSS was positively correlated with change in BPRS positive symptoms (r=0.31, p=0.02) and total BPRS (r=0.34, p=0.008), but not with change in YMRS (r=0.22, p=0.11) (Table 3). The largest magnitude correlation was between the BPRS positive symptom scale and YMRS (r=0.63, p<0.001). There were no significant correlations between vital sign changes and either CADSS, YMRS, BPRS total, or BPRS positive symptom changes. Changes in ketamine and norketamine levels were not significantly related to changes in depression or dissociation.
Table 3.
Rating scale, vital sign, and drug level correlations (from baseline to 40-min post-infusion).
| CADSS
|
BPRS total
|
BPRS positive symptoms
|
YMRS
|
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| BPRS total | .34 | .008 | ||||||
| BPRS positive symptoms | .31 | .02 | .57 | <.001 | ||||
| YMRS | .22 | .11 | .28 | .02 | .63 | <.001 | ||
| Systolic blood pressure | .13 | .34 | .05 | .69 | .01 | .91 | .13 | .29 |
| Diastolic blood pressure | .26 | .051 | .11 | .39 | .13 | .30 | .08 | .54 |
| Pulse | .20 | .15 | .05 | .69 | .11 | .39 | .08 | .53 |
| Ketamine | .23 | .17 | .22 | .19 | .22 | .19 | .09 | .60 |
| Norketamine | −.03 | .86 | .11 | .53 | −.08 | .65 | −.02 | .91 |
Abbreviations: BPRS=Brief Psychiatric Rating Scale; CADSS=Clinician-Administered Dissociative State Scale; YMRS=Young Mania Rating Scale.
4. Discussion
In agreement with previous reports (Driesen et al., 2013; Gibbs, 1970; Krystal et al., 1994), data from 108 depressed MDD or BD participants demonstrated that ketamine increased pulse, blood pressure, psychotomimetic and dissociative side effects. Dissociative side effects, but not psychotomimesis or sympathomimetic effects, correlated with change in depression on the day of infusion and seven days post-infusion. The present correlation suggests dissociative side effects as a clinical biomarker to predict ketamine’s efficacy.
Different underlying mechanisms may explain why dissociation predicts ketamine’s antidepressant effect, but blood pressure, pulse, and psychotomimetic effects do not. Increases in blood pressure and psychotomimetic effects may be due to increases in dopamine. Microdialysis studies showed low-dose ketamine stimulated dopamine release in the conscious rat’s prefrontal cortex (Moghaddam et al., 1997). However, Adams et al. (2002) found that ketamine did not stimulate dopamine release in non-human primates. In humans, ketamine’s effect on dopaminergic neuro-transmission is even more controversial (Aalto et al., 2005, 2002; Breier et al., 1998; Smith et al., 1998; Kegeles et al., 2002; Vernaleken et al., 2013). With regard to psychotomimetic effects, (Sos et al., 2013) BPRS subscales were not correlated with antidepressant response in depressed subjects. Similarly, we did not find correlations between positive symptoms or blood pressure and antidepressant response; these are presumably the behavioral and physiological correlates of a hyperdopaminergic state.
In contrast, dissociation may result from ketamine’s enhancement of glutamate release. Based on the predominant theory of ketamine’s antidepressant effect, i.e. the inhibition of GABAergic cortical interneurons leading to the depolarization of cortical projection (pyramidal) neurons, increased long-term potentiation-like synaptic glutamate release (“glutamate surge”) and greater AMPA-to-NMDA postsynaptic receptor throughput (Dwyer and Duman, 2013; Maeng et al., 2008), subjects with greater dissociation may also have greater presynaptic glutamate release, and vice versa, in response to subanesthetic dose ketamine (Anand et al., 2000). In addition, some evidence suggests psychotomimetic and dissociative symptoms following ketamine affect different areas of the brain. These data suggest possible avenues to pursue in mechanistic studies attempting to disentangle these factors.
Contrary to our results, a prior report found no correlation between maximum CADSS and HDRS response at any time following ketamine infusion (Valentine et al., 2011). Also, (Sos et al., 2013) found that more intense psychotomimetic symptoms (as assessed by BPRS total) correlated with improved mood ratings on the MADRS 7 days post-ketamine infusion. These discrepant findings may be attributed to: (1.) small sample sizes, (2.) inclusion of bipolar patients, (3.) mandatory treatment-resistance, and/or (4.) different doses in some studies.
A limitation of this study is the combination of unipolar and bipolar diagnostic groups and the use of open-label and randomized, placebo-controlled studies. Another major caveat is the adequacy of blinding. Ketamine-induced psychotomimetic and vital sign alterations may have been so noticeable to subjects and researchers as to compromise blinding. Future investigations might employ an active control medication with hypertensive and/or dissociative effects but without antidepressant activity. Midazolam served as an active control in a trial supporting the efficacy of ketamine in treatment-resistant MDD (Murrough et al., 2013). Nonetheless, improved blinding would not clarify the question of necessary or causal relationships between ketamine antidepressant and psychotomimetic/dissociative effects. Finally, although statistically significant, the CADSS change from baseline explained only a fraction of the variance in ketamine’s antidepressant response. Thus, it remains unclear whether intra-infusion dissociation is necessary for ketamine’s antidepressant response.
In conclusion, ketamine’s dissociative adverse effects significantly correlated with antidepressant response, but only explained a fraction of the variance in response.
Acknowledgments
Role of funding source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH), by a NARSAD Independent Investigator Award to CAZ, and by the Brain & Behavior Mood Disorders Research Award to CAZ.
We would like to thank the 7-SE Unit nursing staff, research assistants, and patients for the invaluable contributions to the protocol.
Footnotes
Conflict of interest
Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. The remaining authors have no conflict of interest to disclose, financial or otherwise.
References
- Aalto S, Hirvonen J, Kajander J, Scheinin H, Nagren K, Vilkman H, Gustafsson L, Syvalahti E, Hietala J. Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacol (Berl) 2002;164:401–406. doi: 10.1007/s00213-002-1236-6. [DOI] [PubMed] [Google Scholar]
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Nagren K, Vilkman H, Gustafsson LL, Syvalahti E, Hietala J. Cortical glutamate–dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacol (Berl) 2005;182:375–383. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Aan Het Rot M, Zarate CA, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BW, Bradberry CW, Moghaddam B. NMDA antagonist effects on striatal dopamine release: microdialysis studies in awake monkeys. Synapse. 2002;43:12–18. doi: 10.1002/syn.1114. [DOI] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, Malhotra AK, Pickar D. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse. 1998;29:142–147. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry. 2013;73:1189–1198. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders-Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Gibbs JM. Initial experiences with ketamine anaesthesia. N Z Med J. 1970;72:166–169. [PubMed] [Google Scholar]
- Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19:1033–1046. doi: 10.1016/s0196-0644(05)82569-7. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41:21–24. [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Kochan LD, Hwang DR, Huang Y, Mawlawi O, Suckow RF, Van Heertum RL, Laruelle M. NMDA antagonist effects on striatal dopamine release: positron emission tomography studies in humans. Synapse. 2002;43:19–29. doi: 10.1002/syn.10010. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, Aan Het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer M, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psycho-pharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, Simkowitz P, Hurley A, Cooper T, Volkow ND, Cancro R. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol Lett. 2013;34:287–293. [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1) H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I, Klomp M, Moeller O, Raptis M, Nagels A, Rosch F, Schaefer WM, Cumming P, Grunder G. Vulnerability to psychotogenic effects of ketamine is associated with elevated D(2/3)-receptor availability. Int J Neuropsychopharmacol. 2013;16:745–754. doi: 10.1017/S1461145712000764. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]