Abstract
Social isolation (SI) is increasingly recognized as a risk factor for stroke. Individuals with lack of social support systems have an increased incidence of stroke, poorer recovery, and greater functional decline after injury compared to individuals with social support. Attesting to the importance of social factors in stroke outcome is that these same effects can be reproducibly demonstrated in animals; social interaction improves behavioral deficits and reduces damage after experimental stroke, whereas SI enhances injury. The mechanism by which SI exacerbates injury is unclear. We investigated the role of nuclear factor-kappaB (NF-κB) signaling in male mice that were pair housed (PH) with an ovariectomized female prior to random assignment into continued PH or SI for 7 days prior to middle cerebral artery occlusion. The effects of SI on infarct volume and functional recovery were assessed at 72 h post-stroke. Nuclear NF-κB levels and activity were assessed by Western blot and transcriptional assays. SI significantly exacerbated infarct size in both male and female mice compared to PH mice. SI mice had delayed functional recovery compared to PH mice. An elevation of systemic IL-6 levels, increased nuclear NF-κB transcriptional activity, and enhanced nuclear translocation of NF-κB was seen in SI stroke animals. Interference with NF-κB signaling using either a pharmacological inhibitor or genetically engineered NF-κB p50 knockout mice abolished the detrimental effects of SI on both infarct size and functional recovery. This suggests that NF-κB mediates the detrimental effects of SI.
Keywords: Pair housing, Isolation, Middle cerebral artery occlusion, NF-kappaB, Neuroinflammation
Introduction
Loneliness has dramatic physiological and psychological consequences on physical and mental health [3, 15, 16]. Existing and emerging data from pre-clinical and clinical studies suggests that SI is an important contributing risk factor to mortality and morbidity in patients with established cardiovascular and cerebrovascular disorders [14– 16, 19]. Conversely, high neighborhood cohesion and social interactions are associated with reduced mortality rates compared to isolated individuals [4]. The mechanism involved in the detrimental effects of social isolation on clinical disease expression remains unknown [16, 53, 57]. Considerable evidence from experimental studies has shown that stroke damage and recovery are strongly influenced by SI [14, 19, 46, 53]. In mice, SI leads to enhanced histological damage and mortality, and PH ameliorates these effects.
Stroke is the primary cause of adult disability and a leading cause of death [45]. Advances in knowledge and treatment of risk factors, increasingly rapid acute stroke care, public awareness, and the aging of the population will lead to a growing number of stroke survivors in our communities [8, 45]. Stroke survivors are at increased risk of for SI, which contributes to both poor outcomes and high recurrent stroke rates [2, 4]. SI also is an increasingly accepted risk factor for stroke-related mortality [1, 2, 4] and the magnitude of its effect on mortality equals or surpasses the effects of known risk factors like obesity, hypertension and lack of physical activity [1, 2, 14, 16]. Behavioral disorders such as depression and anxiety increase stroke risk, and SI is associated with depression and anxiety [1, 44]. Currently available interventions for acute stroke are limited to thrombolysis and other reper-fusion therapies, which have short therapeutic time windows [25, 41]. As a result there is a compelling urgency to accelerate efforts to identify potential targets that could lead to improvements in stroke outcome.
Activation of the innate immune system and enhanced production of pro-inflammatory cytokines is a widely recognized response to ischemic stroke [41]. Recent work has implicated inflammatory processes as key mediators of the effects of social environment on stroke outcome [19, 20, 54]. Clinical studies have demonstrated an increase in C-reactive protein (CRP), a biomarker of stroke risk [23], in healthy populations with poor social cohesion [9, 52]. In experimental studies, SI mice demonstrated greater serum CRP, IL-6, and IL-1β levels compared to PH mice [6, 19]. NF-κB is a well-described upstream transcription factor that regulates cytokine signaling, and has an important role in the regulation of the innate immune response [35]. NF-κB refers to the complete p50/p65 heterodimer complex, which is translocated to the nucleus from the cytoplasm in response to injury and can regulate both pro-inflammatory and anti-inflammatory pathways [10, 39–41, 47, 50, 54]. Although p65 deletion (KO) is embryonic lethal, p50 KO mice develop normally with only a mild phenotype of reduced B-cell proliferation in response to liposaccharides [10]. These mice have a targeted disruption of exon 6 which encodes the p105 precursor of the p50 subunit, producing a truncated polypeptide that cannot bind with DNA, dimerize with itself or with other kappaB binding motifs [50]. P50 KO mice might thus offer a potential mechanistic tool to investigate for the role of NF-κB in SI-mediated neuroinflammation after ischemic stroke.
It has been shown that SI can enhance ischemic damage and delay functional recovery in both male and female mice, but the mechanisms involved in this effect are largely unknown. The goals of this study were to determine the role of NF-κB in the detrimental effects of SI. This was investigated by examining NF-κB nuclear translocation, assessing NF-κB transcription levels, and determining levels of IL-6, a downstream target of NF-κB. To directly assess the mechanistic contribution of NF-κB to SI-induced damage, the effect of loss of NF-κB on SI-induced ischemic damage was assessed.
Materials and methods
Experimental animals
All animal protocols were approved by The University's Institutional Animal Care and Use Committee at The University of Connecticut Health Center and were performed in accordance with National Institutes of Health guidelines. Six-week-old C57Bl/6 mice were purchased from Charles River laboratories (Wilmington, MA); NF-κB1 KO mice (006097-B6.Cg-Nfkb1tm1Bal/J) were purchased from JAX laboratories. These mice have a complete deletion of the NF-κB p50 heterodimer generated by a targeting vector containing the PGK-neo resistance gene in opposite transcriptional orientation designed to disrupt exon 6 of the endogenous gene [50]. Mice have been backcrossed to C57BL/6JIco for 12 generations. All mice were maintained in a temperature and humidity controlled vivarium with ad libitum access to food and water for 2 weeks prior to any experimental manipulations.
Housing conditions
Experimental animals were screened for baseline laterality deficits and locomotor activity and pair housed (male with an ovariectomized female) for 2 weeks. Mice were then randomly assigned to either individual housing (SI) or pair housing (PH) in standard mouse cages (11”L, 6”W, 6”H) with a 12-hour light/dark schedule. The assigned housing condition was maintained for 7 days prior to middle cerebral artery occlusion (MCAO) and throughout the reperfusion period until being killed [19, 20, 57]. Animals had ad libitum access to chow and water. All animals were fed with wet mash for 72 h after stroke.
Ovariectomy
For ovariectomy (Ovx), female mice weighing ~18–20 g were anesthetized with isoflurane and the ovaries were surgically removed 10 days before assigning them into their allocated housing. Thus, the female had received an additional exposure to anesthesia. Uterine weights were measured at the time of killing [29] to confirm loss of estrogenic effects.
Middle cerebral artery occlusion
Cerebral ischemia was induced by 90 min of reversible MCAO under isoflurane anesthesia, as described previously [26, 32, 56]. In brief, a midline ventral neck incision was made, and unilateral right MCAO was performed by advancing a 6.0 silicone-coated nylon monofilament (Doccol Corporation, CA) into the internal carotid artery 6 mm from the internal carotid–pterygopalatine artery bifurcation via an external carotid artery stump. Rectal temperatures were monitored with a temperature control system (Fine science tools, Canada) and temperature was maintained with an automatic heating pad at ~37 °C during surgery and ischemia. Cerebral blood flow measurements by laser Doppler flowmetry (DRT 4/Moor Instruments Ltd, Devon, UK) confirmed ischemic occlusion (reduction to 85 % of baseline) during MCAO and restoration of blood flow during reperfusion. Surgical controls are used for molecular analysis, a sham surgery in which the suture was not advanced into the internal carotid artery (controls). All mice are allowed to emerge from anesthesia after the initial suture advancement into the MCA and placed back into their home cage until re-anesthetized for reperfusion. This allows for the assessment of intra-ischemic behavioral deficits to confirm successful suture placement.
Behavioral scores
Neurological deficit scores (NDS) were obtained during the intra-ischemic period and at 72 h post-stroke. Our standard scoring system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling as described previously [26, 34, 56].
Open field
All the mice were acclimatized to the testing room for 1 h before the beginning of the test. Each testing session was 20 min long. Testing was performed during the light phase of the circadian cycle, between 9:00 am and 12:00 pm under normal fluorescent room lights. For testing, mice were individually placed in the open field chamber (15” × 15”) equipped with 16 infrared beam emitting LEDs on each side. The total number of beam breaks was automatically collected by a computer-operated PAS Open Field system (San Diego Instruments, San Diego, CA). The 3 LEDs on each corner of arena were set as periphery and the 10 center LEDs record the beam interceptions in center. Beam breaks in the center of the chamber were analyzed as a measure of anxiety-like behavior (thigmotaxis test); this is presented as percentage [(beam breaks in center/total beam breaks) × 100]. The open field chambers were cleaned after each individual test session using 70 % ethanol [34].
Cylinder test
The cylinder test was used to assess asymmetry in forelimb usage as described previously [28]. For this each mouse was individually placed in a transparent plexiglass cylinder of 9 cm diameter and 15 cm height during the test. After the mouse was put into the cylinder, forelimb use of the first contact against the cylinder wall after rearing and during lateral exploration was analyzed by the following criteria: a total of 20 limb placements on cylinder wall were recorded during the 10-min test session. A mirror was placed behind the cylinder with an angle to enable the rater to view forelimb movements when the mouse was turned to other side. The final score = (nonimpaired forelimb use (right) - impaired forelimb use (left))/(nonimpaired forelimb use + impaired forelimb use + both limbs movement).
Infarct analysis
TTC staining/infarct analysis
After 72 h of reperfusion both PH and SI WT mice were euthanized by cervical dislocation, brains were collected and frozen at –20 °C for 5 min to harden the tissue for subsequent slicing, and then cut into five 2-mm coronal sections and stained with 1.5 % 2,3,5-triphenyltetrazolium chloride (TTC) for 8 min at 38 °C. Slices were formalinfixed (4 %), images were obtained and infarct volumes analyzed using Sigma Scan Pro software as previously described [26, 31, 56]. The final infarct volumes are presented as a percentage of the volume of the contralateral structure (with correction for edema) as in [26, 31, 56].
Cresyl violet staining for infarct analysis
After 72 h of reperfusion NF-κB1 KO mice in both housing conditions were deeply anesthetized with pentobarbital, then transcardially perfused with ice-cold heparinized (0.1 %) phosphate buffered saline (1× PBS) followed by 4 % paraformaldehyde for 15 min, brains were extracted and fixed for 4 h in 4 % paraformaldehyde and were then cryoprotected in 30 % sucrose for overnight, 30-μm sections were cut by using a microtome, sections were mounted on Fisherbrand Superfrost Plus charged slides (Fisher scientific) and then stained with cresyl violet as in [28]. Infarct volumes were quantified from digitalized section images using Sigma Scan Pro software as previously described [28, 34]. The final infarct volumes were presented as percentage volume (percentage of contralateral structures with correction for edema). TTC and cresyl violet assessments of infarct size are well correlated in this model [55]
Immunohistochemistry procedures
To perform immunohistochemistry, we used 30-μm sections obtained at 72 h of reperfusion. Slices were slide mounted and incubated in blocking solution followed by microwave irradiation for 5 min in a 0.1 M, pH 6 citrate buffer solution. NF-κB translocation was visualized by co-labeling with NF-κB, NeuN and DAPI. Sections were incubated overnight with mouse anti-NeuN (1:200, Milli-pore, Billerica, MA), rabbit anti-NF-κB (1:250, Abcam, Cambridge, MA), and subsequently incubated for 60 min with fluorescein-conjugated anti-mouse and rhodamineconjugated anti-rabbit secondary antibodies. The slides were then dipped in DAPI solution (1:1,000) for 5 min and co-localization examined. To examine apoptotic cell death following MCAO an in situ cell death detection kit, TUNEL immunofluorescence assay (Roche Applied Science, Indianapolis, IN) was performed as per manufacturer's instructions. To assess the glial scar, sections were incubated overnight in rabbit GFAP primary antibody (1:200, DAKO, Carpinteria, CA). The sections were subsequently incubated in fluorescein-conjugated anti-rabbit secondary antibody and visualized utilizing an inverted light Zeiss axiovert fluorescence microscope as in [34]. Five sections from each brain were visualized at 20× magnification at the core/penumbra junction, n = 4/group SI versus PH.
Subcellular fractionation
Samples were obtained from separate cohorts of animals at 6 h post-stroke by rapidly removing the brains and flash freezing in 2-methyl butane on dry ice and stored at –80 °C. Samples were homogenized using dounce homogenizers with cold lysis solution (10 mmol/L Tris– HCl, pH 7.5; 5 mmol/L MgCl2; 0.1 mmol/L EDTA; 1.5 mmol/L CaCl2; 0.25 mmol/L sucrose; 1 mol/L DDT; 10 % Triton X-100; 1:50 protease inhibitor). Homogenates were centrifuged at 800 g for 10 min at 4 °C. The pellet contained the nuclear fraction; while supernatant contained cytosolic and mitochondrial fractions. The pellet was re-suspended in lysis buffer and run through a sucrose gradient composed of 1.8 and 2.3 mol/L sucrose with ultracentrifugation at 30,000 g for 45 min. The extracted pellet was transferred into nuclei pure storage buffer (Sigma-Aldrich) and centrifuged at 2,300 rpm for 10 min. The nuclear pellet was resolved with extraction buffer (Sigma-Aldrich), sonicated for 10 s three times, and stored at –80 °C as described in [29]. The nuclear samples were utilized for Western blots and transcription factor assays. Each sample point reflects pooled samples (2 brains/sample).
Western blots
The protein concentration of the fractionated nuclear sample was determined by BCA Protein Assay Kit (Thermo Fisher Scientific Inc) to achieve the equal loading of 10 μg/well and subjected to Western blotting as previously described [29]. Sample proteins were resolved on 4–15 % SDS electrophoresis gels and transferred to a polyvinylidene difluoride membrane. NF-κB protein levels were detected using antibodies (1:200; abcam) and histone H3 (1:4,000; Sigma) was used as loading control for nuclear fraction. All blots were blocked with 5 % milk and incubated overnight in primary antibodies at 4 °C in Tris-buffered saline containing 4 % bovine serum albumin and 0.1 % Tween 20. Secondary antibodies (goat antirabbit IgG 1:5,000 for NF-κB, donkey antigoat IgG 1:1,000 for his-tone; Santa Cruz) were diluted, and ECL detection kit (Amersham Biosciences) was used for signal detection. The densitometry of Western blotting images was performed with computer software (Adobe).
NF-κB transcription factor assay
Nuclear extracts from 6 h post-stroke/sham brain samples were also analyzed for NF-κB transcription activity using non-radioactive NF-κB p65 transcription activity colori-metric assay kit (Millipore, MA). Assay was performed as per manufacturer's instructions. Results were obtained by reading the plate in a spectrophotometric plate reader as optical density (OD) at 450 nm.
ELISA for IL-6 levels
An additional cohort of mice was killed at 24 h post-stroke and interleukin-6 (IL-6) levels were examined. Serum was collected at 24 h after stroke from both male and female mice and an enzyme linked immune absorbent (ELISA) assay (eBiosciences, San Diego, CA) was utilized to assess serum IL-6 levels as per manufacturer's instructions.
PDTC treatment
Pyrrolidine dithiocarbamate ammonium salt was purchased from Sigma-Aldrich. PDTC was dissolved in saline (vehicle). An acute dose of PDTC was injected at 2 and 12 h after the onset of ischemia (200 mg/kg; final volume of 200 μl/20 g body weight injected i.p.), a dose determined to inhibit NF-κB nuclear translocation in earlier studies [41, 42]. Control animals were treated with vehicle.
Statistics
Data are presented as mean ± SEM except for NDS, which was presented as median (interquartile range). Student's t test was used to compare the two groups, ANOVA with housing condition and/or drug treatment and/or genotype as between factors and test day as a repeated measure. A probability value p < 0.05 was considered to be statistically significant. Investigators performing behavioral tests and infarct size analysis were blinded to treatment conditions.
Results
SI enhances ischemic injury
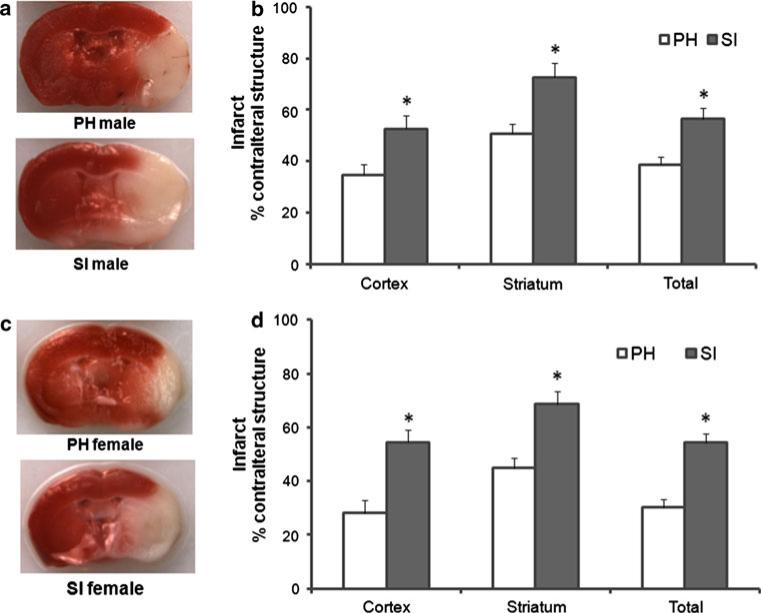
Ischemic occlusion was confirmed by laser Doppler flowmetry. Significantly larger infarct volumes were seen in male SI mice at 72 h of reperfusion compared to PH group in all the three brain regions examined, total hemisphere t(19) = 3.57, p < 0.01; cortex t(19) = 2.81, p < 0.05; and striatum t(19) = 3.92, p < 0.01 (Fig. 1b). A significant increase was also seen in infarct volume in SI female mice compared to PH cohorts; total hemisphere t(19) = 5.06, p < 0.01; Cortex t(19) = 3.76, p < 0.01 and striatum t(19) = 3.78, p < 0.01 (Fig. 1d). Increased mortality was seen in both male and female SI mice (male: 17 % in SI vs. 0 % in PH cohorts; females: 25 % in SI vs. 0 % in PH cohorts).
Fig. 1.
SI mice had significantly increased infarct volumes compared to PH mice after MCAO. a Representative TTC-stained coronal sections of male brain. b Infarct size is expressed as a percent of the contralateral structure at 72 h after 90 min MCAO. Mean (+SEM) shows a significantly larger infarct volumes in SI mice (n = 9) for total, cortical and striatal areas compared to PH cohorts (n = 12). c Representative TTC-stained coronal sections of SI and PH housed female brain. d Infarct size is expressed as a percent of the contralateral structure at 72 h after 90 min MCAO. Mean (+SEM) shows significantly larger infarct volumes in SI cohorts (n = 9) in total, cortical and striatal areas in female brains compared to PH cohorts (n = 12). *p < 0.05 versus PH (Student's t test)
Neurological deficit scores
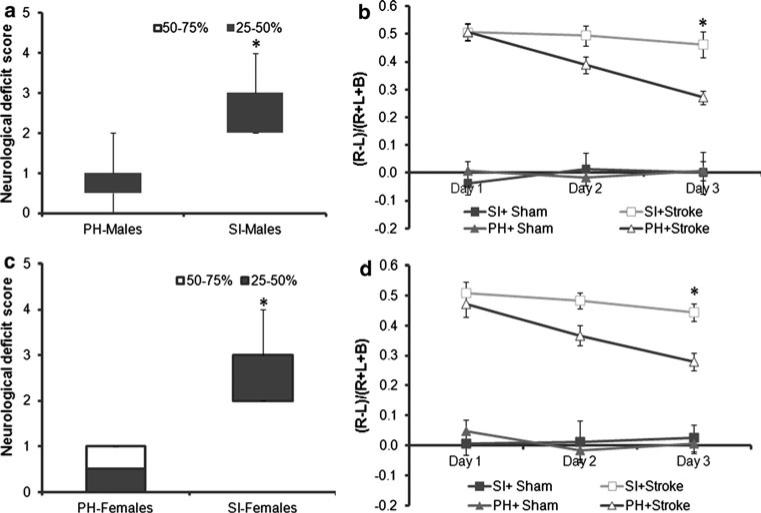
The detrimental effect of SI were also reflected in the NDS, males (SI 3[0.25] n = 9 vs. PH 1[0.25]; n = 12; p < 0.05) (Fig. 2a); females (SI 3[1] n = 9 vs. PH 0.5[1]; n = 12; p < 0.05) (Fig. 2c). Surgical sham mice showed no baseline differences between SI and PH groups (data not shown).
Fig. 2.
SI mice exhibited poorer functional recovery at 72 h compared to PH mice after MCAO. a SI mice have significantly higher neurological deficit scores (NDS) than the PH cohorts, data presented as box-and-whisker plot from minimum to maximum. These effects were evident in both males and b females *p < 0.05 SI (n = 9) versus PH (n = 12) (Mann–Whitney U test). c Cylinder test in the males. The scores are presented as the ratio of (R – L/ R + L + B); where R right forelimb usage, L left forelimb use and B simultaneous usage of both forelimbs. ANOVA with repeated measures showed a significant recovery in PH mice from day 1 to day 3 but not in SI mice *p < 0.05. Similar effects were seen also in d female animals. Data are expressed as mean ± SEM
Cylinder test
There were no significant asymmetries noted in the cylinder test in males or females in sham-treated animals, regardless of housing condition. After stroke, male mice had reduced left forelimb (contralateral) use compared to sham mice at day 1, day 2 and day 3 in both housing groups. There was an overall significant effect of stroke on ipsilateral forelimb use, F(1, 37) = 275.6, p < 0.01. A significant stroke × housing interaction, F(1, 37) = 4.4, p < 0.001 was also seen, due to the fact that PH in stroke mice reduced the limb use difference (Fig. 2b). There was also a significant stroke × day interaction, F(2, 74) = 4.9, p = 0.01, as well as a day × housing interaction, F(2, 74) = 3.7, p < 0.05. Female stroke mice also showed reduced contralateral limb use compared to sham mice.
There was a significant effect of stroke, F(1, 37) = 249.6, p < 0.01, and a significant stroke × housing interaction, F(1, 37) = 4.2, p < 0.05 (Fig. 2d). Additionally, there was a significant effect of day, F(2, 74) = 3.7, p < 0.05, and a trend towards a stroke × day interaction (p = 0.056).
Open field activity
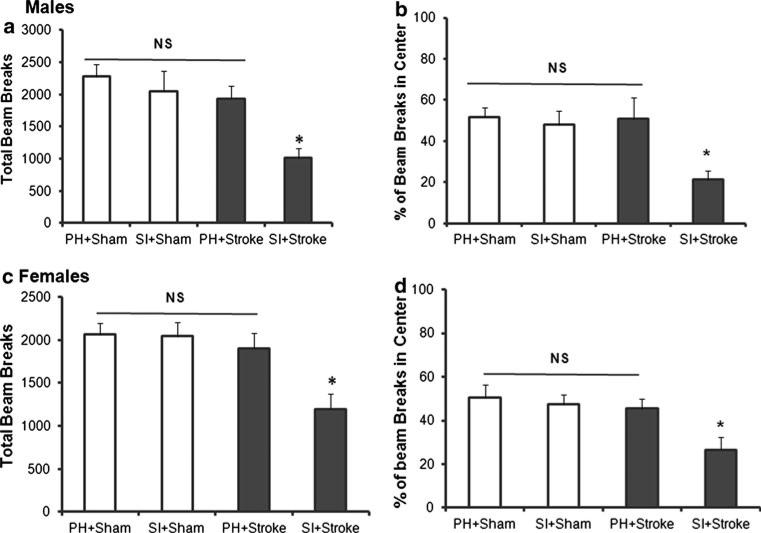
In male mice, there were a reduced percentage of beam breaks in the center in SI stroke animals compared to PH stroke mice at 72 h after stroke. There were overall significant effects of stroke, F(1, 37) = 7.8, p < 0.01, and housing F(1, 37) = 16.6, p < 0.001, as well as a significant stroke × housing interaction, F(1, 37) = 11.4, p < 0.01, suggesting anxiety-like behavior in SI housed stroke males (Fig. 3b). Significantly reduced locomotor activity was observed in SI stroke mice at 72 h post-stroke effects; this was confirmed by independent t tests, t(19) = 2.9, p < 0.05; compared to PH stroke mice], while housing had no significant effect on sham, t(18) 0.65, p > 0.05 (Fig. 3a). This suggests that the reduced percentage of beam breaks in the center seen in the SI mice was not simply due to reduction in total number of beam breaks (overall locomotion). Similarly, female stroke SI mice showed a reduced percentage of beam breaks in the center than PH stroke mice. There were overall significant effects of stoke, F(1, 37) = 9.6, p < 0.001, and housing F(1, 37) = 7.5, p < 0.01] and a stroke × housing interaction, F(1, 37) = 4.3, p < 0.01, suggesting significant anxiety-like behavior in SI housed stroke females (Fig. 3d). Significantly reduced locomotor activity in SI stroked mice at 72 h post-stroke effects were confirmed by independent t tests, t(19) = 2.7, p < 0.05; compared to PH-stroked mice], while in sham mice there was no significant effect of housing, t(18) = 0.89, p > 0.05 (Fig. 3c).
Fig. 3.
Spontaneous locomotor activity and percent of center beam breaks were reduced in isolated mice at 72 h after MCAO. a At 72 h post-stroke, SI male mice made fewer beam breaks than PH mice (*p < 0.05). b SI male mice showed a significantly reduced percent of center beam breaks in SI compared to PH male mice. c Reduced spontaneous locomotor activity and d reduced beam breaks in center were also significant in females (*p < 0.05). Data presented as mean (+SEM)
IL-6 levels, GFAP immunoreactivity and TUNEL-positive cells
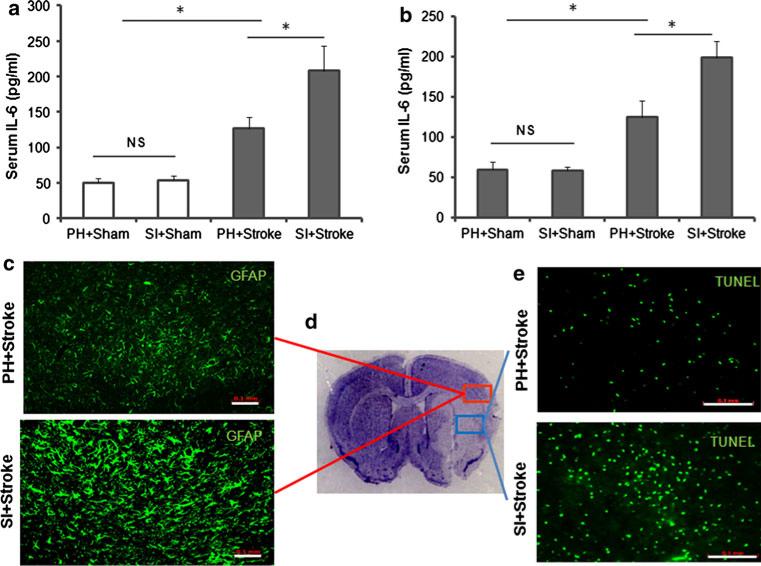
Higher IL-6 levels were observed in serum of post-stroke SI mice compared to PH mice. The ANOVA of males yielded a significant effect of stroke, F(1, 20) = 38.4, p < 0.001, and of housing, F(1, 20) = 6.2, p < 0.001, and a significant stroke × housing interaction, F(1, 20) = 4.9, p < 0.05 (Fig. 4a). ANOVA of female cohorts revealed similar results a significant effect of stroke, F(1, 20) = 39.9, p < 0.001, and of housing, F(1, 20) = 6.1, p < 0.05, and a significant stroke 9 housing interaction, F(1, 20) = 4.8, p < 0.05, suggesting that the elevation of serum IL-6 in SI stroke mice is significantly greater than in PH stroke mice in both males and females (Fig. 4b). Furthermore, a significant reduction in brain IL-6 levels was observed in SI male mice compared to PH male mice F(1, 20) = 22.2, p < 0.05, and of housing, F(1, 20) = 8.7, p < 0.05, and a significant stroke × housing interaction, F(1, 20) = 4.1, p < 0.05. After stroke, higher GFAP immunoreactivity (Fig. 4c) was seen in the ischemic penumbra (as illustrated in Fig. 4d) with more TUNEL-labeled cells (Fig. 4e) in the core (as illustrated in Fig. 4d) in SI mice compared to PH mice.
Fig. 4.
Stroke in SI mice increased serum IL-6 levels, glial immunoreactivity in penumbra and TUNEL-positive cells compared to stroke in PH mice at 24 h post-stroke. a ELISA analysis of serum IL-6 levels collected from sham and stroke mice that were either PH or SI, results for males and in b females. n = 6/grp, *p < 0.05. c Immunohistochemistry for GFAP showed increased levels of GFAP immunoreactivity in SI mice compared to PH mice after stroke in penumbra. Five sections/brain were visualized at 209 magnification in the penumbra/core junction. n = 4/grp. d CV-stained stroke brain section, representing core (blue) and penumbra (red). e Increased TUNEL-positive cells after MCAO are observed in SI cohorts compared to PH mice. Five sections/brain were visualized under 20× magnification in the core. n = 4/grp
Western blot
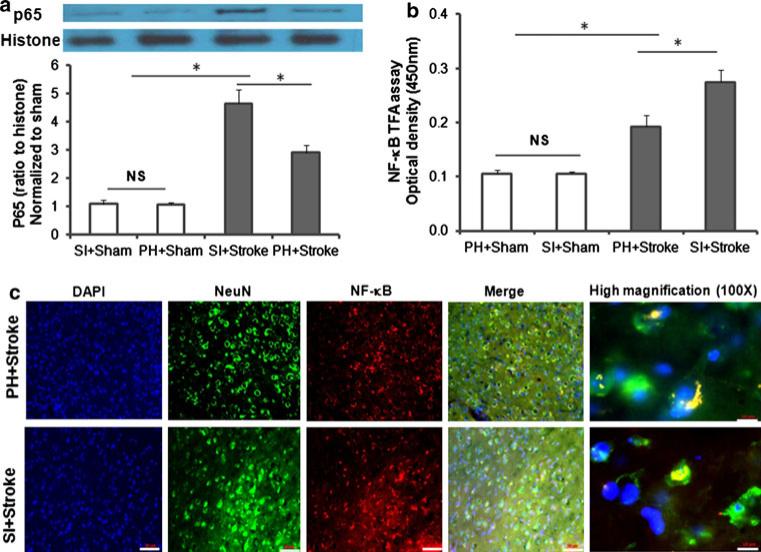
ANOVA for changes in NF-κB protein levels yielded a significant effect of stroke, F(1, 20) = 89.6, p < 0.001, and housing F(1, 20) = 9.3, p < 0.01, and a significant stroke × housing interaction, F(1, 20) = 9.2, p < 0.01] suggesting stroke alone significantly increased NF-κB protein levels compared to shams and that the elevation of NF-κB protein levels in SI stroked mice were significantly greater than in PH stroke mice (Fig. 5a).
Fig. 5.
Stroke in SI mice increased NF-κB expression, analyzed for protein, activity at 6 h and translocation at 72 h compared to PH mice. a Western blot analysis of NF-κB protein expression in nuclear fractions obtained from ipsilateral hemispheres (hemispheres from two mice were pooled to obtain adequate nuclear sample) at 6 h post stroke or sham surgery (n = 6/grp), *p < 0.05. Figure shows a representative western blot with histone as loading control. b NF-κB transcriptional activity in nuclear fractions obtained from ipsilateral hemispheres at 6 h post-stroke or sham surgery in SI and PH mice. Data were obtained as optical density at 450 nm from four independent pooled samples. (n = 4/grp); *p < 0.05. Data presented as mean (+SEM). c NF-κB translocation was visualized by immunohistochemistry co-labeling with DAPI (blue), NF-jB (red) and NeuN (green) images are taken at 20× magnification scale bar 50 μm and 100× magnification scale bar 10 μm
Isolated mice expressed increased nuclear transcription activity and translocation of NF-κB
ANOVA for changes in NF-κB nuclear transcriptional activity yielded a significant effect of stroke, F(1, 12) = 48.7, p < 0.001, housing, F(1, 12) = 5.1, p < 0.05 and a significant stroke × housing interaction, F(1, 12) = 5.0, p < 0.05, suggesting stroke alone significantly increased NF-κB transcription activity and that this increase was greater in SI housed stroke mice than in PH-stroked mice (Fig. 5b). Immunohistochemical analysis revealed preferential co-localization of NF-κB with neurons (at 209 magnification) and demonstrated enhanced NF-κB nuclear translocation in SI mice compared to PH mice (1009) where signal remained cytoplasmic (Fig. 5c).
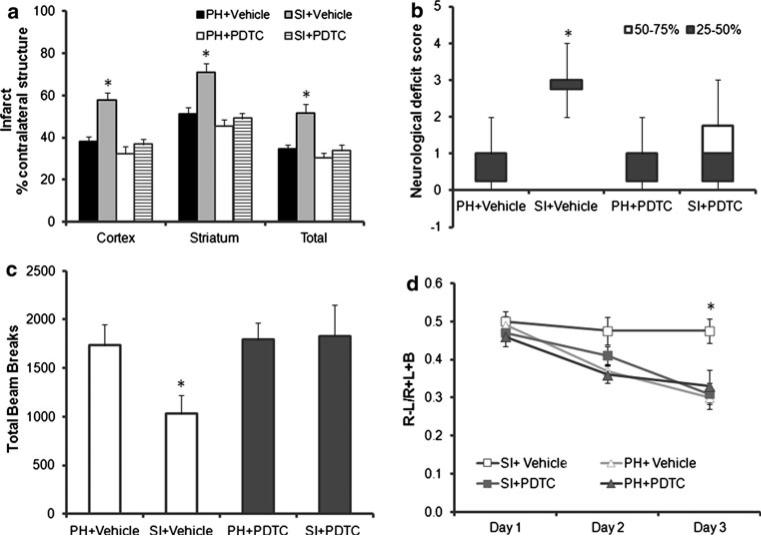
PDTC treatment reverses SI effects
To investigate the contribution of NF-κB activation to the detrimental effects of SI after stroke, a NF-κB inhibitor was utilized. Administration of PDTC abolished the detrimental effects of SI on stroke size (Fig. 6a). Two-way ANOVA yielded a significant effect of housing, F(1, 33) = 15.2, p < 0.001), and a significant effect of drug, F(2, 33) = 11.2, p < 0.001, significant interaction between housing and drug, F(1, 33) = 6.6, p < 0.05 in the cortex. Similarly, a two-way ANOVA yielded a significant effect of housing, F(1, 33) = 9.3, p < 0.01, and of drug, F(2, 33) = 9.6, p < 0.01, and a significant interaction between housing and drug, F(1, 33) = 4.5, p < 0.05 in the striatum. Analysis of total infarct size (two-way ANOVA) demonstrated a significant effect of housing, F(1, 33) = 12.1, p < 0.01) and drug, F(2, 33) = 8.1, p < 0.01, and a significant interaction between housing and drug, F(1, 33) = 5.3, p < 0.05.
Fig. 6.
The NF-κB inhibitor PDTC abolished the detrimental effects of SI. a Mice that were SI or PH housed were subjected to 90 min MCAO and treated with either vehicle or PDTC (200 mg/kg) at 2 and 12 h after surgery. Infarcts were quantified from brains obtained at 72 h after stroke. SI + vehicle (n = 8), PH + vehicle (n = 10), SI + PDTC (n = 10), PH + PDTC (n = 10); *p < 0.05. b Neurological deficit scores were assessed prior to killing from post-stroke vehicle and drug-treated groups, SI + vehicle (n = 8), PH + vehicle (n = 10), SI + PDTC (n = 10), PH + PDTC (n = 10); *p < 0.05. c Spontaneous locomotor activity was assessed at 3 days after stroke vehicle and drug-treated groups, PDTC treatment showed improved activity in SI housed mice. SI + vehicle (n = 8), PH + vehicle (n = 10), SI + PDTC (n = 10), PH + PDTC (n = 10); *p < 0.05. d Cylinder test was performed in all four groups of post-stroke mice from day 1–3 showed an improved recovery in SI + PDTC group compared to SI + vehicle group. SI + vehicle (n = 8), PH + vehicle (n = 10), SI + PDTC (n = 10), PH + PDTC (n = 10); *p < 0.05
The beneficial effects of NF-κB inhibition were also observed on behavioral tasks. The significant difference in the NDS in SI + vehicle compared to PH vehicle mice was abolished with PDTC administration, (SI + vehicle 3[0.25] n = 8 vs. PH 1[0.25]; n = 10; p < 0.05; SI + drug 1[1.5] n = 10 vs. PH + drug 1[0.75]; n = 10; p [ 0.05)) (Fig. 6b).
Figure 6c shows the effects of housing condition and drug on the total beam breaks in the open field 72 h after stroke. There were no significant main effects or interactions: housing, F(1, 34) = 2.0, p > 0.05; treatment with PDTC, F(1, 34) = 3.3, p > 0.05, housing × treatment, F(1, 34) = 2.5, p > 0.05; suggesting PDTC treatment abolished the effects of SI on reduced locomotion in open field.
Significant recovery of contralateral forelimb usage was noted in the cylinder test at day 3 in SI + PDTC treated animals. There were overall significant between-subjects effects of treatment, F(1, 34) = 4.95; p < 0.05, housing F(1, 34) = 7.40; p < 0.05 and treatment 9 housing interaction F(1, 34) = 4.24; p < 0.05. Significant effect of day, F(2, 68) = 29.1; p < 0.01 and a significant day × treatment × housing interaction F(2, 68) = 4.6; p < 0.05. This interaction was due to the fact that both PH groups and the SI + PDTC groups showed recovery over days while the SI + vehicle group did not (Fig. 6d).
Social isolation has no effect in NF-κB knockout mice
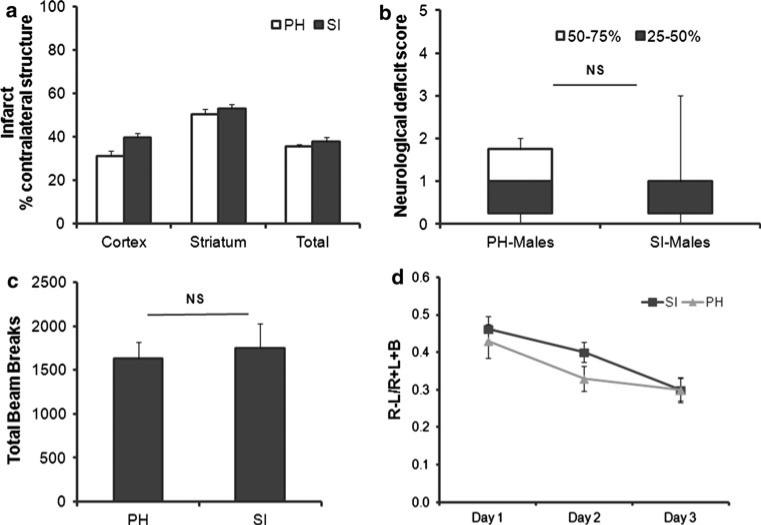
To confirm our hypothesis that NF-κB activation mediates the detrimental effects of SI, we investigated the effects of housing conditions in NF-κB1 KO mice. We found that SI had no effects in NF-κB1 KO mice. Infarct volume were not significantly different between SI and PH NF-κB1 KO mice (t(16) = 2.76, p > 0.05; in cortical infarct areas). Similar findings were seen in striatum (t(16) = 0.86, p > 0.05) and total hemisphere (t(16) = 0.93, p > 0.05) (Fig. 7a). Cerebral blood flow (CBF) was monitored by LDF and an equivalent degree of CBF reduction was seen in all groups (data not shown). No mortality was seen in any of these cohorts.
Fig. 7.
NF-κB1 deficient mice showed no significant detrimental effects to SI. a NF-jB1 KO mice that were SI and PH housed were subjected to 90 min MCAO. Infarcts were quantified from brains obtained at 72 h after MCAO. SI (n = 9), PH (n = 9). b Neurological deficit scores were assessed at 3 days of reperfusion in both SI and PH groups (n = 9/grp). c Spontaneous locomotor activity was assessed at 3 days after stroke. No significant differences in SI compared to PH mice were observed. (n = 9/grp). d Cylinder test was performed in mice from both housing conditions from day 1–3 post-stroke. Housing in either SI or PH showed no differences in recovery of forelimb use (n = 9)
SI NF-κB1 KO mice did not show any additional functional impairment compared to PH NF-κB1 KO mice. No significant difference in the NDS was seen in SI compared to PH NF-κB1 KO mice, (SI 1[0.75] n = 9 vs. PH 1[1]; n = 9; p > 0.05) (Fig. 7b).
When tested for differences in spontaneous locomotor activity. There was no significant interaction in the total beam breaks in SI versus PH NF-κB1 KO mice after stroke. Student's t test confirmed a lack of significant effects of housing [t(15) = 0.38, p > 0.05]. This suggests lack of housing-induced effects on locomotion activity in open field chambers in animals lacking NF-κB1 (Fig. 7c). There were no significant differences in the cylinder test in post-stroke NF-κB1 KO animals, regardless of housing conditions. There were no significant changes in right forelimb (ipsilateral) usage in SI stroke animals compared to PH mice at day 1, day 2 and day 3 in both housing groups. ANOVA with repeated measures showed lack of housing effects on recovery [F(1, 15) = 2.01; p > 0.05]. A similar degree of recovery is seen in NF-κB1 KO mice in both housing conditions (Fig. 7d).
Discussion
Emerging evidence strongly suggests that social isolation is detrimental and predicts morbidity and mortality from a multitude of health conditions, including cancer, stroke and cerebrovascular disease [13, 15–17, 48]. Isolation enhances tissue injury after experimental stroke and contributes to both stroke risk and disability in clinical populations [1, 2, 4, 6, 18, 19, 53]. The detrimental effects of social isolation (SI) are independent of race, ethnicity, age and gender [4, 6, 19, 53, 57]. In contrast, supportive social relationships are associated with improved health and healthy aging and an overall decrease in mortality [4, 19, 53, 57]. The influences of environmental factors on the phenotypic expression of vascular diseases such as stroke are becoming increasingly recognized. The mechanisms involved in the detrimental effects of social isolation remain unclear, but several studies [12] have implicated an enhancement of pro-inflammatory signaling in SI individuals. In the present study, we investigated the effects of pre-stroke SI in male and female mice isolated prior to stroke and report several important new findings. Consistent with previous work [6], SI significantly enhanced brain injury and delayed functional neurological recovery in both males (Fig. 1b) and females (Fig. 1d). SI enhanced stroke-induced IL-6 levels in the serum (Fig. 4a, b), and reduced brain IL-6 levels but had no effect in uninjured animals. SI also increased post-ischemic glial immunoreactivity levels (Fig. 4c), TUNEL-positive cells, and immunohistochemical evidence of nuclear translocation of NF-κB in SI animals (Fig. 4d). Both NF-κB levels and activity as measured by nuclear NF-κB translocation by Western (Fig. 5a) and transcriptional activity (Fig. 5b) increased in SI animals but interestingly SI had no effect on NF-κB signaling in sham mice. This suggests that SI may be a priming stimulus that only manifests its detrimental effects with exposure to subsequent injury or stress [49]. Importantly this is the first report that demonstrates that the detrimental effects of SI could be abolished by interference of NF-κB signaling with a pharmacological inhibitor or deletion of NF-κB p50 subunit, which led to improved post-stroke recovery.
Experimental evidence suggests that post-stroke social interactions have the potential to hasten functional recovery [1, 4, 6, 18]. In this work, SI mice had a significant delay in recovery on several well-validated behavioral tests in addition to significantly higher mortality rates compared to PH mice [6, 18–20, 53]. We found a significant reduction in general locomotor activity in SI mice (Fig. 3a, c), which were tested during the light cycle (when rodents are less active). In contrast, previous work reported decreased spontaneous activity selectively during the dark cycle [18] in mice exposed to SI versus PH housing conditions at 72 h [19] with no effects of housing on total locomotor activity or exploratory behavior. Additionally, no effect was seen on contralateral paw use in the cylinder test in previous work, which we found to be significantly decreased in SI versus PH mice (Fig. 2b, d). In this work, stroke-reduced overall locomotor activity was reduced in both SI and PH mice at 24 h (data not shown); however, PH mice had recovered to levels comparable to shams by 72 h (Fig. 3a, c). Enhanced recovery of locomotion may be secondary to reduced neuronal damage, improvements in residual neuronal function, or enhanced plasticity and synaptogenesis. Studies examining more chronic endpoints are needed to investigate the underlying mechanisms mediating the beneficial effects of PH [18] as this work only examined short time points after injury. Physical activity has been associated with improved clinical outcome in stroke patients [7]. Contrary to the lack of effect in an earlier study [19], in the current study the cylinder test also demonstrated significant differences in recovery in SI mice as early as 72 h post-stroke compared to PH cohorts (Fig. 2b, d). Although the smaller infarct volumes in the PH mice may also be a factor contributing to this faster recovery, previous studies suggests that infarct size does not necessarily correlate to behavioral functional deficits. Consistent with this concept is that the deficits at 24 h after stroke were similar in both SI and PH mice and improvement in forelimb was observed over time. This observation further strengthens the possibility that post-stroke housing conditions are important factors for functional recovery.
Increasing evidence from both pre-clinical and clinical studies strongly implicates SI as a contributor to post-stroke mortality and functional disability, and this occurs in both men and women. Post-stroke recovery is a complex process, influenced by many factors including sex [18, 28, 30, 34]. We designed our studies to investigate the effects of SI in both males and their paired OVXed female partners. As estrogen is neuroprotective in most induced experimental stroke models [27–29, 36], we ovariectomized animals to reduce the hormone-mediated sex differences in infarct size [19, 20, 29]. SI in young females also is known to shorten ovarian cycle length, increasing the days spent in high estrogen proestrus [24]. This model (ovx) may also be more translationally relevant, as stroke is most prevalent in post-menopausal women [27, 29].
Despite conflicting data on functional role of cytokines in cell survival and cell death and differential effects of IL-6 in periphery and central systems, peripheral elevations of IL-6 are widely accepted as pro-inflammatory as its signaling stimulates C-reactive proteins (CRP) production, a widely accepted marker of stress or stroke in humans. Thus, IL-6 is currently considered a target for clinical use for stroke patients [19, 51]. The findings of increased serum IL-6 levels (Fig. 4a) in this study is in agreement with a previous report that demonstrated elevated serum IL-6 and CRP in SI compared to PH mice [6, 19, 20]. Conversely, down regulation of central IL-6 after stroke is detrimental in animal studies and an increase in systemic mRNA expression of IL-6 has been observed with stroke and social isolation [19, 20]. Inhibition of central IL-6 by intracerebroventricular injection using a neutralizing antibody increases lesion volume in PH mice suggesting that central IL-6 expression mediates the beneficial effects of social housing [19], implicating a pivotal role for enhanced neuroinflammatory signaling after SI. SI significantly increased infarct size and serum IL-6 in females (Fig. 4b) which was independent of acute serum estrogen exposure [27] consistent with earlier studies [6]. Interestingly in clinical populations, men and women respond differently to SI as regards to their cytokine profiles, in that social networks inversely correlate with serum IL-6 levels in men, but not in women [33]. Epidemiological data show higher levels of IL-6 and hs-CRP protein in SI individuals, although this relationship was more notable in men. In this pre-clinical work, both sexes had significant stroke-induced increases in IL-6, suggesting other factors influencing cytokine expression may be present in clinical populations.
Studies have indicated that NF-κB at the blood–brain interface is an important molecule in transmitting cytokine signals to the brain [11, 38] and as NF-κB is responsible for IL-6 activation, we decided to focus on this signaling pathway. Despite conflicting data on the beneficial effects of NF-κB inhibition on cell death [35, 39, 43, 47], studies have demonstrated that early inhibition of NF-κB is neuroprotective after stroke [35, 39–42]. Cerebral ischemia and stress result in NF-κB activation [35, 39–42, 47]. NF-κB is upstream regulator of several signaling pathways that includes both IL-6 and oxytocin, another potential mediator of the effects of SI [19, 20, 57]. In this study, sham PH and sham SI mice showed equivalent levels of nuclear NF-κB, suggesting that housing conditions alone do not “activate” NF-κB signaling. Stroke increased neuronal NF-κB transcriptional activity and this increase was greatest in SI mice (Fig. 5b). This suggests that similar to IL-6, SI primes NF-κB signaling in the brain, leading to enhanced sensitivity to subsequent insults. NF-κB is activated by cytokines but can also directly regulate cytokines [35, 38, 47]. NF-jB is activated by stroke when the inhibitory factor IκB undergoes proteomic degradation allowing for the subsequent nuclear translocation of NF-κB. Transcription of NF-κB leads to an enhancement of over 150 genes [43] including TNF-α, ICAM-1, COX-2, iNOS, IL-1β and IL-6 [35, 38, 43, 47, 52]. In a recent study using a neonatal ischemia model, it was found that NF-κB can also regulate cell death by modulating apoptotic pathways [39]. Consistent with this, we found increased apoptotic cell death in SI mice compared to PH mice (Fig. 4e).
To investigate whether these detrimental effects of SI were mediated by NF-κB, we first used the potent pharmacological inhibitor PDTC, which has been shown to be protective in both the MCAO model and neonatal ischemia [40, 41]. SI mice treated with PDTC had infarcts that were comparable to the PH mice treated with either vehicle or PDTC, suggesting that NF-κB inhibition is sufficient to abolish the detrimental effects of SI. Although we did not directly explore the neuroprotective effects of NFκB inhibition (independent of housing effects), based on our data pharmacological NFκB inhibition is neuroprotective. Beneficial effects of PDTC have been previously documented in hypertensive animals, permanent models, and in neonatal models of hypoxic-ischemia [40, 41]. This protective effect of NFκB inhibition was not seen in paired mice. This suggests that the protection seen by affiliative housing is mediated by inhibition of NFκB, as there were no additive effects of housing + drug (Fig. 6a). It is also possible that this is a qualitative or threshold effect, in that the additional elevation of NFκB seen in socially isolated mice leads to exacerbation of injury, whereas the smaller (but significant) MCAO-induced increase in pair-housed mice is not sufficient to induce this additional damage. This could explain the lack of PDTC effects on infarct in pair-housed mice. However, housing conditions were not explicitly stated in many of the previous studies on PDTC. As PDTC may also act as an iron chelator and reactive oxygen scavenger [10], these effects of housing conditions were confirmed in NF-κB1 KO mice. NF-jB1 KO mice have improved outcomes compared to wild-type mice after cerebral or myocardial injury [10, 47], and in this study loss of NF-κB1 abolished the detrimental effects of social isolation. Taken together, these studies provide strong evidence that NF-κB signaling plays a key role in the detrimental effects of SI on infarct volume. NF-κB regulates the synthesis of several cytokines including TNF-α, IL-6, IL-1β, IL-8 and cyclooxygenase-2 [38, 43]. Stress-induced activation of brain inflammation can also lead to the release of other pro-inflammatory cytokines including IL-1, which inhibits neural plasticity and neurogenesis [21, 22, 49]. Interestingly, microarray analyses of peripheral blood monocytes have shown a heightened expression of gene transcripts with response elements for NF-βB [37] in lonely versus non-lonely individuals, consistent with the concept that increased inflammation contributes to the negative impact of SI [5]. Effects on neurogenesis may have contributed to the enhanced recovery seen that was seen in PH mice [22], but evaluation of chronic endpoints is required as functional recovery was only assessed at 72 h post-stroke in this study. One major limitation of this study is that all SI animals were isolated for only 1 week prior to stroke. In clinical settings, socially isolated individuals may not be identified until after the stroke occurs and the patient comes to the attention of health care providers. Thus, our findings need to be confirmed and expanded in future studies that manipulate the post-stroke housing environment. Despite this limitation, our work has identified a potential novel signaling molecule by which SI mediates its detrimental effects on infarct size and post-stroke recovery.
In conclusion, social isolation enhances ischemic injury and delays behavioral recovery. These effects are evident in both males and females. Increased post-stroke mortality was seen in isolated mice of both sexes. NF-κB signaling mediates these detrimental effects which may have important translational relevance. Isolation appears to prime the brain to respond to injury with an exacerbated neuroinflammatory response. Thus, further elucidation of the role of NF-κB and development of pharmacological tools to inhibit NF-κB might offer a potential therapeutic strategy to abolish the detrimental effects of SI in stroke survivors as residual inflammation may further compromise quality of life. From a rehabilitation perspective, efforts to encourage social interactions among stroke survivors could be a major opportunity to enhance post-stroke recovery and to reduce the substantial financial burden of post-stroke care.
Acknowledgments
This work was supported by National Institutes of Health grants R01 NSO77769 and NS055215 (to L.D.M.) and American Heart Association grant 11POST7430045 to V.R.V, 09SDG2261435 to J.L.
Contributor Information
Venugopal Reddy Venna, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA.
Gillian Weston, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA.
Sharon E. Benashski, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA
Sami Tarabishy, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA.
Fudong Liu, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA.
Jun Li, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA.
Lisa H. Conti, Department of Psychiatry, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA
Louise D. McCullough, Department of Neuroscience, University of Connecticut Health Center, Farmington, CT 06030, USA Department of Neurology, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA; The Stroke Center at Hartford Hospital, 85 Jefferson Street, Hartford, CT 06102, USA.
References
- 1.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64(11):1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 2.Boru UT, Ozturk E, Tasdemir M, Sur H. Living alone following first-ever stroke: a prospective study in Turkey identifying the risk factors and evaluating their effects. NZ Med J. 2007;120(1255):U2559. [PubMed] [Google Scholar]
- 3.Cacioppo JT, Fowler JH, Christakis NA. Alone in the crowd: the structure and spread of loneliness in a large social network. J Pers Soc Psychol. 2009;97(6):977–991. doi: 10.1037/a0016076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark CJ, Guo H, Lunos S, Aggarwal NT, Beck T, Evans DA, Mendes de Leon C, Everson-Rose SA. Neighborhood cohesion is associated with reduced risk of stroke mortality. Stroke. 2011;42(5):1212–1217. doi: 10.1161/STROKEAHA.110.609164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craft TK, Glasper ER, McCullough L, Zhang N, Sugo N, Otsuka T, Hurn PD, DeVries AC. Social interaction improves experimental stroke outcome. Stroke. 2005;36(9):2006–2011. doi: 10.1161/01.STR.0000177538.17687.54. [DOI] [PubMed] [Google Scholar]
- 7.Deplanque D, Bordet R. Physical activity: one of the easiest ways to protect the brain? J Neurol Neurosurg Psychiatry. 2009;80(9):942. doi: 10.1136/jnnp.2009.174490. [DOI] [PubMed] [Google Scholar]
- 8.Deplanque D, Masse I, Lefebvre C, Libersa C, Leys D, Bordet R. Prior TIA, lipid-lowering drug use, and physical activity decrease ischemic stroke severity. Neurology. 2006;67(8):1403–1410. doi: 10.1212/01.wnl.0000240057.71766.71. [DOI] [PubMed] [Google Scholar]
- 9.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48(11):2235–2242. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantz S, Hu K, Bayer B, Gerondakis S, Strotmann J, Adamek A, Ertl G, Bauersachs J. Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. FASEB J. 2006;20(11):1918–1920. doi: 10.1096/fj.05-5133fje. [DOI] [PubMed] [Google Scholar]
- 11.Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169(1–2):97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Hafner S, Emeny RT, Lacruz ME, Baumert J, Herder C, Koenig W, Thorand B, Ladwig KH, Investigators KS. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: results from the MONICA/KORA study. Brain Behav Immun. 2011;25(8):1701–1707. doi: 10.1016/j.bbi.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Hawkley LC, Cacioppo JT. Loneliness and pathways to disease. Brain Behav Immun. 2003;17(Suppl 1):S98–S105. doi: 10.1016/s0889-1591(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 14.Hermes GL, Rosenthal L, Montag A, McClintock MK. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am J Physiol Regul Integr Comp Physiol. 2006;290(2):R273–R282. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- 15.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki M, Otani T, Sunaga R, Miyazaki H, Xiao L, Wang N, Yosiaki S, Suzuki S. Social networks and mortality based on the Komo-Ise cohort study in Japan. Int J Epidemiol. 2002;31(6):1208–1218. doi: 10.1093/ije/31.6.1208. [DOI] [PubMed] [Google Scholar]
- 18.Karelina K, Norman GJ, Zhang N, DeVries AC. Social contact influences histological and behavioral outcomes following cerebral ischemia. Exp Neurol. 2009;220(2):276–282. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci USA. 2009;106(14):5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, Devries AC. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke. 2011;42(12):3606–3611. doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4(6):371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- 24.LeFevre J, McClintock MK. Isolation accelerates reproductive senescence and alters its predictors in female rats. Horm Behav. 1991;25(2):258–272. doi: 10.1016/0018-506x(91)90055-m. [DOI] [PubMed] [Google Scholar]
- 25.Leys D, Deplanque D. Thrombolysis beyond the three-hour time window. Clin Exp Hypertens. 2006;28(3–4):313–316. doi: 10.1080/10641960600549371. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Benashski SE, Venna VR, McCullough LD. Effects of metformin in experimental stroke. Stroke. 2010;41(11):2645–2652. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. 2011;31(2):413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu YP, Lang BT, Baskaya MK, Dempsey RJ, Vemuganti R. The potential of neural stem cells to repair stroke-induced brain damage. Acta Neuropathol. 2009;117(5):469–480. doi: 10.1007/s00401-009-0516-1. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179(1):1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 33.Loucks EB, Sullivan LM, D'Agostino RB, Sr, Larson MG, Berkman LF, Benjamin EJ. Social networks and inflammatory markers in the Framingham Heart Study. J Biosoc Sci. 2006;38(6):835–842. doi: 10.1017/S0021932005001203. [DOI] [PubMed] [Google Scholar]
- 34.Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 36.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab TEM. 2003;14(5):228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 37.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadjar A, Tridon V, May MJ, Ghosh S, Dantzer R, Amedee T, Parnet P. NFkappaB activates in vivo the synthesis of inducible Cox-2 in the brain. J Cereb Blood Flow Metab. 2005;25(8):1047–1059. doi: 10.1038/sj.jcbfm.9600106. [DOI] [PubMed] [Google Scholar]
- 39.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39(7):2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 40.Nurmi A, Goldsteins G, Närväinen J, Pihlaja R, Ahtoniemi T, Gröhn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40(10):1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35(4):987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- 42.Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjanheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004;91(3):755–765. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- 43.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 44.Pan A, Okereke OI, Sun Q, Logroscino G, Manson JE, Willett WC, Ascherio A, Hu FB, Rexrode KM. Depression and incident stroke in women. Stroke. 2011;42(10):2770–2775. doi: 10.1161/STROKEAHA.111.617043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54(12):1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- 47.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5(5):554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 48.Schoenbach VJ, Kaplan BH, Fredman L, Kleinbaum DG. Social ties and mortality in Evans County, Georgia. Am J Epidemiol. 1986;123(4):577–591. doi: 10.1093/oxfordjournals.aje.a114278. [DOI] [PubMed] [Google Scholar]
- 49.Serra M, Sanna E, Mostallino MC, Biggio G. Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol. 2007;17(1):1–11. doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80(2):321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 51.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soloff MS, Izban MG, Cook DL, Jr, Jeng YJ, Mifflin RC. Interleukin-1-induced NF-kappaB recruitment to the oxytocin receptor gene inhibits RNA polymerase II–promoter interactions in cultured human myometrial cells. Mol Hum Reprod. 2006;12(10):619–624. doi: 10.1093/molehr/gal067. [DOI] [PubMed] [Google Scholar]
- 53.Stuller KA, Jarrett B, Devries AC. Stress and social isolation increase vulnerability to stroke. Exp Neurol. 2011;233(1):33–39. doi: 10.1016/j.expneurol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Timmers L, van Keulen JK, Hoefer IE, Meijs MF, van Middelaar B, den Ouden K, van Echteld CJ, Pasterkamp G, de Kleijn DP. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res. 2009;104(5):699–706. doi: 10.1161/CIRCRESAHA.108.189746. [DOI] [PubMed] [Google Scholar]
- 55.Türeyen K, Vemuganti R, Sailor KA, Dempsey RJ. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Methods. 2004;139(2):203–207. doi: 10.1016/j.jneumeth.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 56.Venna VR, Li J, Benashski SE, Tarabishy S, McCullough LD. Preconditioning induces sustained neuroprotection by downregulation of adenosine 5′-monophosphate-activated protein kinase. Neuroscience. 2012;10(201):280–287. doi: 10.1016/j.neuroscience.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venna VR, McCullough LD. “Won't You Be my Neighbor?”: deciphering the mechanisms of neuroprotection induced by social interaction. Stroke. 2011;42(12):3329–3330. doi: 10.1161/STROKEAHA.111.632570. [DOI] [PMC free article] [PubMed] [Google Scholar]