Abstract
Liver predominant small cell carcinoma is rare, but often presents as hyper-acute liver failure with unknown primary and is a medical emergency. We present 2 autopsy and 7 biopsy cases of liver predominant small cell carcinoma and demonstrate that these patients present with liver failure and identifiable hepatomegaly, but lack discrete lesions on imaging, as well as no mass lesions identified in other organs including lung. Compared to the multiple nodules of metastatic small cell carcinoma in the liver, unique morphologic feature of liver predominant/primary small cell carcinoma in autopsy and biopsy specimens was a diffuse infiltration of small, blue, neoplastic cells predominantly in the sinusoidal space in the liver parenchyma. Prior to diagnosing liver predominant/primary small cell carcinoma, other infiltrating small blue cell neoplasms including lymphoma and peripheral neuroectodermal tumor need to be ruled out through immunohistochemistry (IHC). We therefore demonstrate that liver biopsy together with a rapid panel of immunostains is necessary to firmly establish a diagnosis of liver predominant small cell carcinoma and allow clinicians to immediately implement potentially lifesaving chemotherapy.
Keywords: Small cell carcinoma, hyperacute liver failure, acute liver failure, hepatomegaly
Introduction
Hyper-acute (<7 days) and acute (7-28 days) liver failure is due to rapid loss in hepatocyte function based on the interval from initial symptom onset to onset of encephalopathy [1, 2]. In general, acetaminophen toxicity results in hyper-acute failure while viral hepatitis leads to slower, acute or subacute onset [1]. Although acetaminophen or drug toxicity and viral hepatitis are the most common etiologies that result in hyper-acute and acute liver failure, there are reports secondary to hepatic infiltration by malignancies including Hodgkin's lymphoma [3-5], non-Hodgkin's lymphoma [6-8], adenocarcinomas [9-14], melanoma [9-12, 14], anaplastic tumors [9-12, 14] and small cell carcinoma of the prostate [15] or lung [10, 12, 16].
Small cell carcinoma is one of the most common primary malignancies in the lung and demonstrates extremely aggressive malignant behavior and early metastasis [16]. Small cell carcinoma of the lung has been reported to rarely manifest as acute hepatic failure when it metastasizes to liver [16]; however, extra-pulmonary small cell carcinoma is rare, and there are only a few case reports for liver primary/predominant small cell carcinoma [17-23]. Recently, we have identified a few cases of hyper-acute or acute liver failure secondary to diffuse infiltration of the liver primary/predominant small cell carcinoma. We therefore reviewed all liver biopsy and autopsy cases of small cell carcinoma over the past 20 years at our institution to evaluate pathologic, clinical and radiologic findings.
Our data demonstrates that primary/predominant liver small cell carcinoma results in a distinct histologic, clinical and radiologic presentation in comparison to metastatic small cell carcinoma to the liver. Liver primary/predominant small cell carcinoma exhibited diffuse sinusoidal infiltration of small blue neoplastic cells almost entirely replacing the hepatic parenchyma, while metastatic small cell carcinoma demonstrated a nodular pattern histologically. Patients with liver primary/predominant small cell carcinoma also had diffuse hepatomegaly and hyperacute or actue liver failure with no distinct liver nodules identified on radiologic imaging or on autopsy, while all patients with metastatic small cell carcinoma had normal liver function and a nodular pattern on imaging. Liver small cell carcinoma patients often presented emergently with rapid progress to death secondary to hyper-acute or acute liver failure making a prompt diagnosis difficult. We therefore demonstrate the need for liver biopsy in rapid, accurate diagnosis in patients with hyper-acute or acute liver failure, diffuse hepatomegaly and no discrete lesions on imaging.
Materials and methods
Case Selection and Review
With approval of the Institutional Review Board from Northwestern University, all autopsy and biopsy cases diagnosed as small cell carcinoma in the liver between 1992 and 2012 were identified from the pathology database at Northwestern Memorial Hospital. Two independent, blinded gastrointestinal surgical pathologists reviewed all slides associated with each case previously diagnosed as small cell carcinoma in the liver. Diagnosis of small cell carcinoma on autopsy or biopsy specimens was based on morphologic findings and confirmed by immunohistochemical stains in all cases. Retrospective chart review was performed to identify any known primary source of small cell carcinoma or clinical diagnosis of hyper-acute, acute or sub-acute liver failure. When a history of liver failure was present, confirmation of the timeframe of symptom onset to encephalopathy and subsequent diagnosis of hyper-acute, acute or sub-acute liver failure was performed. Two independent, blinded radiologists reviewed all previous imaging to evaluate liver size, presence or absence of liver lesions and/or metastatic disease in other organs.
Antibodies and Immunohistochemistry
Immunohistochemical staining for synaptophysin, chromogranin, CD56, cytokeratin AE1/AE3 and Cam5.2, CD45 and/or CD99, as well as Hep Par 1 and EBER were performed on formalin-fixed, paraffin-embedded, 5-μm-thick tissue sections. Tissue sections were deparaffinized in xylene for a total of 15 min and subsequently rehydrated. Immunostaining was performed using a Ventana XT (Ventana Medical Systems, Inc, Tucson, AZ) automated immunostainer for synaptophysin (Cellmarque, clone NA-polyclonal) and chromogranin (Ventana, clone LK2H10). Cytokeratin AE1/AE3 (DAKO, clone AE1/3,) and Cam5.2 (Becton–Dickinson Biosciences, Franklin Lakes, NJ), CD45 (DAKO, clone 2B11/PD7/26) and CD99 (a generous gift from the lab of Dr. William A. Muller, Northwestern University, clone hec 2.2), Hep Par 1 (clone OCH1E5.2.10, 1:80 dilution, DAKO, Carpinteria, CA), CD56 (Vector, clone CD564, Burlingame, CA) and EBER In situ hybridization, (DAKO, Carpinteria, CA) were analyzed by A DAKO Autostainer (Dako North America, Inc, Carpinteria, CA).
In brief, antigen retrieval was carried out at 97°C for 20 minutes in citrate buffer. After blocking the endogenous peroxidase activity with 3% hydrogen peroxidase for 10 minutes, the primary antibody incubation was carried out for 60 minutes at room temperature. Bound primary Abs were detected with a EnVision Horse Radish Peroxidase (HRP) System (DAKO Corporation, Carpinteria, CA) according to the manufacturers' instructions. Staining was considered positive when tumor cells showed cytoplasmic/nuclear reactivity (chromogranin, synaptophysin, cytokeratin AE1/AE3, CD45, CD56 and CD99). Negative controls (substituting Tris-buffered saline for primary antibody) were run simultaneously. Two blinded pathologists assessed the slides.
Results
Unique autopsy findings of liver predominant/primary small cell carcinoma
Two autopsy cases were performed on patients that presented to outpatient clinic with symptoms of vague abdominal pain, jaundice or icterus. One patient also had recent mental status changes reported by a family member. For both patients, elevated AST (741 U/L and 12574 U/L), ALT (291 U/L and 5768 U/L), total bilirubin (25.6 mg/dL and 10.8 mg/dL) and direct bilirubin (10.0 mg/dL, performed on one patient only) were identified on initial laboratory work up. Both patients were admitted and rapidly decompensated with AST progressing from 741 U/L to 22867 U/L and ALT progressing from 291 U/L to 3882 U/L in one patient within 2 days of presentation. Ultrasound (US) imaging obtained the day prior to death in one case revealed marked hepatomegaly and a heterogenous nodular contour to the liver with hypo-echoic areas and no evidence of a definitive mass lesion. No imaging was performed in the other case. Despite aggressive efforts, the patients expired 7 and 2 days from the time of presentation in hyper-acute liver failure.
Autopsies revealed livers with marked hepatomegaly, weighing 4380 and 5360 grams, respectively, which contained multiple, scattered, small nodules ranging from 0.1 to 0.6 cm in size (Figure 1A). Histologically, liver sections in both cases demonstrated all of the salient morphological features of small cell carcinoma including small, blue cells with scant cytoplasm, nuclear molding and a finely granular “salt and pepper” chromatin pattern, as well as absent or inconspicuous nucleoli. The small neoplastic cells diffusely infiltrated the sinusoidal spaces and almost completely replaced the liver parenchyma (Figure 1B). Abundant necrosis and only rare viable hepatocytes were also identified. Despite extensive examination and sampling of the lung and peribronchial parenchyma, a primary source of the small cell carcinoma or evidence of further metastasis was not detected in either case. Further review of the clinical history revealed no prior diagnosis of liver dysfunction, small cell carcinoma or other malignancies. Immunohistochemical stains were obtained in both cases and revealed that the residual hepatocytes were highlighted by cytokeratin Cam 5.2 (Figure 1C), and neoplastic small cells were strong positive for CD56 (Figure 1D) and AE1/AE3, at least focally positive for chromogranin (Figure 1E) and synaptophysin, and negative for TTF-1, CD99, Hep Par 1 and EBER.
Figure 1.
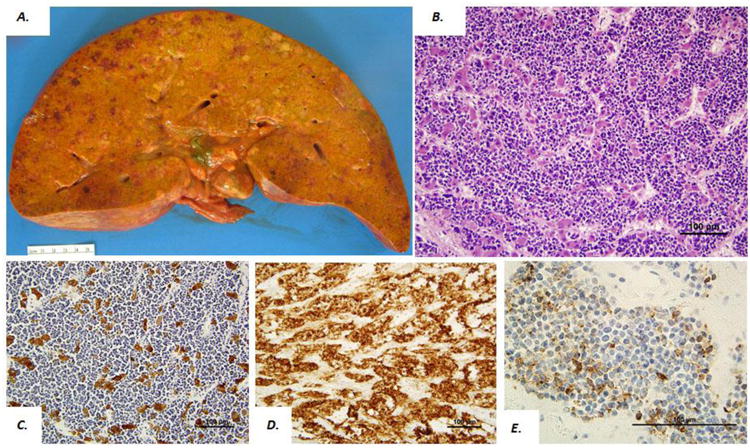
Gross and microscopic findings in an autopsy case of liver small cell carcinoma. Gross photo of liver cut surface (A) in a patient presenting with hyperacute liver failure and hepatomegaly (liver weight 5360 gm). Microscopic findings from the same case demonstrate a diffuse pattern of small blue cells diffusely infiltrating through the hepatic parenchyma (B). Hepatocytes were positive for Cam 5.2 (C) and lesional cells were positive for CD56 (D) and chromogranin (E).
Morphologic features of liver predominant/primary small cell carcinoma compared to metastatic small cell carcinoma in liver needle core biopsies
Total 13 patients with diagnosis of liver small cell carcinomas in liver core biopsies were retrieved over past 20 years at our institution. Histologically, two distinct histologic patterns were identified in these biopsies. One group (n=6) demonstrated a diffuse pattern of small, blue, neoplastic cells infiltrating into the hepatic parenchyma (mainly in the sinusoidal spaces) and almost completely replacing the liver parenchyma (Figure 2A and B), identical to our initial autopsy cases. The other group (n=7) demonstrated a nodular growth pattern of small, blue neoplastic cells with abundant necrosis adjacent to the well-delineated background hepatic parenchyma consistent with metastatic disease (Figure 3A and B).
Figure 2.
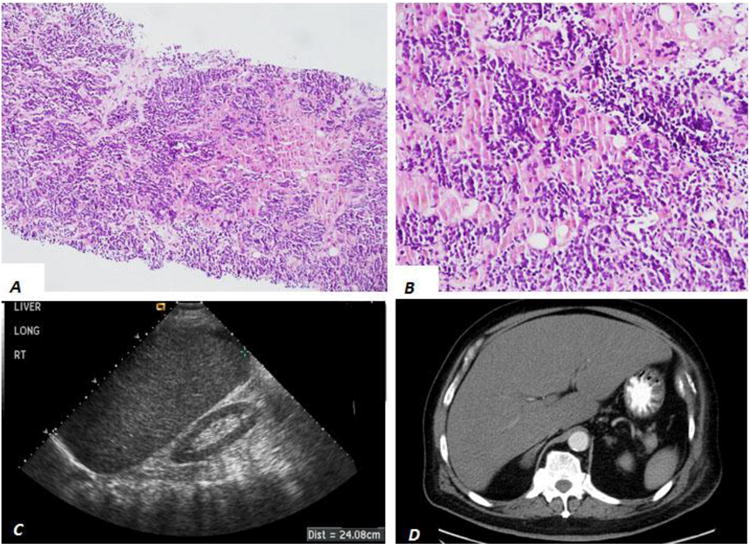
Liver imaging and histology from patients with diffuse liver small cell carcinoma. H&E stained liver core biopsies (A and B) demonstrate a diffuse pattern of small blue cells in the sinusoid space of liver parenchyma. Grayscale longitudinal ultrasound of the liver demonstrates hepatomegaly without discrete mass lesion (C). Axial contrast-enhanced computed tomography (D) demonstrates hepatomegaly without discrete mass lesion.
Figure 3.
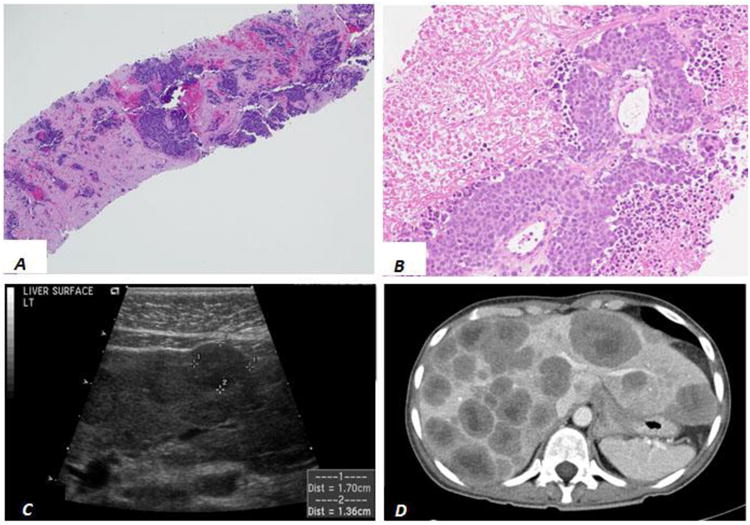
Liver imaging and histology from patients with metastatic small cell carcinoma. H&E stained liver core biopsies (A and B) demonstrate a nodular pattern of small blue cells and abundant necrosis adjacent to background hepatic parenchyma. Grayscale longitudinal ultrasound of the liver (C) and axial contrast-enhanced computed tomography (CT, D) demonstrate well-defined round masses throughout the liver. Central necrosis is evident by CT.
Immunohistochemistry (IHC) was performed in all cases. Both the diffuse infiltrating and nodular growth patterns of small blue neoplastic cells showed that neoplastic cells were positive for cytokeratin AE1/AE3 (Figure 4A) and the residual hepatocytes were highlighted by Cytokeratin Cam 5.2 (Figure 4B) and variably positive for TTF-1, as well as neuroendocrine markers including at least focally positive for chromogranin (Figure 4C), synaptophysin (Figure 4D) and CD56. CD45 and CD99 were negative in all cases to rule out other possible small blue cell malignancies including peripheral neuroectodermal tumor/Ewing's and lymphoma. The overall IHC patterns of expression confirmed a diagnosis of small cell carcinoma in all cases.
Figure 4.
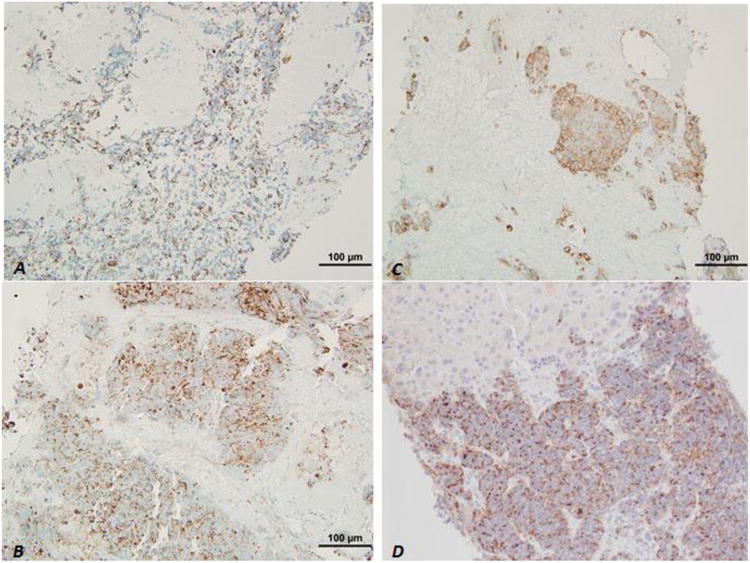
Immunohistochemistry of liver core biopsies. Neoplastic cells in both the nodular and diffuse patterns were positive for AE1/AE3 (A), and residual hepatocytes were positive for CK Cam5.2 (B); and, neoplastic cells were also highlighted by synaptophysin (C) and chromogranin (D).
Clinical presentation and imaging features of liver predominant/primary small cell carcinoma compared to metastatic small cell carcinoma
Chart review revealed that the two distinct histological patterns of liver small cell carcinom correlated to different clinical presentations and imaging patterns, as summarized in Table 1. In patients with the liver primary/predominant, diffuse infiltrating pattern of liver small cell carcinoma (n=6), all reported prodromal symptoms included vague abdominal discomfort, nausea or vomiting for 7-30 days prior to presentation. All patients rapidly progressed to hyper-acute (n=4) or acute liver failure (n=2). Review of all ultrasound (US) and CT imaging within 14 days of liver core biopsy or autopsy revealed hepatomegaly (Figure 2C)—defined by a liver measuring greater than 15 cm craniocaudal at the midhepatic line by US or CT in 4 of the 6 cases with available imaging. None of the 6 cases had a definitive liver mass lesion on US or CT (Figure 2C and D).
In contrast, none of the patients with the nodular growth pattern (metastasis) of small cell carcinoma presented with liver failure or hepatomegaly and were often asymptomatic with only 3 of 7 patients presenting with vague abdominal pain or discomfort. However, US and CT imaging demonstrated discrete, round nodules in the liver consistent with metastatic malignancy in all 7 cases (Figure 3C and D). In all cases a primary lesion was present in the lungs and in 3 of the 7 cases additional metastases were present in the hilar lymph nodes and/or chest wall. 18Flourine-deoxyglucose (FDG) Positron Emission Tomography (PET Scan) was performed in 2 patients with nodular liver lesions on CT (Figure 5A) and confirmed hyper-metabolic foci in the lung, liver and/or hilar lymph nodes consistent with metastatic disease (Figure 5B and C).
Figure 5.
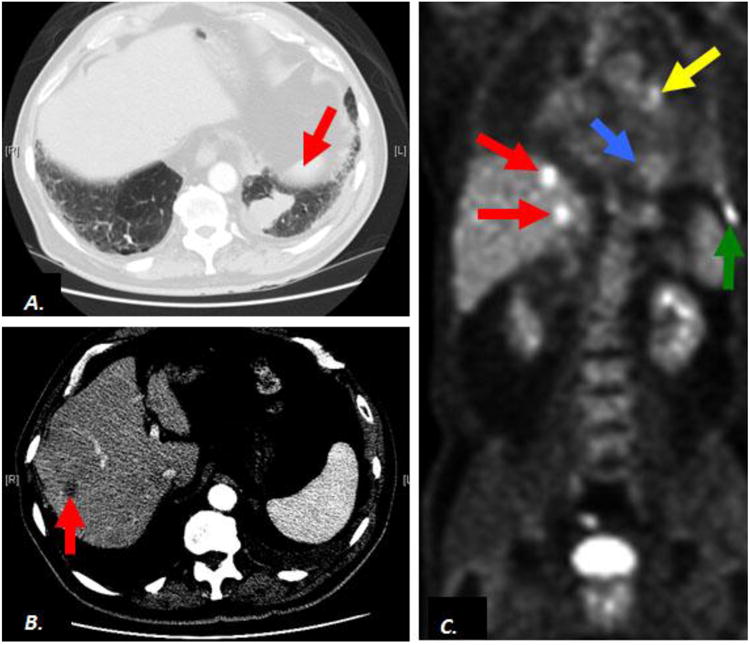
Contrast-enhanced computed tomography (CT) and 18Flourine-deoxy-glucose (FDG) Positron Emission Tomography (PET scan) in a patient with metastasis of small cell carcinoma to the liver. Axial CT demonstrates a suspicious pulmonary mass In left lower lobe of lung (A, arrow) and a nonspecific liver lesion (B, arrow). Coronal FDG PET scan images confirm hypermetabolism in the lung mass (C, blue arrow), consistent with malignancy as well as hypermetabolic liver lesions (C, red arrows) consistent with metastatic disease. Additional metastatic activity corresponds to a left hilar lymph node (C, yellow arrow) and left chest wall lesion (C, green arrow).
Discussion
Small cell carcinoma is a highly malignant neoplasm, is commonly seen in the lung. Ex-pulmonary small cell carcinoma is rare, and there are only a few case reports on liver small cell carcinoma [17-23]. In the present study, we have reported the autopsy and biopsy case series of liver predominant/primary small cell carcinoma and identified the unique morphologic and clinical features. Particularly, compared to multiple nodules/mass of metastatic small cell carcinoma in the liver, liver predominant/primary small cell carcinoma exhibited a diffusely sinusoidal infiltrative pattern in the liver parenchyma that was well correlated with clinical hepatomegaly and highly aggressive behavior – hyperacute liver failure or acute liver failure.
It is critical to define liver predominant or primary small cell carcinoma pathologically and clinically. Obviously, pathological definition of liver predominant or primary small cell carcinoma is histogenic origin of small cell carcinoma primarily from liver, and further clinically rule out the metastasis or prove that there is no small cell carcinoma in other organs. The possibility of histogenic origin of liver primary small cell carcinoma include: 1) hepatic stellate cell origin, 2) cancer-initiating cell or malignant stem cells; 3) ectopic heterotopic pancreatic or adrenal tissue located in the liver; and 4) neuroendocrine cells in the intrahepatic biliary ductal epithelium. Extensive anatomic analysis of two autopsy cases in our case series provided a strong evidence of liver predominant/primary small cell carcinoma that confirmed the hepatomegaly with diffuse sinusoidal infiltration of small cell carcinoma and no tumors were identified in other organs, providing the evidence of liver primary small cell carcinoma. Furthermore, liver small cell carcinoma in a serial biopsy cases provide further support of liver original tumor that the unique morphologic features of sinusoid diffusely infiltrating pattern with hepatomegaly and with no other mass lesions in other organs by body imaging studies. Thus, because of lack of extrahepatic disease, our case series confirms that these lesions should be considered liver predominant or primary small cell carcinoma.
The crucial morphologic features are the diffusely sinusoidal infiltrating small cell carcinoma, leading to hematomegaly with no discrete nodule. Thus unique morphologic features are well correlated with clinical presentation of hyper-acute or acute liver failure as initial clinical presentation. Imaging investigation including CT, US or magnetic resonance imaging (MRI) together with biopsy appears crucial clinical procedures to differentially diagnosing liver predominant/primary and metastatic small cell carcinoma. Obviously, the diagnosis of hepatic metastasis is usually straightforward on CT, US or magnetic resonance imaging (MRI). A discrete, solid hepatic mass lesion in the setting of a known primary neoplasm is presumed to be a metastasis in the absence of convincing features of benignity. Multiple focal lesions within the liver increase the probability that these lesions are metastatic. However, the imaging characteristics of an enlarged liver without focal lesions or discrete mass are nonspecific. Hepatomegaly can be caused by non-neoplastic diseases including viral/autoimmune hepatitis, metabolic or vascular disease, and storage disorders, or infiltrative processes including metastatic disease such as myloid leukemia. Though rare, malignant infiltration of the liver should be considered in the face of unexplained hepatomegaly seen by radiographic study. In these patients, needle biopsy warrants further definitive diagnosis.
In conclusion, we demonstrate that small cell carcinoma can present with two discrete histologic patterns in the liver. Patients demonstrating the diffusely sinusoidal infiltrative pattern may present with hepatomegaly and no evidence of mass lesion on liver imaging. Additionally, no evidence of a primary neoplasm may have been previously established or even identifiable on full body imaging in the subset with the diffusely infiltrative pattern. Our data, therefore, supports the importance of rapid immunohistochemical evaluation of liver biopsies in patients presenting with liver failure and evidence of a diffusely infiltrative small blue cell neoplasm histologically. Once a diagnosis is confirmed and communicated to clinicians the appropriate chemotherapy treatment plan can be rapidly implemented in hopes of preventing development of hyper-acute or acute liver failure. Although data available on the effectiveness of chemotherapy in treating small cell carcinoma in the liver needs more investigation, studies have shown that chemotherapy may occasionally reverse hepatic failure [24, 25].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WM. Recent developments in acute liver failure. Best Pract Res Clin Gastroenterol. 2012;26:3–16. doi: 10.1016/j.bpg.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canbay A, Tacke F, Hadem J, et al. Acute liver failure: a life-threatening disease. Dtsch Arztebl Int. 2011;108:714–720. doi: 10.3238/arztebl.2011.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairbank WH. Three atypical cases of Hodgkin's Disease, presenting with liver failure. Can Med Assoc J. 1953;69:315–317. [PMC free article] [PubMed] [Google Scholar]
- 4.Trewby PN. Liver disease as presenting manifestation of Hodgkin's disease. Q J Med. 1979;48:137–150. [PubMed] [Google Scholar]
- 5.Lefkowitch JH, Falkow S, Whitlock RT, et al. Hepatic Hodgkin's disease simulating cholestatic hepatitis with liver failure. Arch Pathol Lab Med. 1985;109:424–426. [PubMed] [Google Scholar]
- 6.Woolf GM, Petrovic LM, Rojter SE, et al. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med. 1994;121:729–735. doi: 10.7326/0003-4819-121-10-199411150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Braude S, Gimson AE, Portmann B, et al. Fulminant hepatic failure in non-Hodgkin's lymphoma. Postgrad Med J. 1982;58:301–304. doi: 10.1136/pgmj.58.679.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salo J, Nomdedeu B, Bruguera M, et al. Acute liver failure due to non-Hodgkin's lymphoma. Am J Gastroenterol. 1993;88:774–776. [PubMed] [Google Scholar]
- 9.Eras P, Sherlock P. Hepatic coma secondary to metastatic liver disease. Ann Intern Med. 1971;74:581–583. doi: 10.7326/0003-4819-74-4-581. [DOI] [PubMed] [Google Scholar]
- 10.Harrison HB, Middleton HM, Crosby JH, et al. Fulminant hepatic failure: an unusual presentation of metastatic liver disease. Gastroenterology. 1981;80:820–825. [PubMed] [Google Scholar]
- 11.Myszor MF, Record CO. Primary and secondary malignant disease of the liver and fulminant hepatic failure. J Clin Gastroenterol. 1990;12:441–446. doi: 10.1097/00004836-199008000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Krauss EA, Ludwig PW, Sumner HW. Metastatic carcinoma presenting as fulminant hepatic failure. Am J Gastroenterol. 1979;72:651–754. [PubMed] [Google Scholar]
- 13.Nouel O. Severe hepatic failure and portal hypertension due to metastatic carcinoma of the liver (author's transl) Gastroenterol Clin Biol. 1979;3:135–137. [PubMed] [Google Scholar]
- 14.Bouloux PM. Fulminant hepatic failure secondary to diffuse liver infiltration by melanoma. J R Soc Med. 1986;79:302–303. doi: 10.1177/014107688607900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleichner JC, Chun B, Klappenbach RS. Pure small-cell carcinoma of the prostate with fatal liver metastasis. Arch Pathol Lab Med. 1986;110:1041–1044. [PubMed] [Google Scholar]
- 16.McGuire BM, Cherwitz DL, Rabe KM, et al. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72:133–139. doi: 10.4065/72.2.133. [DOI] [PubMed] [Google Scholar]
- 17.Isobe T, Yanai S, Kusaba H, et al. Effective monotherapy with amrubicin for a refractory extrapulmonary small-cell carcinoma of the liver. Case Rep Med. 2009;2009:538081. doi: 10.1155/2009/538081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert J, Rutledge H, Koch A. Diffuse malignant infiltration of the liver manifesting as a case of acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2008;5:405–408. doi: 10.1038/ncpgasthep1154. [DOI] [PubMed] [Google Scholar]
- 19.Choi SJ. Extrapulmonary small cell carcinoma of the liver: clinicopathological and immunohistochemical findings. Yonsei Med J. 2007;48:1066–1071. doi: 10.3349/ymj.2007.48.6.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YH, Kwon R, Jung GJ, et al. Extrapulmonary small-cell carcinoma of the liver. J Hepatobiliary Pancreat Surg. 2004;11:333–337. doi: 10.1007/s00534-004-0904-9. [DOI] [PubMed] [Google Scholar]
- 21.Zanconati F, Falconieri G, Lamovec J, et al. Small cell carcinoma of the liver: a hitherto unreported variant of hepatocellular carcinoma. Histopathology. 1996;29:449–453. doi: 10.1046/j.1365-2559.1996.d01-514.x. [DOI] [PubMed] [Google Scholar]
- 22.Park CH, Chung JW, Jang SJ, et al. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27:1306–1311. doi: 10.1111/j.1440-1746.2012.07117.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia MT, Bejarano PA, Yssa M, et al. Tumor of the liver (hepatocellular and high grade neuroendocrine carcinoma): a case report and review of the literature. Virchows Arch. 2006;449:376–381. doi: 10.1007/s00428-006-0251-0. [DOI] [PubMed] [Google Scholar]
- 24.Rice K, Schwartz SH. Lactic acidosis with small cell carcinoma. Rapid response to chemotherapy. Am J Med. 1985;79:501–503. doi: 10.1016/0002-9343(85)90038-5. [DOI] [PubMed] [Google Scholar]
- 25.Colman LK, Baker TM. Lactic acidosis with extensive oat cell carcinoma of the lung--not necessarily a poor prognostic sign: case report. Mil Med. 1983;148:440. [PubMed] [Google Scholar]


