Abstract
Nicotine-induced hypothermia is well established, but the nicotinic receptor actions underlying this effect are not clear. Nicotine causes activation and desensitization at a variety of nicotinic receptor subtypes. Sazetidine-A [6-(5(((S)-azetidine-2-yl)methoxy)pyridine-3-yl)hex-5-yn-1-ol] is a novel compound that potently and selectively desensitizes α4β2* nicotinic receptors. The main goal of this study was to investigate the effects of sazetidine-A, on core body temperature (Tc) in mice and rats. Sazetidine-A effects on Tc and the interactions of sazetidine-A with nicotine and selective nicotinic antagonists were investigated to determine the receptor actions underlying nicotine-induced hypothermia. Adult male mice were injected with different dose of nicotine (0.2, 0.4 and 0.8 mg/kg), sazetidine-A (0.3, 1, and 3 mg/kg), a mixture of nicotine (0.4 or 0.8 mg/kg) and sazetidine-A (0.3 or 0.6 mg/kg) or saline and Tc was monitored telemetrically. In another set of experiments, the interaction between sazetidine-A and dihydro-β-erythroidine (DHβE), an α4β2* nicotinic receptors antagonist, and methyllycaconitine (MLA), an α 7 antagonist, was investigated. Tc of mice was monitored following DHβE (1, 3 and 6 mg/kg), a combination of DHβE (3 mg/kg) and sazetidine-A (0.6 mg/kg), MLA (1.5, 3 or 6 mg/kg) or combination of MLA (6 mg/kg) and sazetidine (0.6 mg/kg) or saline. The acute effect of sazetidine-A (1, 3, and 6 mg/kg) on rats Tc was also studied. Acute sazetidine-A caused a pronounced and long-lasting hypothermia in mice; Tc decreased to about 28 °C at 100 min and recovered within 230 min. The hypothermic effect of sazetidine in rats was much less in magnitude (about 3°C) and shorter in duration compared with that in mice. Nicotine co-administration with low doses of sazetidine potentiated the magnitude and duration of hypothermia in mice. The α4β2* nicotinic receptors antagonist DHβE significantly prolonged sazetidine-A-induced hypothermia but did not increase its depth. The α7 antagonist MLA caused a modest degree of hypothermia with relatively short duration in mice. MLA failed to counteract the sazetidine-A-induced hypothermia. Overall, our results show that pharmacological modulation of α4β2* nicotinic receptors elicits changes in body temperature that may involve desensitization of these receptors.
Keywords: Hypothermia, Thermoregulation, Nicotine, Telemetry, DHβE, MLA, Body temperature
1. Introduction
Nicotinic receptors have been shown to be involved in many vital functions, including regulation of body temperature. The hypothermic effects of nicotine have been documented in several species, including rodents (Gordon, 2001; Knox et al., 1973; Marks et al., 1984; Overstreet, 1995; Overstreet et al., 1998; Ruskin et al., 2008; Sack et al., 2005; Salminen and Ahtee, 2000) as well as primates (Hall and Myers, 1972). While the neuronal mechanism(s) of thermoregulation has been extensively studied (Gordon, 2005), it is not fully understood. It is likely that in addition to other transmitter systems, distinct nicotinic receptors play a part in this complex process. It has been shown that administration of mecamylamine, a non-specific nicotinic receptor antagonist, can block or decrease nicotine-induced hypothermia in mice (Nordberg and Sundwall, 1983; Zarrindast et al., 2001). Studies in inbred mouse strains have demonstrated that the high-affinity nicotine-binding sites, which are predominantly α4β2 receptors (Flores et al., 1992) may mediate the hypothermic effects of nicotine (Marks et al., 1989) Presuming that nicotine exerts its effects on body temperature through nicotinic cholinergic receptors, we decided to study the role of desensitization vs. activation of nicotinic receptors in the regulation of core body temperature.
Recently, a new compound, sazetidine-A, with selective desensitizing action on α4β2* nicotinic acetylcholine receptors has been developed. Sazetidine-A has a very high binding affinity for rat α4β2* nicotinic receptors, approximately >10,000-fold and 3,500-fold higher than its affinity at rat α3β4 and rat α7 nicotinic receptors, respectively (Xiao et al., 2006). Consistent with its high selectivity for α4β2* receptors in binding assays, sazetidine-A is a much weaker desensitizer of α3β4 or α7 nicotinic receptor subtypes (Liu et al., 2010; Xiao et al., 2006; Xiao et al., 2008). Thus, sazetidine-A is a highly selective and potent α4β2* nicotinic receptor desensitizer (Xiao et al., 2006).
The goal of this study was to investigate the involvement of nicotinic acetylcholine receptors containing β2 or α7 nicotinic receptors in the regulation of body temperature in mice and rats. Based on existing literature on the role of neuronal nicotinic receptors on thermoregulation, it was hypothesized that desensitization of α4β2* receptors by sazetidine-A would significantly reduce body temperature and would enhance the hypothermic effect of nicotine.
2. Materials and methods
2.1. Animals
Adult male C57BL/6J mice weighing 25±2 (S.E.M.) g. and adult male Sprague Dawley rats weighing 294± 4.2 (S.E.M.) g. were used for these experiments. Animals were housed in groups of three for two weeks before surgery and were single housed after the surgery. Wood chip bedding was used throughout. Animals had free access to food and water in an ambient temperature of 22 ± 1°C and 12L: 12D photoperiod (lights on at 1800 h). After surgery animals were disturbed only for injections and once weekly for the replacement of food, water and bedding during the dark phase of the light/dark cycle. All injections were given subcutaneously about the same time during the dark phase when animals were active. All experiments were approved by the International Animal Care and Use Committee at Duke University.
2.2. Surgery and telemetry system
To measure body (core) temperature without disturbing the animals, we used an automated telemetry system (Data Sciences Int., St. Paul, MN, USA). A transmitter weighing about 3.5 g (Model TA-F20 for mice) and 7 g (Model TA-F40 for rats) was surgically implanted into the abdominal cavity of the animal. Animals were anesthetized with a dose of 60 mg/kg Ketamine (Fort Dodge Animal Health, Fort Dodge, Iowa, USA) mixed with 15 mg/kg domitor (Pfizer Animal Health, NY, NY, USA) injected ip. The fur over the ventral abdominal cavity was shaved and the area was cleaned with beta iodine first and a solution of 70% (v/v) alcohol. After the alcohol dried, a 2-cm longitudinal incision was made in mice and rats along the midline of the abdomen about 1 cm below the sternum, and the transmitter was inserted into the abdominal cavity. Before inserting the transmitter, it was soaked in 70% alcohol for 30 min and rinsed with saline. The incision was sutured with 4-0 silk thread, and the outer skin was closed with wound staples. Animals remained on a heating pad until they recovered fully from anesthesia. After surgery animals were housed singularly in cages with wood chip bedding and maintained at Ta of 22±1° and relative humidity of about 50%. Ten days for recovery was allowed before testing commenced. Cages were positioned on a receiver board, which decoded the temperature of the transmitter, and body temperature was collected automatically every 5 min and stored in a PC computer using the Data Quest IV software (Data Science, Inc., St. Paul, MN).
2.3. Experimental protocol for mice
The study consisted of several phases:
2.3.1. Dose-response Function for Nicotine
Mice were injected sc with 0.2, 0.4 and 0.8 mg/kg nicotine or an equal volume of saline following a cross-over design with random assignment. The intervals between injections were 3 days. Fifteen animals were used for this study.
2.3.2. Dose-response function for sazetidine-A
Mice were injected sc with a dose of 0.3, 1 and 3 mg/kg sazetidine-A or saline following a cross-over design with random assignment. Similar to the phase 1, the intervals between injections were 3 days.
2.3.3. Interaction of nicotine and sazetidine-A
Mice were injected sc with 0.4 mg/kg nicotine, 0.3 or 0.6 mg/kg sazetidine-A, mixture of 0.4 mg nicotine and 0.3 mg sazetidine-A, mixture of 0.4 mg/kg nicotine ± 0.6 mg/kg sazetidine-A or an equal volume of saline. The intervals between injections were at least 2 days. To expand the doses of nicotine and sazetidine-A, two weeks after completion of phase 3, the same mice were injected with a dose of 0.8 mg/kg nicotine, 0.6 mg/kg sazetidine-A, saline or a mixture of 0.8 mg/kg nicotine and 0.6 mg/kg sazetidine-A.
2.3.4. Interaction of DHβE and sazetidine-A
First a dose-response function for DHβE (0, 1, 3 and 6 mg/kg) was established using a new group of mice (n=9). Then, the interaction of sazetidine-A with DHβE was determined. Mice were injected with saline, DHβE (3 mg/kg), sazetidine-A (0.6 mg/kg) or a mixture of 3 mg/kg DHβE ± 0.6 mg/kg sazetidine-A and their body temperature was monitored telemetrically (Gordon et al., 2002).
2.3.5. Interaction of MLA and sazetidine-A
First, a dose response for MLA (1.5, 3 and 6 mg/kg) was established. Then, the interaction of MLA (6 mg/kg) with sazetidine-A (0.6 mg/kg) was investigated. All animals in each experiment received all treatments following a random order design with at least two days interval between injections (N=8).
2.4. Experimental protocol for rats
To compare the effect of sazetidine-A in rats with that of in mice, similar to experiment in mice a dose-response function for sazetidine-A was also established. After recovery from the telemetry surgery, and the establishment of a stable baseline for body temperature, rats were injected sc with 1, 3 or 6 mg/kg sazetidine-A or saline, following a cross-over design with random assignment and body temperature was monitored telemetrically. The intervals between injections were at least 3 days.
2.5. Drug preparation
Solutions of sazetidine-A, nicotine, DHβE and MLA were prepared weekly in sterilized isotonic saline and were injected sc. Volume of the drug injected was 10 ml/kg in mice and 1 ml/kg in rats. The doses of nicotine used were calculated as a function of the nicotine tartrate salt. Nicotine solutions were kept refrigerated in the dark between experiments. Sazetidine-A was synthesized at Research Triangle Institute (Research Triangle Park, NC, USA).
2.6. Statistical analysis of data
The temperature data were assessed with analysis of variance for repeated measures. The factors in the design were drug treatment including sazetidine-A, nicotine, dihydro-β-erythroidine (DHβE) and methyllycaconitine (MLA). Greenhouse-Geiser correction was used to protect against non-sphericity of the data. Planned comparisons of the treated groups to control were done using Fisher’s Least Significant Difference test.
3. Results
3.1. Sazetidine-A effects on body temperature in mice
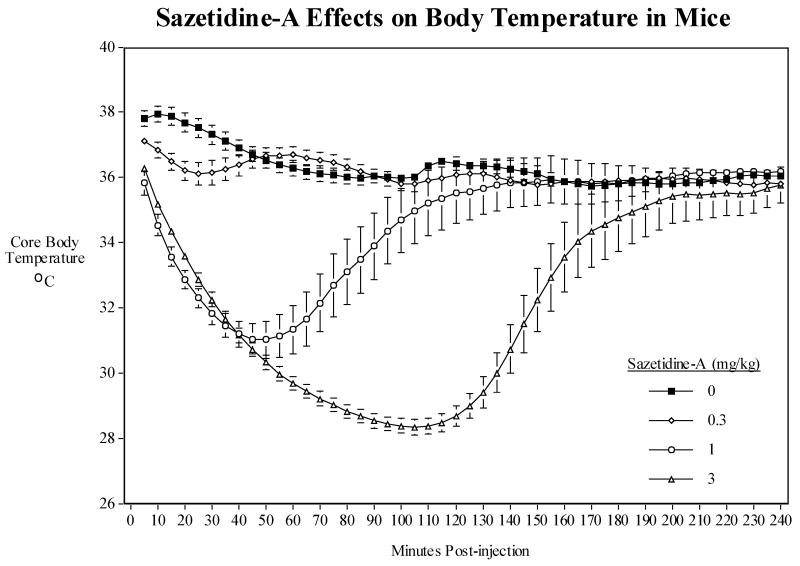
Acute sazetidine-A caused a pronounced and long-lasting hypothermia, after which the temperature of the subjects returned to normal (Fig. 1). The effect of sazetidine-A treatment on body temperature was significant lasting over ~ 3 hrs after injection (F(3,42)=39.55, P<0.0005). Sazetidine-A doses of 1 mg/kg (P<0.005) and 3 mg/kg (P<0.0005) caused a significant hypothermia relative to control averaged over the four-hour period after injection. There was also a significant sazetidine-A × time interaction ((F(141,1972)=21.28, P<0.0005) indicating a differential effect of sazetidine-A over the time course of measurement. The 0.3 mg/kg sazetidine-A dose caused a modest, though significant (P<0.05), hypothermia during the first 35 min. after injection. The dose of 1 mg/kg sazetidine-A produced a more pronounced (P<0.05-P<0.005) hypothermia that lasted ~2 hrs after injection, with the peak effect at ~ 60 min. The dose of 3 mg/kg sazetidine-A resulted in a marked (P<0.05-P<0.0005) and highly consistent hypothermia lasting for ~ 3 hrs after injection, with the peak effect at ~ 120 min. A few mice showed a prolonged hypothermia before returning to baseline temperature, with one mouse not returning to baseline temperature until more than seven hours with body temperature of about 29 °C after the acute treatment. Interestingly, gross observation of the animal’s behavior did not show any impairment or sedation during hypothermia.
Fig. 1.
Sazetidine-A dose-effect function (0.3-3 mg/kg) on core body temperature of mice over 4-hours post injection. The mean baseline temperature for the mice before drug dosing was 36.8±0.2°C. Data represent group mean values ± S.E.M. N=15/treatment.
3.2. Dose-effect function of nicotine
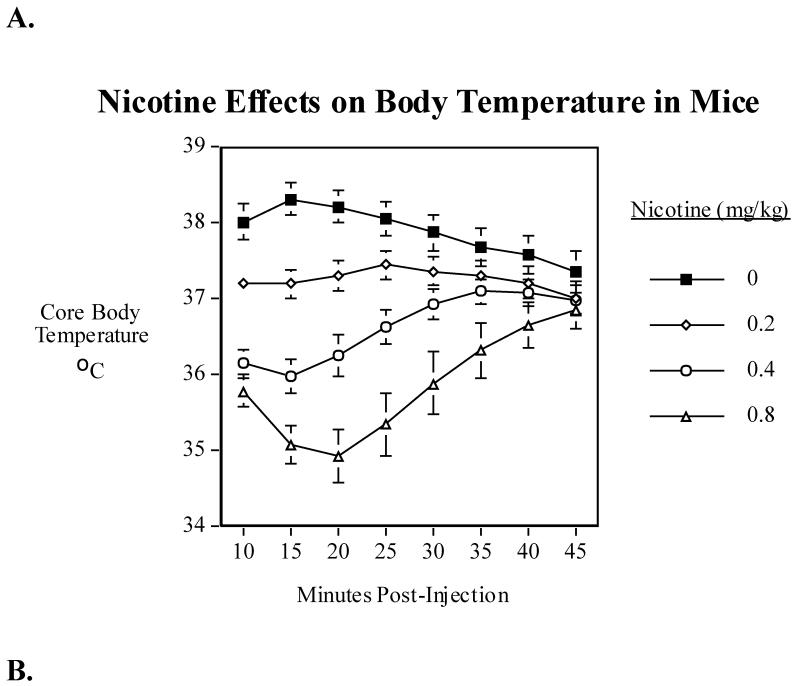
Acute administration of nicotine (0.4 and 0.8 mg/kg) caused a brief and small but significant (F(3,42)=20.94, P<0.0005) dose-related decrease in body temperature over a period of ~ 45 min (Fig. 2A).
Fig. 2.
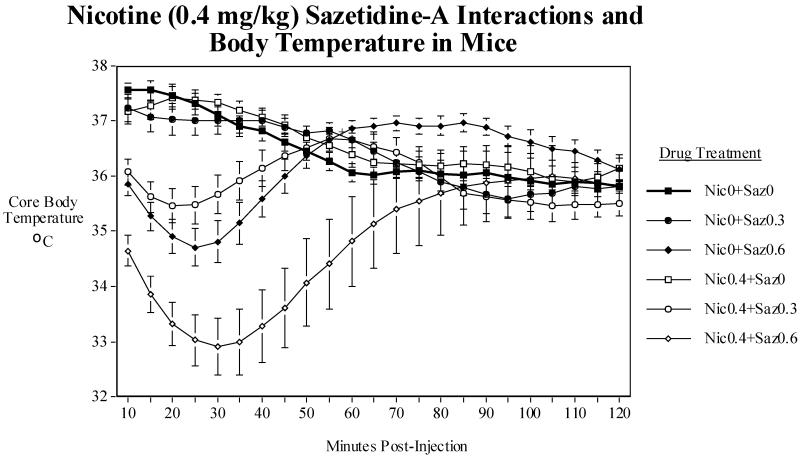
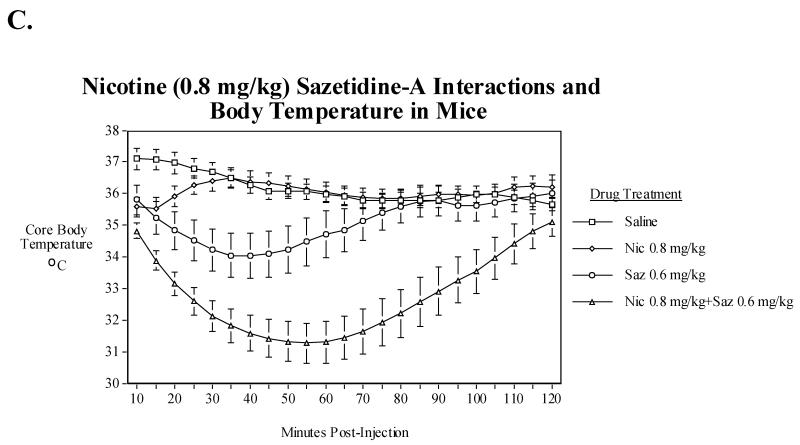
A) Effects of acute administration of nicotine (0.2, 0.4 and 0.8 mg/kg) on core body temperature in mice. Data represent means ± S.E.M. N=15. The mean baseline temperature for the mice before drug dosing was 37.1±0.1°C. B) Sazetidine-A (0.3 and 0.6 mg/kg) interactions with nicotine (0.4 mg/kg) and core body temperature in mice. Data represent means ± S.E.M. N=14. The mean baseline temperature for the mice before drug dosing was 37.0±0.1°C. C) Sazetidine-A (0.6 mg/kg) interactions with nicotine (0.8 mg/kg) and core body temperature in mice. Data represent means ± S.E.M. N=13. The mean baseline temperature for the mice before drug dosing was 36.7±0.2 °C.
3.3. Interactions of nicotine with sazetidine-A
The first study of the interactions of 0.3 and 0.6 mg/kg of sazetidine-A with 0.4 mg/kg of nicotine detected a significant interaction of nicotine and sazetidine-A (Fig. 2B). There were significant main effects of nicotine (F(1,13)=8.33, P<0.05) and sazetidine-A (F(2,26)=10.32, P<0.005) showing hypothermia. Dose comparisons showed that the higher (0.6 mg/kg) but not the lower (0.3 mg/kg) sazetidine-A dose caused a significant (P<0.005) average decrease in body temperature over the two hours after injection. The nicotine × sazetidine-A interaction was also significant (F(2,26)=4.54, P<0.05). Follow-up tests of the simple main effects showed that averaged over the two-hour post-injection period neither dose of sazetidine-A or 0.4 mg/kg of nicotine caused a significant hypothermia. However, at certain points they caused significant hypothermia. The combination of 0.4 mg/kg of nicotine and 0.6 mg/kg of sazetidine-A did cause a significant (P<0.005) hypothermia relative to 0.6 mg/kg of sazetidine-A alone (Fig. 2B). There was also a significant nicotine × sazetidine-A × time interaction (F(44,572)=3.76, P<0.0005). As shown in Fig. 2B, the time course analysis showed that both doses of sazetidine-A produced a significant hypothermia at specific time points. Both treatments caused significantly greater effects at specific time points when given with 0.4 mg/kg of nicotine which itself did not produce a significant hypothermia at any time point within the first two hours after injection. The lower sazetidine-A dose (0.3 mg/kg) when given alone caused slight though significant (P<0.05) hyperthermia at 55 and 60 min. post injection relative to saline control. Nicotine co-administration with 0.3 mg/kg of sazetidine-A produced a significantly (P<0.05) greater hypothermia than this dose of sazetidine-A alone during the period of 10-35 min. post-injection. The higher dose of 0.6 mg/kg of sazetidine-A caused a significant (P<0.05-0.0005) hypothermia from 10-45 min. post-injection and then a significant (P<0.05) rebound hyperthermia from 60-115 min. post-injection. Co-administration of 0.6 mg/kg of sazetidine-A with 0.4 mg/kg of nicotine caused a significantly (P<0.05-P<0.005) greater hypothermia than sazetidine-A alone between 10 and 100 min. post-injection.
In a follow-up study, sazetidine-A interactions with the higher dose of nicotine (0.8 mg/kg) were investigated (Fig. 2C). This dose of nicotine decreased body temperature significantly during the first 10 min. after injection. Sazetidine-A, at a dose of 0.6 mg/kg, decreased body temperature more and for a longer duration than nicotine. Co-administration of 0.6 mg/kg of sazetidine-A with 0.8 mg/kg of nicotine caused a significantly (P<0.005) greater hypothermia than this dose of sazetidine-A alone between 10 and 120 min. post-injection.
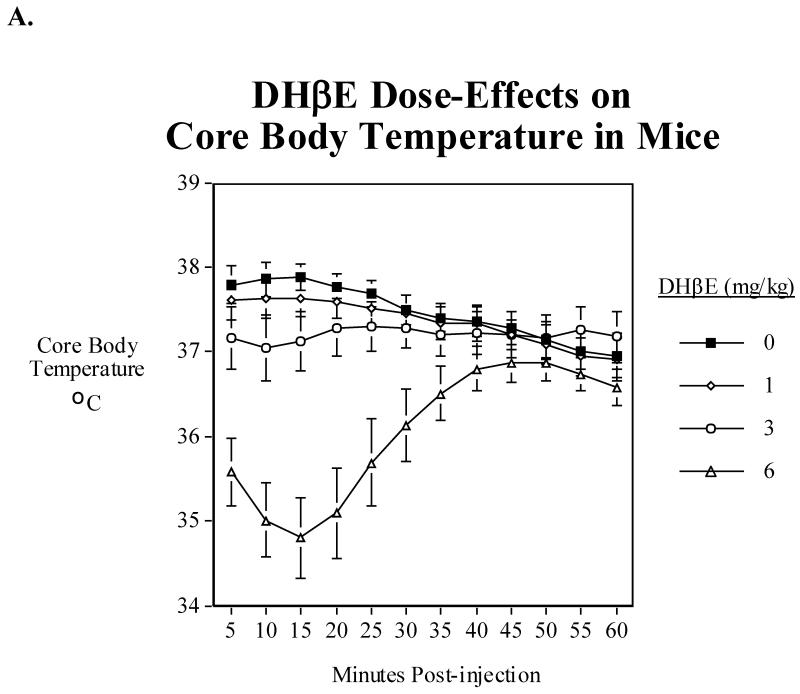
3.4. DHβE dose effect function
Nine drug-naïve mice were used to test the dose effect function of the α4β2 nicotinic antagonist DHβE on body temperature. Over the one hour period after injection there was a significant (P<0.0005) main effect of DHβE and a significant (P<0.0005) interaction of DHβE and time. Tests of the simple main effects of DHβE at each time point indicate a very modest but significant (P<0.05) hypothermia caused by 3 mg/kg of DHβE at 5-20 min. post injection (Fig. 3A). The higher 6 mg/kg dose caused a significant (P<0.05-P<0.0005) effect for 5-40 min. post-injection. The low dose of 1 mg/kg did not have a significant effect.
Fig. 3.
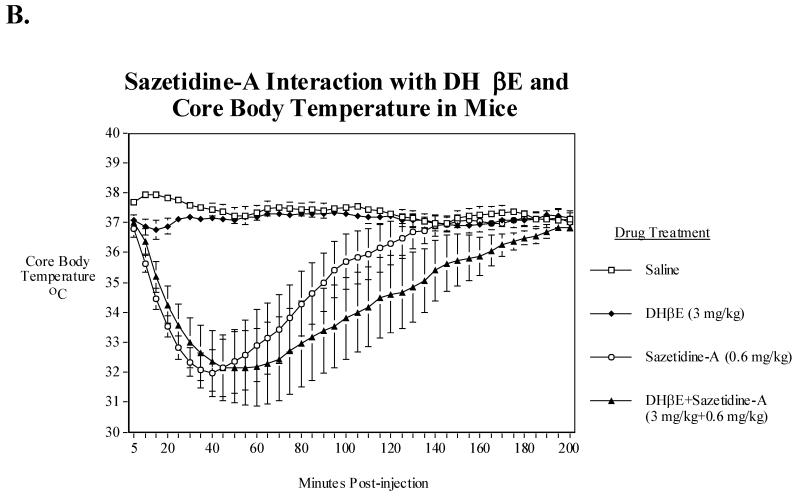
A) Effects of acute administration of DHβE (1, 3 and 6 mg/kg) on core body temperature in mice. Data represent means ± S.E.M. N=9. The mean baseline temperature for the mice before drug dosing was 37.2±0.2°C., B) Sazetidine-A (0.6 mg/kg) interactions with DHβE (3 mg/kg) and core body temperature in mice. Data represent means ± S.E.M. N=13. The mean baseline temperature for the mice before drug dosing was 37.2±0.1 °C.
3.5. Interactions of sazetidine-A with DHβE
Sazetidine-A at 0.6 mg/kg reduced body temperature significantly; while in contrast, the acute sc administration of 3 mg/kg DHβE produced only a brief and very modest hypothermia. In contrast, however, this dose of DHβE, when was given in combination with sazetidine-A significantly (P<0.05-0.01) potentiated the duration, but not the degree of sazetidine-A induced hypothermia (Fig. 3B).
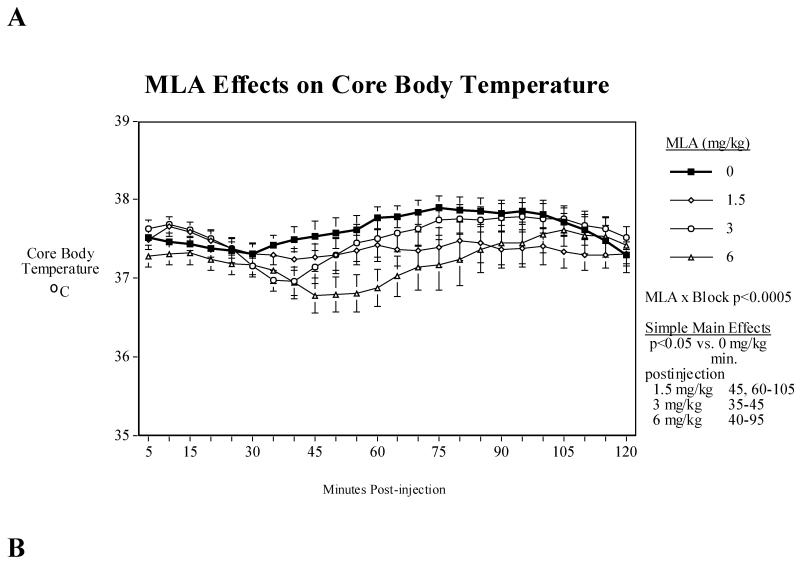
3.6. MLA dose-effect function
Three does of MLA (1.5, 3 and 6 mg/kg) were tested in 11 mice following a repeated measures counterbalanced design. There was a significant (F(69,690)=2.01, P<0.0005) interaction of MLA treatment × 5-minute block after injection over the two hours after testing that encompassed the MLA-induced hypothermia. Tests of the simple main effects showed that all three MLA doses caused significant hypothermia but with differing time courses. The highest MLA dose tested (6 mg/kg) caused a modest, though significant (P<0.05), hypothermia from 40-95 min. after injection. The intermediate MLA dose (3 mg/kg) caused a shorter (P<0.05) hypothermia from 35-45 min. after injection. Interestingly, the lowest MLA dose tested (1.5 mg/kg) caused a very small though long-lasting temperature reduction relative to control, which was initially seen as a significant (P<0.05) at 45 min. after injection, resumed being significant (P<0.05) at 60 min. after injection and persisted until 105 min. after injection All of the MLA effects on body temperature were quite small in magnitude (Fig. 4A).
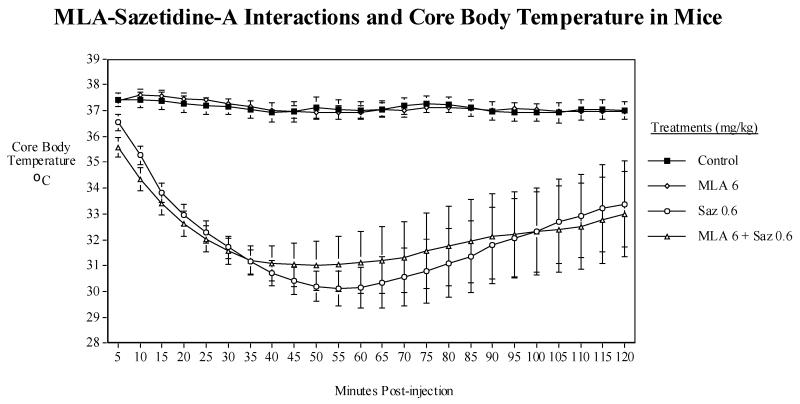
Fig. 4.
A). Dose-effect function of MLA on body temperature (mean±s.e.m.), B) Effects of acute administration of MLA (6 mg/kg) and sazetidine-A (0.6 mg/kg) on core body temperature in mice. Data represent means ± S.E.M. N=8. The mean baseline temperature for the mice before drug dosing was 37.0±0.1°C.
3.7. Interactions of MLA with sazetidine-A
Sazetidine-A (0.6 mg/kg), as previously seen, caused a significant (F(1,7)=64.20,<0.0005) hypothermia, with body temperature falling from 37± 0.1 to approximately 30 °C at 40 min. This hypothermic effect of sazetidine-A was not significantly affected by co-administration of MLA (6 mg/kg) (Fig. 4B).
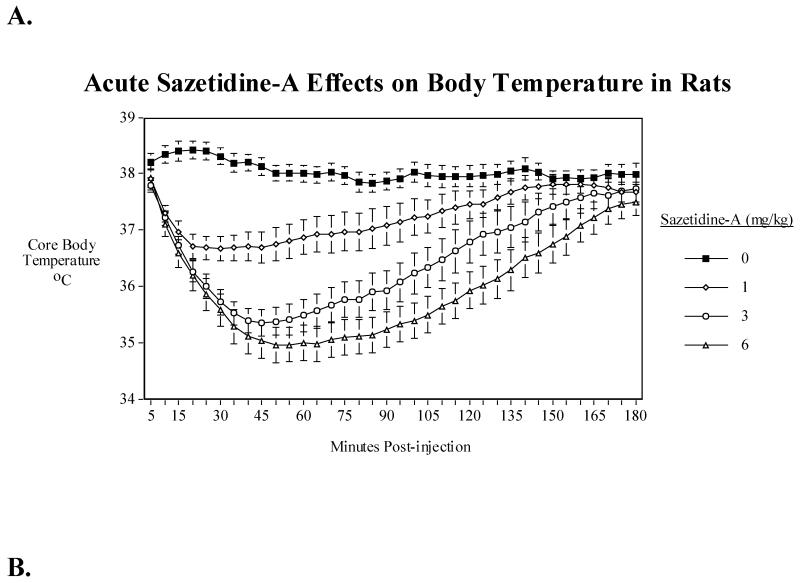
3.8. Sazetidine-A effects on body temperature in rats
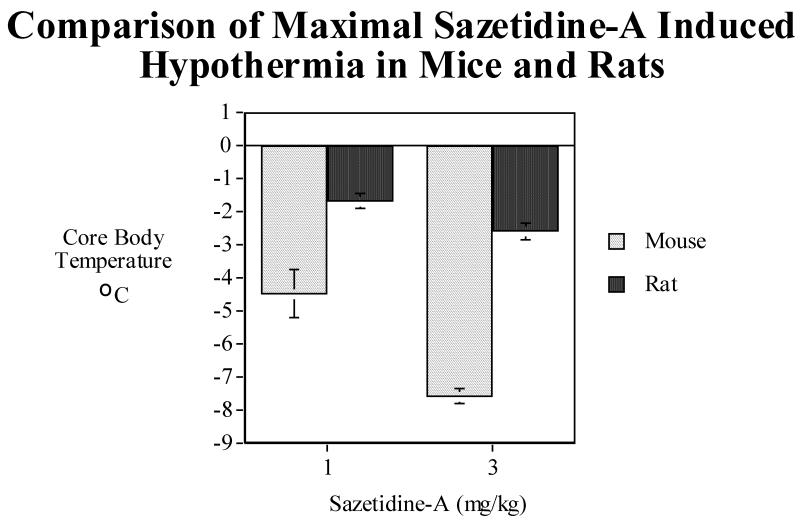
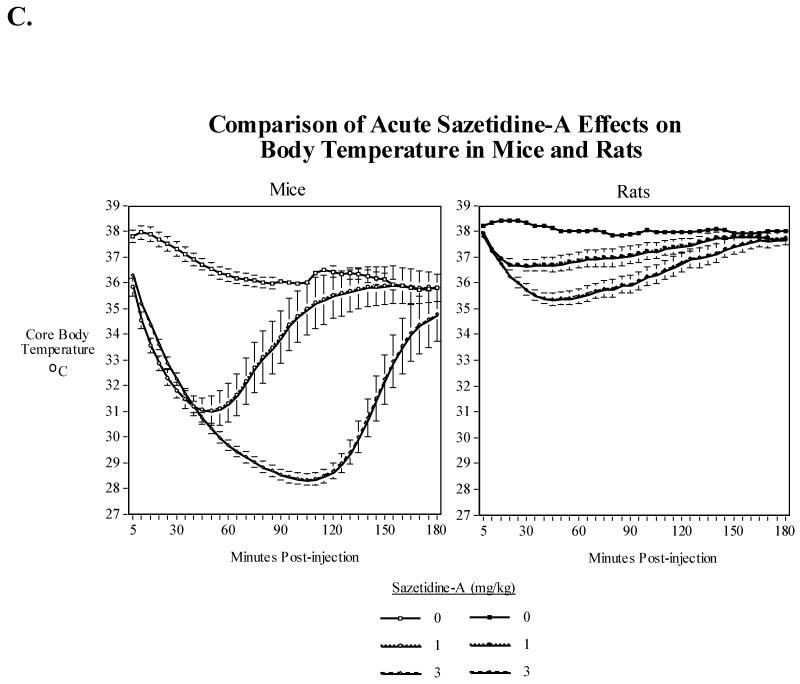
In rats, sazetidine-A also produced a hypothermic effect but to a much lesser extent than in mice. There was a significant main effect of sazetidine-A (F(3,39)=31.64, P<0.0005), with the 1 mg/kg (P<0.005), 3 mg/kg (P<0.0005) and 6 mg/kg (P<0.0005) all causing overall reductions in core body temperature over the first three hours after injection (Fig. 5A). There was also a significant (F(105, 1365)=11.34, P<0.0005) sazetidine-A × time interaction with a dose-related effect of greater and longer-lasting hypothermia from 1 to 3 to 6 mg/kg. Tests of the simple main effects of sazetidine-A at each time point showed that a slight though significant hypothermia was induced by 1 mg/kg for a little more than two hours while the higher doses extended the hypothermia out to three hours. The maximal extent of hypothermia was also greater with 3 and 6 mg/kg than with 1 mg/kg. The maximal extent of hypothermia caused by 1 and 3 mg/kg was much greater in mice than in rats at both doses (Fig. 5B). Fig. 5C shows the relative time course of 1 and 3 mg/kg sazetidine-A effects on body temperature in mice and rats in terms of both extent and duration of the effect.
Fig. 5.
A) Effects of acute administration of sazetidine-A (1, 3 and 6 mg/kg) on core body temperature in rats. The mean baseline temperature for the rats before drug dosing was 38.1±0.1°C. B) Comparison of maximal sazetidine-A induced-hypothermia in rats and mice. C) Comparison of the time course of sazetidine-A induced-hypothermia in rats and mice over three hours. Data represent means ± S.E.M. N= 15 mice and 14 rats.
4. Discussion
Sazetidine-A produce several interesting and potentially important pharmacological effects, including analgesic effects (Cucchiaro et al., 2008); reduction of nicotine and alcohol self-administration (Levin et al., 2010; Rezvani et al., 2010), and induction of behaviors consistent with anxiolytic and possibly antidepressant effects (Caldarone et al., 2011; Kozikowski et al., 2009; Turner et al., 2011). In addition, it improves performance in tests of attention in rats (Rezvani et al., 2011). Here we show for the first time that sazetidine-A when given acutely significantly and dose-dependently lowers core body temperature in mice (Fig. 1) and rats (Fig. 5). The magnitude and duration of the hypothermic effect of sazetidine-A was significantly greater in mice than in rats (Fig. 5B and 5C). As shown previously (Overstreet, 1995; Overstreet et al., 1998; Sack et al., 2005; Zarrindast et al., 2001), an acute systemic administration of nicotine also significantly reduced core body temperature in mice and rats; however, the effects of nicotine at tolerated doses are less pronounced and of shorter duration than those of sazetidine-A (compare Fig.1A and 2A). It has been suggested that nicotine produces hypothermia by interacting with presynaptic nicotinic receptors to release acetylcholine upon muscarinic receptors (Gordon, 1994; Gordon et al., 2002; Overstreet et al., 1998). In addition, β4 nicotinic null mice have been shown to have a lower baseline body temperature and reduced nicotine-induced hypothermia suggesting the involvement of receptors containing the β4 nicotinic subunit in the core body temperature homeostasis and nicotine-induced hypothermia in mice (Sack et al., 2005).
In the current study nicotine and sazetidine-A given in combination appeared to produce a a profound hypothermia that lasted for more than an hour in mice (Fig. 2B and 2C). This effect might be the result of the desensitization β2* nicotinic receptors by both sazetidine-A (Xiao et al., 2006) and nicotine. In the nicotine dose-effect study, 0.4 mg/kg of nicotine moderately but significantly reduced body temperature, while in the nicotine-sazetidine-A interaction study it did not. This inconsistency may have been due to carryover effects in the repeated measures counterbalanced design studies used. Interestingly, the rebound hyperthermic effect of 0.6 mg/kg of sazetidine-A was seen between 50 and 120 min. post-injection when sazetidine-A was given intermittently with a lower dose of 0.4 mg/kg nicotine (Fig. 2B). However, no rebound hyperthermic effect with 0.6 mg/kg of sazetidine-A was seen when given intermittently with a higher dose of nicotine (0.8 mg/kg) or with intermittent doses of the DHβE or when sazetidine-A was given alone. The intermittent dosing of low doses of nicotine may have had some carryover effect that potentiated this rebound. The greater hypothermic effect of 0.6 mg/kg of sazetidine-A in the experiment shown in Fig. 3B compared to Fig. 2B and 2C may also be due to carryover effects from intermittent nicotine treatment in the experiments shown in Fig. 2B and 2C vs. DHβE in Fig. 3B. Contrary to the mice used in experiments 1 and 2 (Fig. 1 and 2), mice used in DHβE experiment (Fig. 3) did not have a history of nicotine exposure and were drug naïve before being exposed to DHβE.
In addition to its ability to cause long lasting desensitization of α4β2 receptors, sazetidine-A showed agonist activity at the receptors (Zwart et al., 2008). It is very interesting that between the two stoichiometries of the α4β2 receptors, sazetidine-A showed full agonist activity at (α4)2(β2)3 receptors but nearly-none agonist activity at (α4)3(β2)2 receptors (Carbone et al., 2009). This raises a possibility that agonist activities at (α4)2(β2)3 receptors may mediate the hypothermic effects of sazetidine-A. However, DHβE, a selective α4β2 nicotinic receptor antagonist, by itself caused a significant drop in body temperature in mice (Fig. 3A), and when given in combination with sazetidine-A prolonged the hypothermic effects of sazetidine-A (Fig. 3B). It is important to note that DHβE is a very potent antagonist of (α4)2(β2)3 receptors with low nM IC50 values (Carbone et al., 2009). The fact that DHβE did not diminish the sazetidine-A-induced hypothermia but instead prolonged it suggests that it is not the agonist activity of sazetidine-A that is responsible for the hypothermia; rather, this experiment further supports desensitization of α4β2 nicotinic receptors as the main mechanism. The hypothermic effect of nicotine also could be associated with the desensitization of α4β2* receptors, because this type of nicotinic receptors is known to respond to even low concentrations of nicotine with a prolonged desensitization (Paradiso and Steinbach, 2003).
To investigate the role of α7 nicotinic receptors in the regulation of body temperature, the acute effect of the specific α7 nicotinic receptor antagonist MLA, by itself and in combination with sazetidine-A was also investigated. Acute administration of MLA by itself caused a very modest but statistically significant reduction in body temperature (Fig. 4A). Interestingly, MLA at 6 mg/kg when given in combination with the highest dose of sazetidine-A (0.6 mg/kg), which significantly reduces body temperature, failed to diminish the hypothermic effect of sazetidine-A (Fig. 4B), suggesting the specificity of sazetidine-A to β2* nicotinic receptors.
Based on the magnitude and duration of the hypothermic response to sazetidine-A, it appears that mice are at least twice as sensitive as rats to this compound. This is consistent with previous studies of the effects of drugs and toxicants on body temperature in mice and rats. However, it is important to note that thermoregulatory stability of mice to drugs and toxicants is typically more labile than rats when tested at a given ambient temperature (Gordon, 2005). As one example, the hypothermic response to cholinesterase inhibitors is much greater in mice compared to larger mammals (Gordon, 1994). The greater hypothermic response in mice may be attributed to their smaller size and, hence, larger ratio of surface area to body mass; thus, mice will cool much faster than rats when given drugs, anesthetics, or toxic chemicals that impair metabolic thermogenesis (Gordon, 2001, Gordon, 1995 #13808; Gordon et al., 1998). All tests in the current study were performed at standard room temperature of 22 ± 1 °C, which is approximately 6-8 °C below the thermoneutral zone for mice and rats (Gordon, 1993). Thus, rats and especially mice may be experiencing cold stress under these ambient conditions plus a drug-induced reduction in metabolic rate will lead to marked hypothermia. Evaluating the thermoregulatory effects of sazetidine-A at a thermoneutral ambient temperature (ca. 30 °C) in both mice and rats would allow us to better compare the interspecies sensitivity of this compound.
In summary, sazetidine-A by desensitization of α4β2* nicotinic receptors caused a significant hypothermia in mice and rats, with mice being much more significantly affected. Our results suggest that α4β2* type of nicotinic receptors is involved in the regulation of the body temperature in rodents and compounds similar to sazetidine-A, which desensitize α4β2* nicotinic receptors, can reduce body temperature.
Acknowledgement
This project was supported by NIH Grant DA027990.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Caldarone BJ, Wang D, Paterson NE, Manzano M, Fedolak A, Cavino K, Kwan M, Hanania T, Chellappan SK, Kozikowsk i.A.P., Olivier B, Picciotto MR, Ghavami A. Dissociation between duration of action in the forced swim test in mice and nicotinic acetylcholine receptor occupancy with sazetidine, varenicline, and 5-I-A85380. Psychopharmacology. 2011;217:199–210. doi: 10.1007/s00213-011-2271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. Pentameric concatenated (a4)2(b2)3 and (a4)3(b2)2 nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br J Pharmacol. 2009;156:970–981. doi: 10.1111/j.1476-5381.2008.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiaro G, Xiao Y, Gonzalez-Sulser A, Kellar KJ. Analgesic effects of Sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology. 2008;109:512–519. doi: 10.1097/ALN.0b013e3181834490. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha4 and beta2 subunits and is up-regulated by chronic nicotine treatment. Molecular Pharmacology. 1992;41:31–37. [PubMed] [Google Scholar]
- Gordon CJ. Temperature regulation in Labrador rodents. Cambridge University Press; New York: 1993. [Google Scholar]
- Gordon CJ. Thermoregulation in laboratory mammals and human exposed to anticholinestrase agents. Neurotoxicology and Teratology. 1994;16:427–453. doi: 10.1016/0892-0362(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg. Med. J. 2001;8:81–89. doi: 10.1136/emj.18.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ. Temperature and toxicology: An integrative, comparative and environmental approach. Taylor & Francis Group; New York: 2005. [Google Scholar]
- Gordon CJ, Mohler FS, Watkinson WP, A.H. R. Temperature regulation in laboratory mammals following acute toxic insult. Toxicology. 1998;53:161–178. doi: 10.1016/0300-483x(88)90211-9. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Rowse PJ, Yang YL. Effects of repeated nicotine exposure on core temperature and motor activity in male and female rats. Journal of Thermal Biology. 2002;27:485–492. [Google Scholar]
- Hall GH, Myers RD. Temperature changes produced by nicotine injected into the hypothalamus of the conscious monkey. Brain Res. 1972;37:241–251. doi: 10.1016/0006-8993(72)90669-5. [DOI] [PubMed] [Google Scholar]
- Knox GV, Campbell C, Lomax P. The effects of acetylcholine and nicotine on unit activity in the hypothalamic thermoregulatory centers of the rat. Brain Res. 1973;51:215–223. doi: 10.1016/0006-8993(73)90374-0. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Eaton JB, Bajjuri KM, Chellappan SK, Chen Y, Karadi S, He R, Caldarone B, Manzano M, Yuen PW, Lukas RJ. Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression. Chem Med Chem. 2009;4:1279–1291. doi: 10.1002/cmdc.200900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells D, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar K. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist reduces nicotine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Liu J, Eaton JB, Caldarone B, Lukas RJ, Kozikowski AP. Chemistry and pharmacological characterization of novel nitrogen analogues of AMOP-H-OH (Sazetidine-A, 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol) as alpha4beta2-nicotinic acetylcholine receptor-selective partial agonists. Journal of Medicinal Chemistry. 2010;53:6973–6985. doi: 10.1021/jm100765u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Miner L, Burch JB, Fulker DW, Collins AC. A diallel analysis of nicotine-induced hypothermia. Pharmacology. Biochemistry and Behavior. 1984;21:953–959. doi: 10.1016/s0091-3057(84)80079-9. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol. Biochem. Behav. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Sundwall A. Pharmacodynamic effects of nicotine and acetylcholine biosynthesis in mouse brain. Acta Pharmacol. Toxicol. 1983;52:341–347. doi: 10.1111/j.1600-0773.1983.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Overstreet D,H. Differential effects of nicotine in inbred and selectively bred rodents. Behav. Genet. 1995;25:179–185. doi: 10.1007/BF02196926. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Daws LC, Schiller GD, Orbach J, Janowsky DS. Cholinergic/serotonergic interactions in hypothermia: implications for rat models of depression. Pharmacology, Biochemistry and Behavior. 1998;59:777–785. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
- Paradiso KG, Steinbach JH. Nicotine is highly effective at producing desensitization of rat alpha4beta2 neuronal nicotinic receptors. J Physiol (Lond) 2003;15:857–871. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Sexton H, Xiao X, Brown ML, Paige MA, McDowell BE, Kellar KL, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent reverses dizocilpine and scopolamine-induced attentional impairments in rats. Psychopharmacology. 2011;215:621–630. doi: 10.1007/s00213-010-2161-8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Li TK, Lumeng L, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Rose JE, Levin ED. Sazetidine-A, a Selective α4β2 nicotinic acetylcholine receptor desensitizing agent and partial agonist reduces both alcohol and nicotine self-administration in selectively-bred alcohol preferring (P) rats. Psychopharmacology. 2010;211:161–174. doi: 10.1007/s00213-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Anand R, LaHoste GJ. Chronic menthol attenuates the effect of nicotine on body temperature in adolescent rats. Nicotine Tob. Res. 2008;10:1753–1759. doi: 10.1080/14622200802443734. [DOI] [PubMed] [Google Scholar]
- Sack R, Gochberg-Sarver A, Rozovsky U, Kedmi M, Rosner S, Orr-Urtreger A. Lower core body temperature and attenuated nicotine-induced hypothermic response in mice lacking the beta4 neuronal nicotinic acetylcholine receptor subunit. Brain Research Bulletin. 2005;66:30–36. doi: 10.1016/j.brainresbull.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Salminen O, Ahtee L. The effects of acute nicotine on the body temperature and striatal dopamine metabolism of mice during chronic nicotine infusion. Neurosci Lett. 2000;284:37–40. doi: 10.1016/s0304-3940(00)00983-6. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob. Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Yasuda RP, Sahibzada N, Horton L, DiPietro JR, Iwueze AF, Paige MA, McDowell B, Brown ML, Wolfe B,B, Kellar KJ. Pharmacological properties of sazetidine-A, a selective ligand of α4β2 nicotinic acetylcholine receptor; Society for Neuroscience 38th Annual Meeting; Washington, DC. 2008. [Google Scholar]
- Zarrindast MR, Barghi-Lashkari S, Shafizadeh M. The possible cross-tolerance between morphine- and nicotine-induced hypothermia in mice. Pharmacology, Biochemistry and Behavior. 2001;68:283–289. doi: 10.1016/s0091-3057(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]