Abstract
The expression and biological consequences of Kaiso, a novel bi-modal transcription factor, in infiltrating ductal carcinomas (IDCs) have not been widely investigated. In the present study, we determined Kaiso expression and subcellular localization in 146 normal tissues, 376 IDCs, and 85 lymph node metastases. In IDCs, there was higher Kaiso expression in both the cytoplasmic and nuclear compartments, which correlated with age <48 (cytoplasmic p<0.0093; nuclear p< 0.0001) and moderate differentiation (cytoplasmic p<0.0042; nuclear p<0.0001), as determined by Chi-square analysis. However, only nuclear Kaiso correlated with poor prognostic factors, i.e., race (African Americans) (p<0.0001), poor differentiation (p<0.0001), and metastases (p<0.0001). Nuclear Kaiso was also associated with worse overall survival (p<0.0019), with African American patients displaying worse survival rates relative to Caucasian patients (p<0.029). MCF-7 (non-metastatic), MDA-MB-468 (few metastases), and MDA-MB-231 (highly metastatic) breast cancer cells demonstrated increasing Kaiso levels, with more nuclear localization in the highly metastatic cell line. Over-expression of Kaiso in MCF-7 cells increased cell migration and invasion, but treatment of MDA-MB-468 and MDA-MB-231 cells with si-Kaiso decreased cell migration and invasion and induced expression of E-cadherin RNA and protein. E-cadherin re-expression was associated with a reversal of mesenchymal associated cadherins, N-cadherin and cadherin 11, as well as decreased vitmenin expression. Further, Kaiso directly bound to methylated sequences in the E-cadherin promoter, an effect prevented by 5-aza-2-deoxycytidine. Immunofluorescence co-staining of poorly differentiated IDCs demonstrated that nuclear Kaiso is associated with a loss of E-cadherin expression. These findings support a role for Kaiso in promoting aggressive breast tumors.
Keywords: Breast cancer, Kaiso, EMT, Metastasis, DNA methylation, African American
Introduction
Infiltrating ductal carcinoma (IDC) is the most commonly diagnosed breast cancer in women [1], with African-American women more likely to be diagnosed with larger and later stage tumors [2]. However, most of the morbidity and mortality for patients with IDC is associated with metastatic disease [3]. The conversion to metastasis has been associated with a loss of cell-cell contacts, in particular, down-regulation of E-cadherin, which further influences the degree of cell morphology and migratory and invasive capacities in vitro and in vivo [4, 5]. Although there are several mechanisms proposed for transcriptional silencing of E-cadherin, hypermethylation of the E-cadherin promoter is thought to be a major mode of down-regulation [6–8]. However, the mechanism associated with hypermethylation-related silencing of E-cadherin is not elucidated.
Epigenetic changes, in particular DNA methylation, are common molecular alterations that promote tumor development and progression. However, DNA methylation alone is insufficient to silence transcription [9]; instead, recognition of methylated DNA by two classes of proteins that contain a methyl-CpG binding domain and/or with C2H2 zinc fingers mediates the repressive effect. Kaiso, a bi-modal transcription factor that belongs to the BTB-POZ (broad complex, tramtrak bric-a-brac/Pox virus and zinc finger) subfamily of zinc-finger proteins, is such a protein (POZ-ZF) [10–12]. Kaiso is expressed in numerous tumor types, with different subcellular patterns. For example, elevated levels of Kaiso are present in the cytoplasm of chronic leukemia cells and in cells of non-small cell lung cancers in late stages [13, 14]. In colorectal and prostate cancers, however, Kaiso is present in both the cytoplasm and the nucleus, with more expression within the nuclear compartment [15, 16]. We reported that nuclear Kaiso is observed predominantly in prostate tumors with high Gleason grades. Furthermore, epidermal growth factor receptor (EGFR)-induced Kaiso subcellular localization to the nucleus caused methylation-dependent silencing of E-cadherin, promoted increased cell migration and invasiveness of prostate cancer cell lines, and induced these cells to undergo an epithelial-mesenchymal transition (EMT) [17]. In other models, Kaiso regulated genes associated with EMT, including E-cadherin [16, 18], Wnt 11 [19], and matrilysin [20]. However, with the exception of Kaiso-regulated expression of cyclin D1 [21], a tumor promoting function for Kaiso in breast cancer has yet to be elucidated. During preparation of this manuscript, a report was published demonstrating that increased expression of Kaiso, in particular its nuclear localization, is associated with high-grade, triple-negative IDCs [22], suggesting that Kaiso promotes aggressive breast tumors. Nevertheless, the mechanism accounting for the repressor activity of Kaiso in breast cancers has not been determined.
Herein, we report a cytoplasmic-to-nuclear shift of Kaiso in late-stage, poorly differentiated IDCs in a large patient cohort. Nuclear expression of Kaiso correlated with clinicopathological features, such as tumor grade/differentiation, clinical stage, and race. Paired samples of normal tissues, primary tumor tissues, and tumor metastases demonstrated an increase in nuclear expression of Kaiso, with African Americans patients having higher levels at each stage (p<0.0001). Nuclear Kaiso is further associated with poor patient survival, with African American women having lower survival rates relative to Caucasian patients. MCF-7, MDA-MB-468, and MDA-MB-231 are cell lines which represent progressive stages of breast cancer. They were found to have increasingly higher Kaiso levels, as well as increased nuclear localization, in the metastatic cell lines. Over-expression of Kaiso in MCF-7 cells increased cell migration and invasion, but depletion of Kaiso in MDA-MB-468 and MDA-MB-231 cells decreased cell migration and invasion and induced expression of E-cadherin mRNA and protein levels. This appears to be a direct regulation, as Kaiso binds to methylated sequences in the E-cadherin promoter, an effect prevented by 5-aza-2-deoxycytidine (5-aza). Lastly, immunofluorescence co-staining of poorly differentiated IDCs demonstrated that nuclear Kaiso is associated with a loss of E-cadherin expression. Collectively, these findings support an oncogenic role for Kaiso in promoting aggressive breast tumors.
Materials and Methods
Patient Populations
Breast cancer tissue microarrays (TMAs) [23] were obtained from US Biomax (Rockville, MD) or from the Anatomic Pathology Division at University of Alabama at Birmingham (UAB). All specimens were originally collected utilizing Institutional Review Board-approved protocols with informed consent. Additionally, the Institutional Review Boards of Tuskegee University and UAB approved the use of tissues for this study.
Immunohistochemistry (IHC)
IHC was performed with the anti-Kaiso clone 6F (Upstate Biotechnology, MA) and the 12H clone (Santa Cruz, CA), which were generated against the same antigenic epitope in a Kaiso-ΔZF fusion protein [24]. Duplicate TMAs were stained for evaluation by IHC [25]. Briefly, cells were blindly, scored by two pathologists who obtained similar results. Individual specimens stained for Kaiso were scored separately for cytoplasmic and nuclear staining. Immunostaining was evaluated by determining the percentage of malignant cells in three random fields that demonstrated staining on a scale of 0–4. Scores of 0 (no staining), 1 (<10%), 2 (10%–50%), 3 (>50%–75%), and 4 (>75%) were assigned as previously described [17, 26].
Cell culture, antibodies, reagents, and transfection
Human breast cancer cell lines, MCF-7, MDA-MB-468, and MDA-MB-231, were obtained from ATCC (Manassas, VA) and routinely cultured in Dulbecco’s modified Eagle’s medium/F-12 supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA and antibiotics in a humidified atmosphere of 5% CO2.
MCF-7 cells over-expressing Kaiso were generated by transient transfection with pLenti-Kaiso red fluorescence protein (RFP) (Applied Biological Materials, Richmond, BC). Small-interfering RNA (siRNA) Kaiso constructs 1 and 2 were obtained from Invitrogen. Transient transfections were performed using Lipofectamine 2000 according to the manufacturer’s protocol (Invitrogen). Primary antibodies were obtained as follows: Kaiso 6F clone (Abcam, Boston, MA); anti-Kaiso clone 6F (Upstate Biotechnology, Billerica, MA); anti-Kaiso 12H (Santa Cruz, CA), anti-E-cadherin, and anti-p120ctn (BD Biosciences, OR); anti-cadherin 11 (Cell Signaling); anti-N-cadherin (BD Biosciences); anti-α-tubulin and anti-TFIID (Santa Cruz); and anti-β-actin (Sigma Aldrich, MO). Secondary antibodies were Alexa 488 and 594 anti-mouse and anti-rabbit (Invitrogen). Nuclear staining was accomplished with Vectashield mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) (Vectorlabs, Burlingame, CA).
5-aza-2-deoxycytidine Treatment
Cells (60% confluent) were treated with 5 µM 5-aza-2-deoxycytidine (5-aza) for 3 days, with fresh media supplemented with 5-aza each day. Cells were harvested and assayed for E-cadherin expression by qRT-PCR and Kaiso binding in ChIP assays.
Immunoblotting
Cells were grown to 80% confluence in six-well plates. Lysates were prepared from cultured cells in a solution containing 50 mM Tris, pH 7.5; 120 mM NaCl; 0.5% Nonidet p-40; 40 µM phenylmethylsulfonylfluoride (PMSF); 50 µg/ml leupeptin; and 50 µg/ml aprotinin (all from Sigma, St. Louis, MO). Cells were allowed to lyse for 1 hr on ice. The lysed cells were centrifuged, the resulting supernatants were collected, and the protein contents were measured by a Bradford assay. Lysates (30 µg of protein) were separated by polyacrylamide gel electrophoresis with 8% sodium dodecyl sulfate, immunoblotted, and analyzed by chemiluminescence (Amersham Biosciences, NJ).
Immunofluorescence microscopy
Cells (3 × 105) were grown to 80% confluence on glass coverslips and then fixed with methanol alone or with 4% paraformaldehyde, permeabilized with 100 mM Tris-HCl (pH 7.4), 150 mM NaCl; 10 mM EGTA; 1% Triton X-100; 1 mM PMSF; and 50 µg/ml aprotinin (all from Sigma), and subsequently blocked with 5% bovine serum albumin for 1 hr at room temperature. Identical results were obtained with both methods. Samples were incubated with indicated primary antibodies diluted in blocking buffer at 4°C overnight. Secondary antibodies were Alexa 488 and 594 anti-mouse and anti-rabbit (Invitrogen). Nuclear staining was accomplished with Vectashield mounting medium containing DAPI, and staining was observed with a DSU confocal microscope (Olympus, New York). To determine the relative intensities, total areas of cytoplasmic and nuclear regions of each image were measured as well as the threshold intensity for each channel utilizing Metamorph Imaging Software (Molecular Devices, LLC, Sunnyvale, CA). Differences between intensities were determined by Excel. Bar graphs represented n = 3 images sectioned and individually analyzed for total area. All quantitative data were normalized to appropriate control images.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with TRIzol (Invitrogen). cDNA was prepared using Superscript III First Strand cDNA Synthesis kits (Invitrogen). Gene expression was quantified by the Taqman assay (Invitrogen), and fold change was calculated using the 2-ΔCt value method. Analyses were performed in triplicate. Taqman assay IDs included Kaiso (Hs00272725_s1), E-cadherin (Hs01023894_m1), and HPRT1 (Hs02800695_m1).
Cell migration assays
Cell migration assays were performed as previously described [17]. In brief, cells treated with siRNA Kaiso or negative control (scrambled siRNA) were plated at 70–80% confluency in complete growth media for 24 hr. A denuded area was generated in the middle of each well with a rubber policeman. The cells were then incubated for 24 hr in dialyzed media. Images were taken at 0 and 24 hr, and the relative distance of migration into the wounded area was determined using Metamorph Imaging Software. The mean values ± SEM of six measurements were recorded for each time point and condition. All measurements were normalized to 0 hr controls.
Invasion assays
Cancer cell invasiveness was determined with 24-well Trans plates (8-µm pore polycarbonate membrane inserts; BD Biosciences, CA) according to the manufacturer’s protocol. For these assays, 5 × 104 cells were plated in the Matrigel-coated chamber inserts. Cells were suspended in medium without serum or growth factors, and medium with 10 ng/mL of epidermal growth factor was used as a chemo-attractant in the lower chamber. After incubation at 37°C for 18 hr, the noninvasive cells on the top of the chambers were removed with cotton swabs. The invading cells on the underside of the membrane were fixed in 100% methanol for 10 min, air dried, stained in 0.1% crystal violet, and counted under a microscope. The number of invading cells in five random, 10× microscopic fields were counted and expressed as percentages of the control. The results presented were the means ± SD of invading cells from three independent experiments, all performed in triplicate.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) experiments were performed with the use of the ChIP-IT kit (Active Motif, Carlsbad, CA). In brief, MDA-MB-231 cells were fixed in 1% formaldehyde at room temperature for 10 min; the fixation reaction was stopped by adding a 1:10 volume of 10× glycine at room temperature for 5 min. The cell pellets were suspended and incubated for 30 min in ice-cold lysis buffer with PMSF and a proteinase inhibitor cocktail. The nuclear pellets were suspended in shearing buffer, and chromatin was sheared to an average size of 200 to 1500 bp by sonication at 25% power for 10 pulses of 20 seconds each, with a 30-second rest on ice between each pulse. Chromatin (10 µL) was saved for input DNA control. Sheared chromatin was incubated in ChIP buffer 1 with 25 µL of protein G magnetic beads, anti-Kaiso antibody (Abcam), mouse RNA Polymerase II (Active Motif), and rabbit IgG antibody (Active Motif, as a negative control), on a rolling shaker at 4°C for 4 hr. The immunoprecipitated chromatin was purified from the chromatin-antibody mixture by washing several times, and the chromatin-immunoprecipitated DNA was eluted in 50 µL of elution buffer AM2 (Active Motif). Crosslinks were reversed by adding 50 µL of reverse-crosslink buffer. After removing proteins by digestion with proteinase K, the purified DNA was used as a template for PCR analysis. In silco analysis of KBS (CTGCNA) and methyl binding sites in the E-cadherin promoter were determined using three databases: PROSCAN, BIOBASE, and Genomatix. The primers used were designed according to Prokhortchouk et al. [18, 27] to amplify a 280-bp methylated fragment of the E-cadherin promoter (−1290 to −1570): 5′- AGCACAGAGACTGGCACAGTAA -3′ and 5′-GATTGAGACCATCCTGGCTAAC -3′.
Statistical analyses
For all experiments, statistical calculations were performed with Microsoft Excel or GraphPad prism software. Independent Student’s t-test was utilized to determine statistical differences between experimental and control values. Tissue correlations were performed with Matlab (Mathworks Inc., Natick, MI). The probabilities of overall survival were calculated using the Kaplan-Meier method and were compared using the log-rank test. For determining factors related to overall survival, a Cox proportional hazard model was utilized. All statistical analyses were performed using SAS for Windows (Version 9.2; SAS Institute, Cary, NC). p values < 0.05 were considered statistically significant.
Results
Subcellular localization of Kaiso in IDCs
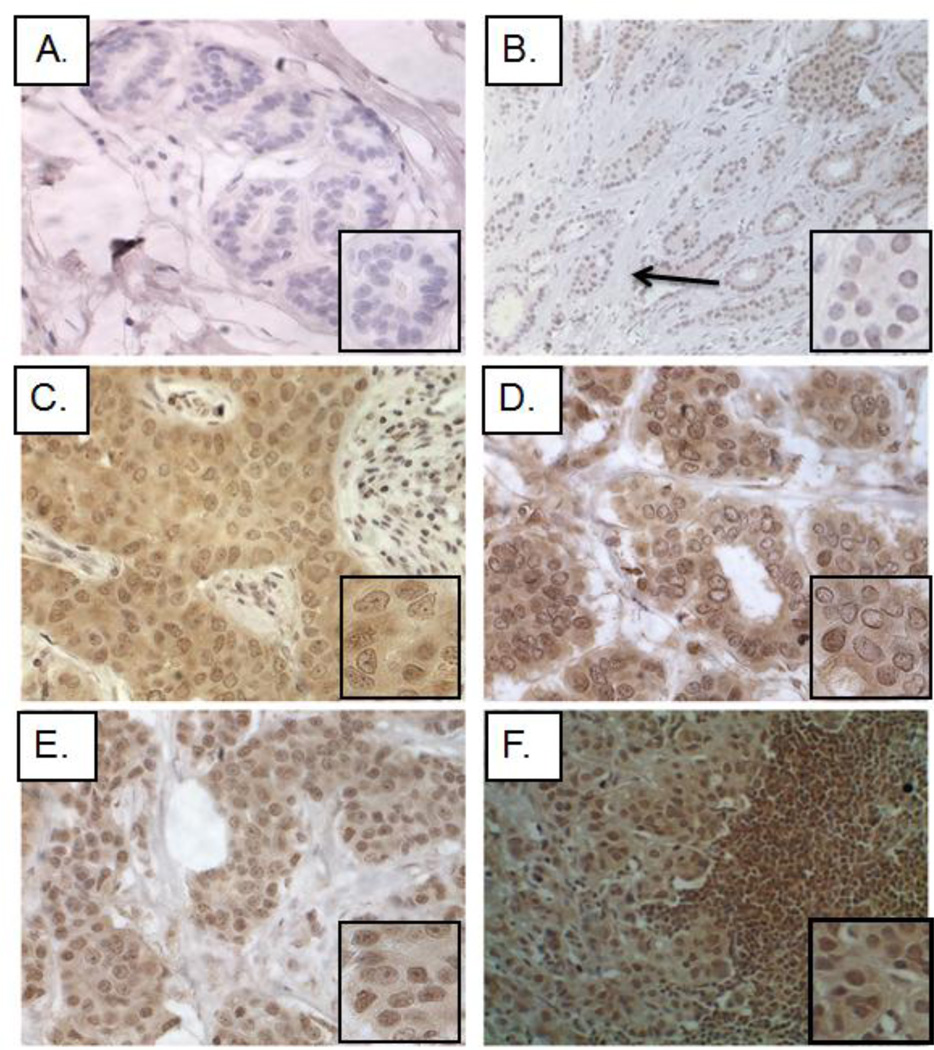
Kaiso expression and subcellular localization were determined for a cohort that included 607 specimens, consisting of normal breast epithelium (146 patients), IDCs (376 patients), and lymph node metastases (85 patients). Data on the patient population included clinicopathological characteristics such as age, histological grade, and ethnicity; however, not all clinical data were available for each patient (supplemental Table 1). As determined with the 6F Kaiso antibody, Kaiso expression in the epithelial cells of normal samples (obtained from healthy volunteers without a breast cancer diagnosis) was low to absent (Figure 1A); however, histologically normal cells adjacent to tumors showed detectable Kaiso expression in the cytoplasm and nucleus (Figure 1B). Similar to previous reports [28], Kaiso expression in IDCs was increased in the cytoplasm of well-differentiated and moderately differentiated tumors, and minimal expression in the nucleus was present (Figure 1C–D). In poorly differentiated tumors and lymph node metastases, however, both cytoplasmic and nuclear Kaiso expression was noted with more intense staining in the nucleus (Figure 1E–F). Similar results were obtained using the 12H Kaiso antibody (Supplemental Figure 1A–F).
Fig. 1.
Subcellular localization of Kaiso in normal tissues, primary tumors, and metastatic breast tumors. With the 6F Kaiso antibody, IHC was performed on multiple breast cancer progression TMAs. Presented are representative images from 607 patients. (A) Normal breast tissue showed low to absent Kaiso expression. (B) Adjacent normal cells demonstrated detectable Kaiso expression in the cytoplasm and nucleus. (C) Well-differentiated tumors revealed a general upregulation of Kaiso expression within the cytoplasm, with nuclear positivity. (D–E) Moderately and poorly differentiated tumors showed intense cytoplasmic and nuclear positivity. (F) Lymph node metastases demonstrated low cytoplasmic Kaiso and intense nuclear Kaiso positivity. There was an incremental trend to an overall increase in nuclear expression of Kaiso in primary tumors and metastases relative to normal tissues. Images in Panel B were taken at an original 100× magnification; all other images were taken at 400× magnification. The inserts are pictures at high magnification. The material quality of each specimen was determined based on hematoxylin and eosin (H&E) staining (data not shown).
To determine the clinical significance of Kaiso expression, semi-quantitative analysis of IHC staining was performed. Fishers Exact test and Chi-Square analyses demonstrated that cytoplasmic and nuclear Kaiso expression correlated with patient age (<48 years of age, p<0.0001) and tumor grade (cytoplasmic, p<0.0042, and nuclear, p<0.0001) (Table 1). However, only nuclear Kaiso correlated with tumors of African-American patients. Cytoplasmic expression of Kaiso correlated with local invasion (p<0.0001), but this was not apparent in lymph node metastases (p=0.8906) (Table 1). Nuclear Kaiso, however, correlated with both local invasion (p<0.0001) and lymph node metastasis (p<0.0001) (Table 1). These analyses support the concepts that there is a progressive enhancement of nuclear Kaiso during the progression of breast cancer and that the extent of this abnormal expression correlates with clinicopathological features commonly utilized to diagnose clinical stages of breast tumors.
Table 1. Correlation of sub-cellular Kaiso expression with patient and tumor characteristics.
Correlation of Kaiso subcellular localization with clinical features. IHC staining of Kaiso in the cytoplasmic and nuclear compartments, determined by semi-quantitative analysis, was examined for correlation with clinical features utilizing Fishers exact test and validated with Chi-square tests. (Patients missing clinical characteristics were excluded from individual correlation analyses.)
| Characteristics | All patients | Cytoplasmic Kaiso Expression | p† | Nuclear Kaiso Expression | p† | ||
|---|---|---|---|---|---|---|---|
| <1.5(median) | ≥1.5 | <1.5median) | ≥1.5 | ||||
| Total (n) | 607 | ||||||
| Age | |||||||
| ≤48(median) | 170 | 104 | 66 | 0.0093 | 167 | 3 | < 0.0001 |
| >48 | 241 | 116 | 125 | 47 | 194 | ||
| Grade | |||||||
| Well-Mod | 137 | 12 | 125 | 0.0042 | 73 | 64 | <0.0001 |
| Mod - Poor | 156 | 2 | 154 | 72 | 154 | ||
| Race | |||||||
| Back | 118 | 117 | 1 | 0.4448 | 45 | 73 | <0.0001 |
| White | 250 | 7 | 243 | 165 | 85 | ||
| Invasion | |||||||
| Non-invasive (Normal) | 146 | 62 | 84 | < 0.0001 | 142 | 4 | < 0.0001 |
| Invasive | 376 | 12 | 364 | 179 | 197 | ||
| Metastasis | |||||||
| Non-metastatic (Normal) | 146 | 84 | 62 | 0.8906 | 142 | 4 | <0.0001 |
| Metastatic | 85 | 50 | 35 | 5 | 80 | ||
P values with * are deemed significant.
Fishers exact test was used and subsequently validated with the c2 test.
NOTE: Patients missing due to the lack of clinical characteristics or tissue damage were excluded from the analysis.
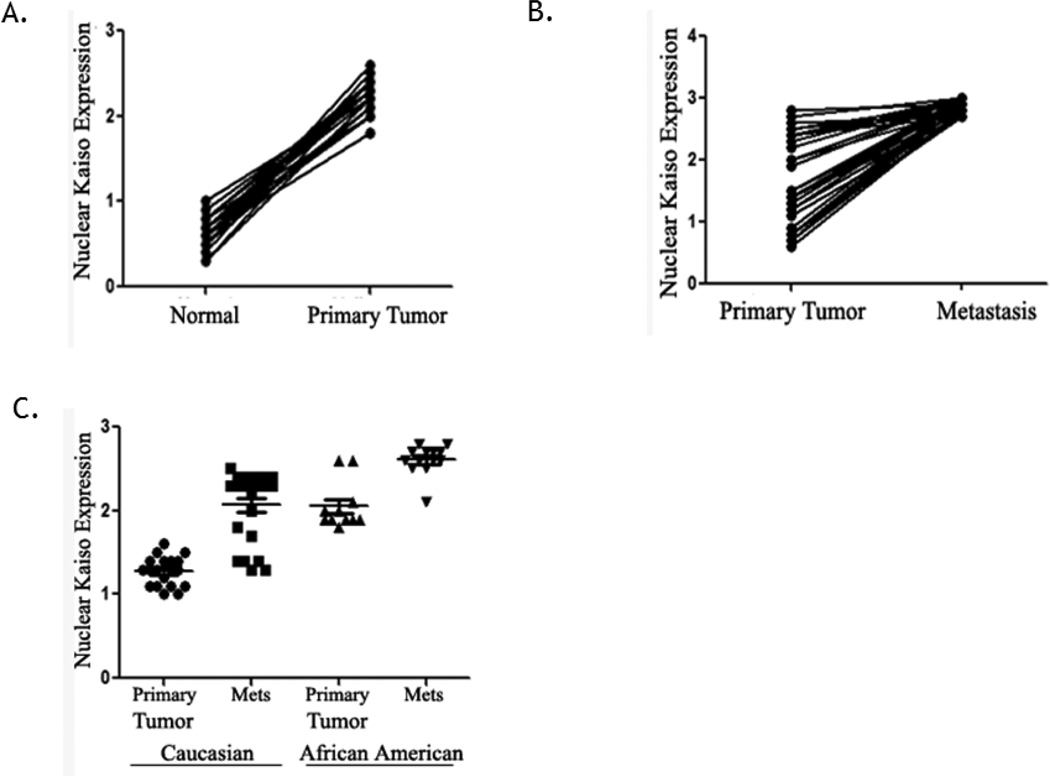
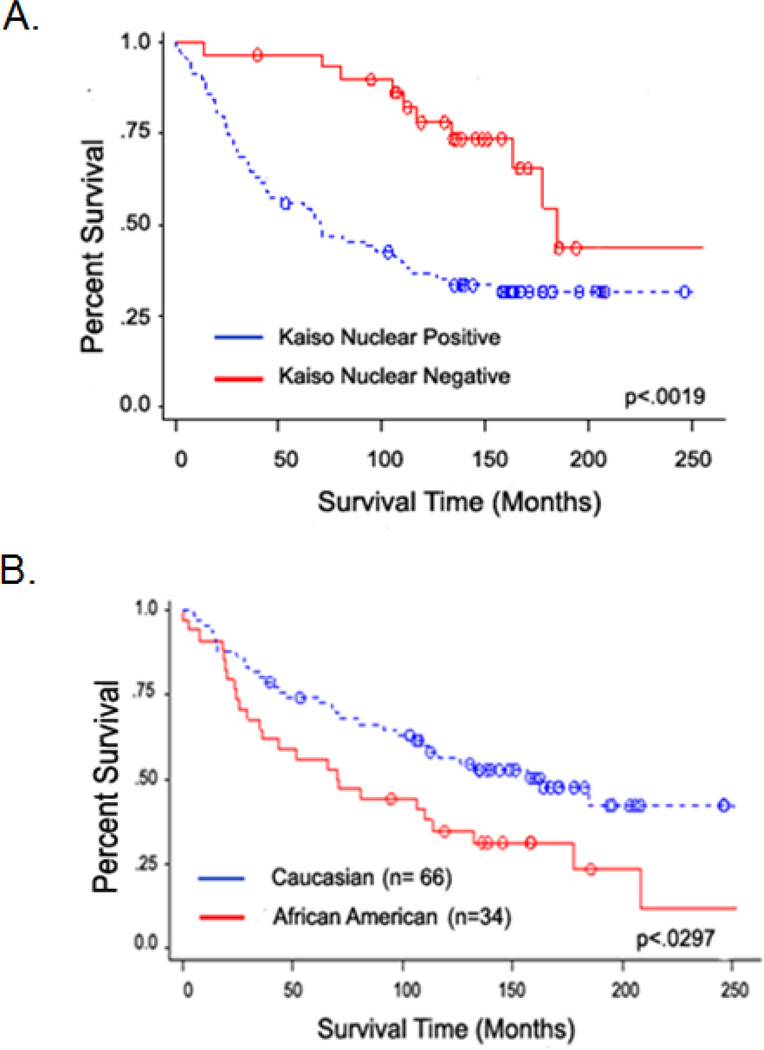
Since there appeared to be an increase in nuclear localization of Kaiso in IDCs, the localization pattern in individual patients was analyzed. Analysis of paired samples from histologically normal epithelium and primary IDCs from individual patients (n=48) demonstrated an increase in nuclear Kaiso (p<0.0001) (Figure 2A). This difference was also found by comparing nuclear expression in primary IDCs with lymph node metastases (n=51) (p<0.0001) (Figure 2B). An analysis of race in a subset of patients with the same histological grade of IDCs demonstrated that, in primary IDCs and metastases, African-Americans have higher expressions of nuclear Kaiso compared to Caucasian patients (Figure 2C). To determine the prognostic significance of Kaiso expression in IDC patients, a Kaplan-Meier survival analysis was performed on a subset of patients with follow-up information (n=100). Patients positive for nuclear Kaiso had an overall poorer survival relative to patients whose tumors were nuclear Kaiso-negative (p<0.0019) (Figure 3A). Since there were significant increases in nuclear Kaiso expression in African American patients, the presence of nuclear Kaiso in relation to survival rates in this population was determined. African-American patients with nuclear Kaiso had a poor survival rate relative to Caucasian patients positive for nuclear Kaiso (p=0.0297) (Figure 3B). These results indicate that nuclear Kaiso is associated with aggressive tumors and that it negatively influences patient survival.
Fig. 2.
Increased nuclear expression of Kaiso associated with clinical features of breast cancer progression. (A) Paired normal and primary breast tumors (n=48) showed higher nuclear Kaiso expression in primary tumors relative to normal tissues from the same patients (p<0.0001). (B) For paired primary IDCs and lymph node metastases (n=51), there was higher nuclear Kaiso expression in the metastases relative to primary tumors from the same patients (p<0.0001). (C) In primary tumors and lymph node metastases from African American patients, there was higher expression of nuclear Kaiso than in similar samples from Caucasians (p<0.0001).
Fig. 3.
Kaplan-Meier analysis showing overall survival among IDC patients, based on their positive or negative expression of nuclear Kaiso. (A) Positive nuclear expression of Kaiso correlated with a poor prognosis (p = 0.0019). (B) Positive nuclear Kaiso expression in samples from African American patients correlated with a poorer prognosis relative to similar samples from Caucasian patients positive for nuclear Kaiso (p= 0.0297).
A functional role for Kaiso expression in models of breast cancer
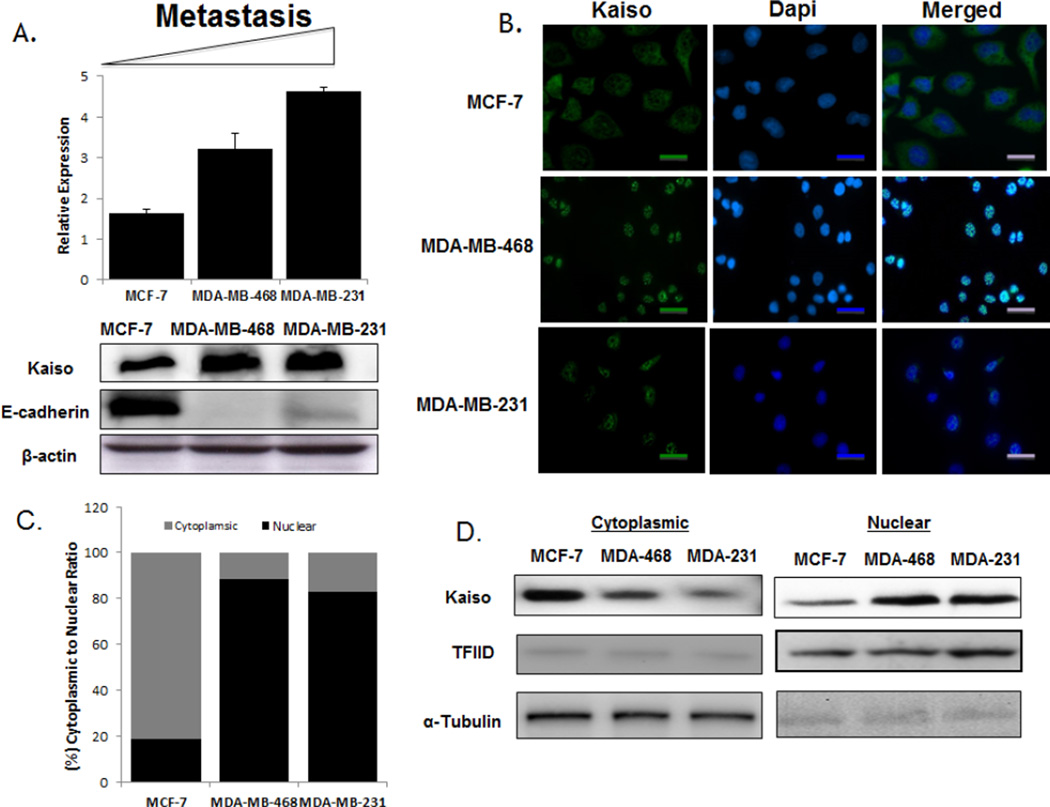
Since there have been no reports of Kaiso expression in non-metastatic relative to metastatic breast cancer cell lines, Kaiso expression and localization was assessed in MCF-7, MDA-MB-468, and MDA-MB-231 cells. qRT-PCR and immunoblots showed that Kaiso levels were elevated at the mRNA and protein levels in MDA-MB-468 and MDA-MB-231 cells relative to MCF-7 cells (Figure 4A). This higher expression correlated with an absence of E-cadherin expression. Confocal images showed that Kaiso was localized predominantly in the cytoplasm of the non-metastatic MCF-7 cells, as opposed to the metastatic MDA-MB-468 and MDA-MB-231 cells, which exhibited intense cytoplasmic and nuclear positivity (Figure 4B). This was confirmed by quantification of fluorescence intensity (Figure 4C) and by immunoblots of subcellular fractionations of cytoplasmic and nuclear compartments (Figure 4D). These findings suggest that Kaiso expression and nuclear localization in the more aggressive breast cancer cells lines were similar to the results for patient samples, and further suggest that this is an appropriate model to determine the functional role of Kaiso in promoting aggressive breast tumors.
Fig. 4.
Kaiso expression and localization in breast cancer cell lines. (A) Kaiso mRNA expression levels, measured by real-time PCR, revealed that increasing expression correlated with aggressiveness for MCF-7, MDA-MB-468, and MDA-MB-231 cells. Immunoblot analyses of cells treated with the anti-Kaiso 6F8 antibody showed increasingly higher expression of Kaiso protein in MCF-7, MDA-MB-468, and MDA-MB-231 cells, correlating with less to more aggressive cell lines. (B) Kaiso protein expression and localization among breast cancer cells, determined by immunofluorescence, showed that MCF-7 cells displayed high cytoplasmic expression relative to the metastatic cell lines, MDA-MB-468 and MDA-MB-231, which have a higher nuclear expression. Anti-mouse Alexa 488 (green) was utilized as secondary antibody and DAPI as a nuclear counter-stain (blue). (C) Bar-graph quantification of Kaiso fluorescent intensity in the individual cytoplasmic and nuclear compartments. (D) Cytosolic and nuclear fractions were isolated by sequential extraction. Anti-α-tubulin was used as a loading control for the cytoplasmic fraction and TFIID as a loading control for the nuclear fraction.
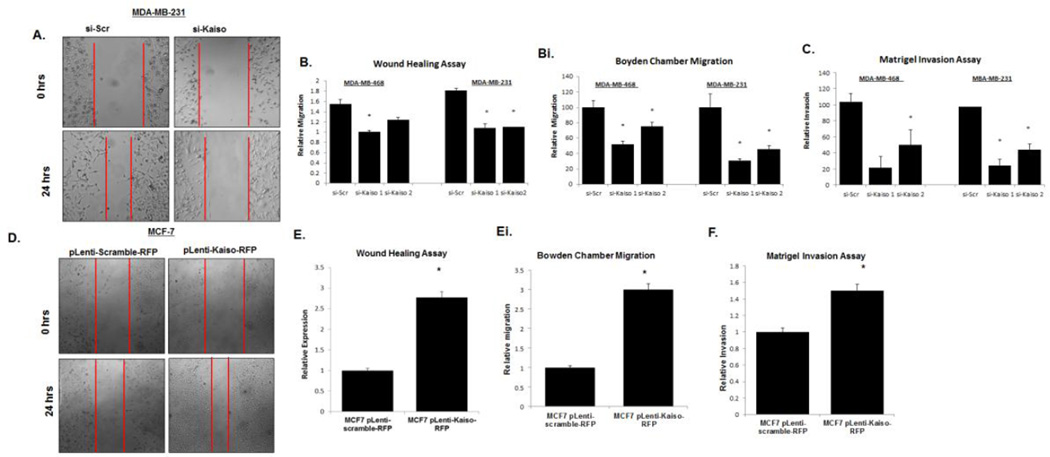
An important attribute of aggressive tumor cells is their capacity to invade the basement membrane of local tissues and arrive at ectopic locations. Tumor cells invading local tissue must gain the capacity to become motile as well as invasive. To accomplish invasion, cells degrade protein barriers and tunnel through the extracellular matrix. Since our data suggested an increase in nuclear Kaiso expression in IDCs and in metastatic tissues, we investigated the role of Kaiso in functional assays of cell migration and invasion. To determine if increased Kaiso expression had an effect on cellular behavior, we first utilized two siRNA Kaiso constructs (constructs 1 and 2) in the highly metastatic MDA-MB-231 cell line. At 100 nM, siRNA Kaiso 1 construct caused an 87% decrease of Kaiso expression, compared to that of siRNA Kaiso construct 2, which was only 63% lower (Supplemental Figure 2A). For MDA-MB-468 cells, siRNA Kaiso construct 1 showed similar results (Supplemental Figure 2B). siRNA Kaiso construct 1 down-regulated Kaiso expression at the protein level (Supplemental Figure 2C), with siRNA Kaiso cells displaying a lack of Kaiso expression in the nucleus, as determined by immunofluorescence (Supplemental Figure 2D). Hence, siRNA Kaiso construct 1 was utilized for the remaining experiments.
Since our results with human tissues suggested that nuclear Kaiso is associated with IDCs and lymph node metastases, we determined if reducing Kaiso levels affected essential features of metastatic cells, i.e., cell migration and invasion, by performing cell migration (scratch) and Boyden chamber migration assays in cells treated with either siRNA Kaiso or scrambled siRNA. After 24 hr, measurements of the denuded areas, starting from the acellular front of both sides (Figure 5A) of MDA-MB-468 and MDA-MB-231 cells treated with siRNA Kaiso showed a decrease in cell migration relative to control (scrambled siRNA) cells (Figure 5B). Similar results were obtained utilizing Boyden chambers (Figure 5Bi, Supplemental Figure 3). Similarly, cells treated with siRNA Kaiso showed a reduced capacity to invade through a layer of Matrigel (Figure 5C). In a related experiment, MCF-7 cells were transfected with pLenti-scramble-RFP plasmid or with pLenti-Kaiso RFP plasmid to over-express Kaiso. There was increased Kaiso expression at the RNA and protein levels in cells transfected with the pLenti-Kaiso RFP plasmid relative to cells transfected with the pLenti-scramble-RFP plasmid (Supplemental Figure 4 A,B). RFP was expressed in the cytoplasm of cells transfected with the scramble plasmid, in contrast to predominantly nuclear expression in cells transfected with Kaiso-RFP (Supplemental Figure 4C). MCF-7 cells transfected with pLenti-Kaiso RFP displayed increased cell migration (Figure 5D,E) and invasion (Figure 5F) relative to cells treated with pLenti-scramble-RFP. Thus, increased Kaiso expression and nuclear localization is associated with features of metastatic cells.
Fig. 5.
Down-regulation of Kaiso expression results in decreases of migration and invasion. (A) Cells treated with siRNA Kaiso 1, siRNA Kaiso 2, or scrambled siRNA were wounded, and cell migration was analyzed after 24 hr. Photos were taken at 100× magnification. MDA-MB-231 images serve as representative images of both MDA-MB-468 and MDA-MB-231 cells. Red vertical bars indicate the starting area migration on Day 0. (B) Quantitative analysis of relative migration also showed that MDA-MB-231 and MDA-MB-468 cells treated with siRNA Kaiso 1 or 2 had delays in migration relative to untreated cells or cells treated with scrambled siRNA (si-Scr). (Bi) Migration rates of MDA-MB-231 and MDA-MB-468 cells treated with siRNA Kaiso 1 or 2 were measured in Boyden cambers and compared to cells treated with si-Scr controls. (C) MDA-MB-468 and MDA-MB-231 cells treated with siRNA Kaiso 1 or 2 showed a decrease in the number of cells invading through a layer of Matrigel relative to control cells and cells treated with si-Scr. (D) MCF-7 cells transfected with pLenti-scramble-RFP plasmid or pLenti-Kaiso-RFP were wounded, and cell migration was measured at 24 hr. Photos were taken at 100× magnification. Red vertical bars indicate the starting area migration on Day 0. (E) Quantitative analysis of relative migration shows that MCF-7 cells treated with pLenti-Kaiso-RFP had an increased capacity to migrate relative to cells treated with the pLenti-scramble-RFP plasmid. (Ei) Migration rates of MCF-7 cells transfected with pLenti-scramble-RFP plasmid or pLenti-Kaiso-RFP were measured at 24 hr in Boyden chambers. (F) MCF-7 cells transfected with pLenti-Kaiso-RFP showed an increase in the number of cells invading through a layer of Matrigel relative to cells treated with the pLenti-scramble-RFP plasmid. All data presented are the means of three independent experiments, performed in triplicate ± s.e. *P<0.05.
Kaiso directly regulates the tumor suppressor, E-cadherin
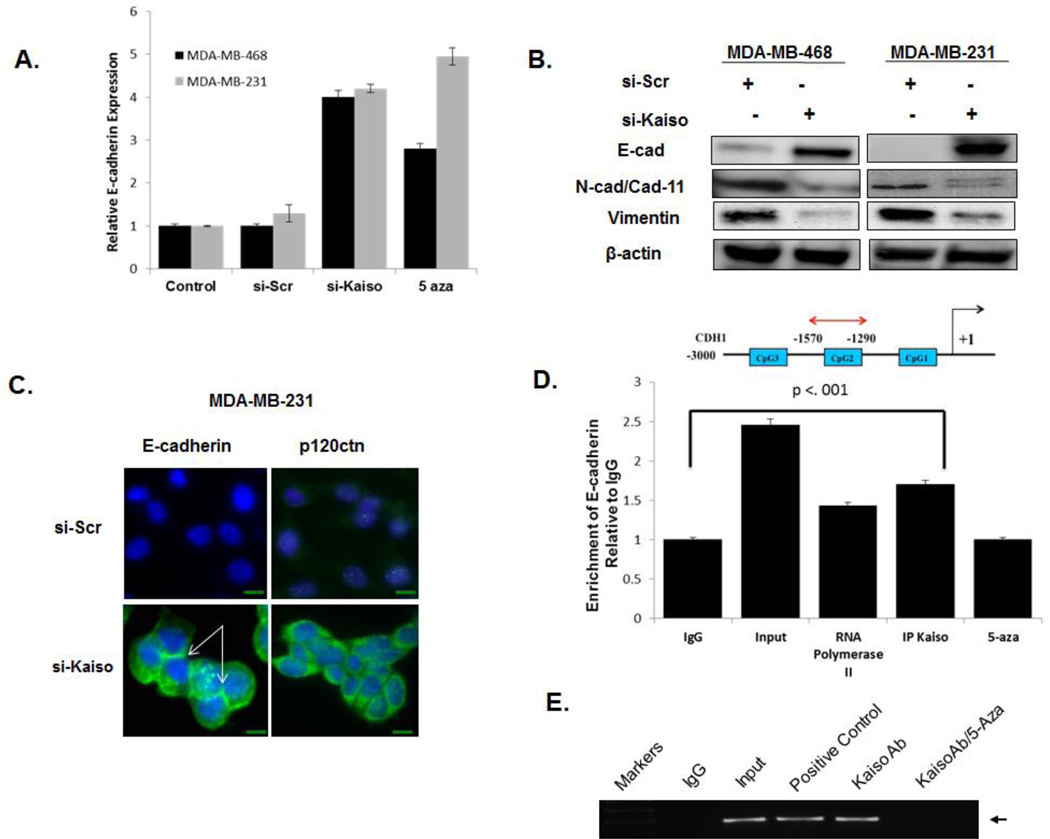
Characteristics of increased metastasis in IDCs are a loosening of cell-cell contact and reduced expression of the tumor suppressor, E-cadherin. In other models, this protein is a transcriptional repressor target of Kaiso [16, 18]. To determine if nuclear expression of Kaiso is related to the lack of E-cadherin expression in MDA-MB-468 and MDA-MB-231 cells, levels of E-cadherin were assessed after treatment of these cells with si-Kaiso. Both cell types expressed E-cadherin at the mRNA and protein levels (Figure 6A,B). E-cadherin re-expression was associated with decreases in mesenchymal markers, N-cadherin expression in MDA-MB-468 cells and cadherin 11 expression in MDA-MB-231 cells (Figure 6B). After si-Kaiso treatment, both cell lines demonstrated decreased vimentin expression. Nevertheless, there were no appreciable changes in the expression of predicted Kaiso target genes, matrilysin or MTA2 (Supplemental Figure 5 A,B). Immunofluorescence staining of cells treated with si-Kaiso demonstrated increased E-cadherin expression in the cytoplasm and at the points of cell-cell contacts. We further determined that p120ctn, which is expressed in the nucleus of E-cadherin deficient cell lines [29, 30], is expressed in the cytoplasm and at the cell membranes in si-Kaiso treated cells, compared to predominantly nuclear expression in si-Scr treated cells (Figure 6C).
Fig. 6.
Down-regulation of Kaiso results in increased expression of E-cadherin in aggressive breast cancer cells. (A) MDA-MB-486 and MDA-MB-231 cells treated with si-Kaiso or 5-aza expressed E-cadherin at higher levels than control cells or cells treated with si-Scr. (B) Immunoblots revealed that knockdown of Kaiso in MDA-MB-231 and MDA-MB-468 cells resulted in expression of E-cadherin. (C) In contrast to cells treated with si-Scr, cells treated with si-Kaiso showed E-cadherin expression at the cellular membrane. E-cadherin localization was determined by immunofluorescence in MDA-MB-231 cells treated with si-Kaiso or si-Scr using anti–E-cadherin (green) or anti-p120ctn (green) and DAPI nuclear stain (blue) (arrows indicate membrane staining). (D) Chromatin immunoprecipitation (ChIP) products were analyzed by realtime PCR with an E-cadherin primer set (−1290 to−1570) to show association of Kaiso with the methylated region of the E-cadherin promoter in MDA-MB-231cells in the presence or absence of 5-aza. The effects of an antibody to Kaiso (6F/6F8 ChIP grade) were compared to effects of antibodies to mouse IgG (negative control) and RNA pol II. Input DNA served as a positive control. (E) Ethidium bromide-stained gel of chromatin immunoprecipitation products obtained in panel 6D.
To determine if Kaiso binds to methyl-CpG dinucleotides in the promoter of the E-cadherin gene, several ChIP primers were utilized to amplify the 230-bp fragment of the E-cadherin promoter. Immunoprecipitated DNA was incubated with Kaiso antibody, RNA polymerase II antibody (positive control), or IgG antibody (negative control) in the presence or absence of the demethylating agent, 5-aza. The Kaiso antibody, as opposed to the negative control IgG antibody, enriched an mCGmCG fragment within the E-cadherin promoter, but this did not occur after treatment of the cells with 5-aza (Figure 6 D). These results indicate that Kaiso binds to the Ecadherin promoter region in a methylation-dependent manner.
Nuclear Kaiso in poorly differentiated IDCs is associated with low E-cadherin
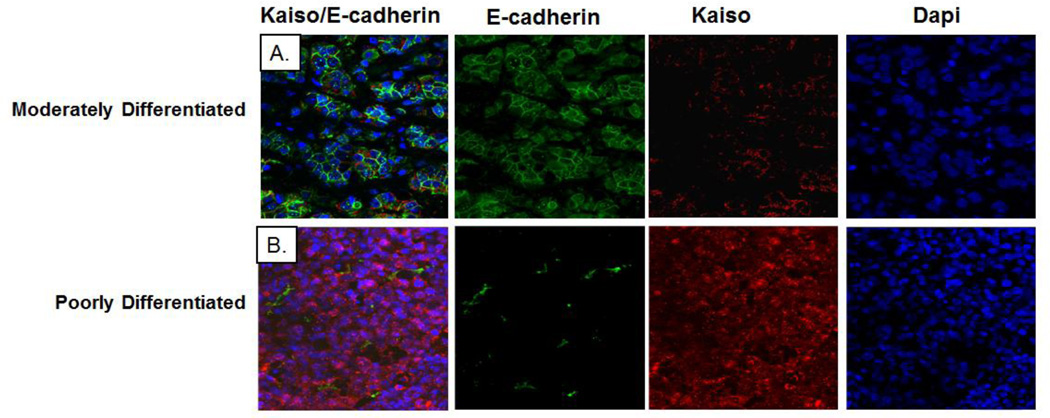
Because Kaiso binds to E-cadherin, indicating regulation of its expression, we investigated the co-expression of Kaiso and E-cadherin in human breast tissues. Double-labeled immunofluorescence staining of Kaiso (red) and E-cadherin (green) showed that, in moderately differentiated, E-cadherin-positive tumors, Kaiso staining intensity was low and localized within the cytoplasm (Figure 7). However, in poorly differentiated, E-cadherin-negative tumors, Kaiso staining was more intense and was localized predominantly within the nucleus (Figure 7). Thus, the increased expression and shift in subcellular localization of Kaiso from the cytoplasm to the nucleus are associated with a loss of E-cadherin, a characteristic of more invasive carcinomas.
Fig. 7.
Lack of E-cadherin expression in IDC tissues correlated with nuclear Kaiso expression. (A) Double immunofluorescence was performed on well differentiated IDCs for E-cadherin and Kaiso using anti-E-cadherin with Alexa 488 secondary (green) and anti-Kaiso with Alexa 594 secondary (red), and with DAPI as a nuclear counter-stain (blue). (B) Double immunofluorescence was performed on poorly differentiated IDCs for E-cadherin and Kaiso using anti-E-cadherin with Alexa 488 secondary (green) and anti-Kaiso with Alexa 594 secondary (red), with DAPI as a nuclear counter-stain (blue). Note: Single channels of E-cadherin expression (green), Kaiso expression (red), and DAPI expression (blue) are shown to demonstrate the cytoplasmic and nuclear localization of Kaiso in relation to E-cadherin expression in well differentiated and poorly differentiated tumors.
Discussion
Nuclear Kaiso is associated with aggressive breast cancer subtypes. For example, evidence of nuclear positivity is evident in aggressive, inflammatory breast tumors [16]; high-grade, invasive IDCs; and basal-derived, triple-negative breast tumors [22]. Our findings in a large patient cohort of IDCs are similar to those of previously published reports for breast tumors [28]; however, the present observations contribute mechanistic insights related to Kaiso function in late-stage tumors. First, there was low to absent Kaiso expression in normal samples from healthy patients, with both cytoplasmic and nuclear expression in normal samples from adjacent tissues. We also observed a significantly higher expression of nuclear Kaiso in lymph node metastases relative to primary tumors. There were similar expression patterns, as determined by both the 6F and 12H Kaiso antibodies. Increases in nuclear Kaiso were most evident in a subset of matched pairs of normal tissues/primary tumors and primary tumors/lymph node metastases (48 and 51 patients, respectively). These samples consistently demonstrated higher nuclear expression in the more advanced tumors. Although there were some increases in cytoplasmic expression, this was not consistent in the more advanced tumors. In both primary and lymph node metastases, there were higher levels of nuclear Kaiso expression in African American patients relative to Caucasian patients. Further, the presence of nuclear Kaiso in breast tumors lowered the 5- and 10-year survival probabilities relative to survival of patients whose tumors lacked nuclear Kaiso. This was most apparent in tumors from African American women, who had a lower survival relative to Caucasian patients whose tumors contained nuclear Kaiso, regardless of grade and differentiation status. African American women diagnosed with IDC commonly have a worse survival compared to otherwise similar Caucasian patients, even after adjusting for within-stage differences in tumor size and lymph node status [31–33]. Thus, in view of the evidence of Kaiso expression and localization in IDCs, it appears that increased nuclear Kaiso is associated with a more aggressive phenotype.
The associations observed here between a shift in Kaiso subcellular localization and tumor aggressiveness could have functional and biological significance. Our findings of intense staining within the nucleus of poorly differentiated tumors and the repressive role of Kaiso led us to speculate that Kaiso has a functional role in promoting this phenotype. An essential feature of poorly differentiated cells is a loss of cell-cell contact, in particular, loss of E-cadherin expression [34, 35]. This accounts for the infiltrating phenotype, which is essential for increased cell migration, local or distant invasion, and EMT. The cell culture models MDA-MB-468 and MDA-MB-231 are derived from IDCs [36], and both are highly migratory and lack expression of Ecadherin [37]. Similar to our observation with patient tumors, MDA-MB-468 and MDA-MB-231 cells exhibited nuclear positivity relative to the more differentiated, E-cadherin-positive MCF-7 cells, which express Kaiso predominantly in the cytoplasm. In both MDA-MB-468 and MDA-MB-231 cells, depletion of Kaiso expression through siRNA resulted in reduced cell migration and invasiveness. Furthermore, cells treated with siRNA Kaiso expressed E-cadherin at both the RNA and protein levels, and expression was localized to the cellular membrane, suggesting a re-establishment of cell cohesiveness. While we did not observe significant changes in expression of Kaiso target genes, matrilysin or metastasis-associated gene 2 (MTA2), there were significant decreases in expression of the mesenchymal markers, N-cadherin in MDA-MB-468 cells, and cadherin 11 in MDA-MB-231 cells. Treated with si-Kaiso, both cell lines demonstrated decreased vimentin expression, suggesting a role for Kaiso during the EMT in breast cancer cells.
A previous report, involving several lobular-derived and ductal-derived MCF10A cell lines, demonstrated that Kaiso is expressed in the nucleus and that E-cadherin is present in the cell membrane [28]. While there are limited data available regarding Kaiso expression/localization in lobular tumors in cell lines, E-cadherin expression in MCF10A cells is dependent on culture density. Sparse cultures of MCF10A cells exhibit fibroblast-like spindle morphology and express N-cadherin, not E-cadherin. E-cadherin is present only after cells become confluent, concurrent with a switch to a more epithelial morphology [38]. Similarly, sparse cultures of MCF10A cells grown on Matrigel demonstrate nuclear Kaiso. After a half-day of culture, however, Kaiso translocates from the nucleus to the cytoplasm and is eventually undetectable at the time typical breast acini are formed. Lack of Kaiso expression correlates with the presence of E-cadherin in the membrane [16]. While it is possible that E-cadherin mediates nuclear translocation of Kaiso in non-cancerous cells, the present data show that Kaiso is present in the nuclei of both MDA-MB-468 and MDA-MB-231 cell lines, even in confluent cultures. Furthermore, in MCF-7 cells transfected with Kaiso cDNA, there is immediate nuclear localization and increased migration and invasiveness. These findings are similar to those for Madin-Darby canine kidney epithelial (MDCK) cells and for MCF-7 breast cancer cells, both of which originally display Kaiso predominantly in the cytoplasm, but show nuclear expression after over-expression induced by Kaiso cDNA [24].
Kaiso regulates several tumor-associated genes such as CDH1 (E-cadherin), MMP7 (matrilysin), MTA2, and Wnt11 [18, 39–41]; and in models of human cancer, it directly binds to methylated sequences in cyclin D1 [42] and CDKN2A [9]. In the case of E-cadherin, both KBS (CTGCNA) sequences and methylated sequences are found in the E-cadherin promoter as determined with in silico databases. Our findings with aggressive breast cancer cells suggest that Kaiso promotes cell migration and invasion through direct binding to CpG-rich regions in the E-cadherin promoter in a methylation-dependent manner and are similar to findings with NIH3T3 cells and PC-3 cells [17, 18]. Furthermore, 5-aza treatment reverses Kaiso binding. The effect of 5-aza treatment on E-cadherin re-expression is documented for E-cadherin deficient cell lines [7, 8, 37, 43]. Thus, the findings presented here are similar to those of previous reports, suggesting that the rate-limiting effect of Kaiso on E-cadherin expression is methylation-dependent.
In IDCs, over-expression of EGFR and loss of expression of estrogen receptor-α correlate with nuclear Kaiso levels [22]. At the molecular level, EGFR regulates E-cadherin expression and cell-cell cohesiveness [44, 45]. We have recently demonstrated, for aggressive prostate cancer cells lines DU-145 and PC-3, that EGFR over-expression regulates Kaiso expression and cytoplasmic-to-nuclear shuttling, and that Kaiso-depleted cells display reduced cell migration and invasion, even in the presence of EGF stimulation [17]. Both MDA-MB-468 and MDA-MB-231 cells have high levels of EGFR expression and a reinforced autocrine signaling network, which, when attenuated by blocking of receptor binding or kinase activity, show less aggressive characteristics [46]. Thus, it is possible that reinforced autocrine signaling in the highly aggressive carcinoma cell lines relative to non-carcinoma and/or early stage carcinoma cell lines is responsible for the maintenance of nuclear Kaiso expression. Nevertheless, we did not observe significant changes in expression of matrilysin and MTA-2, which contain both Kaiso binding sites (KBS) and methylated CpG-dinucleotides [19, 20]. These results are similar to our findings for prostate cancer cells, which have depleted Kaiso. Multiple reports suggest that MDA-MB-231 invasiveness is mediated through matrix metalloproteinases (MMPs)-2 and 9[47–49]. Furthermore, EGFR targets MMP-2 and MMP-9 through the ERK-MAPK pathway [50]. Thus, it is possible that, in breast cancer cells, additional MMPs are responsible for Kaiso mediated invasiveness lines. More work should be accomplished to clarify this role in aggressive breast cancers.
One caveat is that nuclear Kaiso is present in breast tumors with membrane expression of E-cadherin [22]. Across multiple studies with IDCs, the presence of E-cadherin expression or its aberrant expression has been variable [5, 7, 51]. Within the present cohort, we observed that late-stage, poorly differentiated tumors, which have nuclear Kaiso, lack co-expression of E-cadherin, relative to moderately differentiated tumors, which express E-cadherin and lack co-expression of nuclear Kaiso. Loss of E-cadherin is a prognostic factor for triple-negative breast cancers, an aggressive breast cancer subtype [52]. With the currently available data, it is reasonable to conclude that nuclear Kaiso and aberrant E-cadherin expression could be utilized to identify highly aggressive subtypes of breast cancer, particularly in African American patients.
Conclusion
In summary, the cytoplasmic-to-nuclear localization of Kaiso correlates with less differentiated features of IDC progression, including race. Additionally, Kaiso has the potential to regulate multiple tumorigenic events, which could expand the set of markers available to correlate with morphological differentiation. This would be of particular interest for treatment of African American breast cancer patients, who have a high incidence of aggressive, triple-negative breast cancers. Collectively, these findings highlight a role for Kaiso during transcriptional silencing of E-cadherin and support a function for Kaiso as a promoter of aggressive breast tumors.
Supplementary Material
Acknowledgments
This work was supported by grants G12 RR03059-21A1 (NIH/RCMI) [CY] and a pilot project on U54 CA118948 (NIH/NCI) [CY]. Partial support was received from R01 CA87728 (NIH), Susan G. Komen for the Cure, and the National Foundation for Cancer Research [DRW]. Pre-doctoral fellowship for Jacqueline Jones was supported by UNCF/Merck graduate science initiative. We would like to thank members of the Yates laboratory for their comments and discussions.
Abbreviations
- IDC
Infiltrating ductal carcinoma
- BTB-POZ
Broad complex, tramtrak bric-a-brac/pox virus, and zinc finger subfamily of zinc-finger proteins
- CDH1
Cadherin-1 gene
- EMT
Epithelial to mesenchymal transition
- TMA
Tissue microarray
- siRNA
Small-interfering RNA
- RFP
Red fluorescence protein
- PMSF
Phenylmethylsulfonylfluoride
- DAPI
2,4-Diamidino-2-phenylindole
- qRT-PCR
Quantitative real-time PCR
- EGFR
Epidermal growth factor receptor
- 5-aza
5-Aza-2-deoxycytidine
Footnotes
Competing interests
The authors have filed a patent application relating to the content of this manuscript but have no further conflicting financial interests.
Contributor Information
Jacqueline Jones, Department of Biology and Center for Cancer Research, Tuskegee University, Tuskegee, AL 36088.
Honghe Wang, Department of Biology and Center for Cancer Research, Tuskegee University, Tuskegee, AL 36088.
Balasubramanyam Karanam, Department of Biology and Center for Cancer Research, Tuskegee University, Tuskegee, AL 36088.
Shaniece Theodore, Department of Biology and Center for Cancer Research, Tuskegee University, Tuskegee, AL 36088.
Windy Dean-Colomb, Department of Oncology, University of South Alabama Mitchell Cancer Center, Mobile, AL 36688.
Danny R. Welch, Department of Cancer Biology, The University of Kansas Medical Center, The University of Kansas Cancer Center, Kansas City, Kansas 66045
William Grizzle, Department of Pathology, University of Alabama at Birmingham School of Medicine, Birmingham, AL 35205.
Clayton Yates, Department of Biology and Center for Cancer Research, Tuskegee University, Tuskegee, AL 36088.
References
- 1.DeSantis C, et al. Breast cancer statistics, 2011. CA: a cancer journal for clinicians. 2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Amirikia KC, et al. Higher population-based incidence rates of triple-negative breast cancer among young African-American women : Implications for breast cancer screening recommendations. Cancer. 2011;117(12):2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118(2):290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 5.Zou D, et al. Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CDH1) as a target for chemoprevention of lobular neoplasia. The Journal of pathology. 2009;218(2):265–272. doi: 10.1002/path.2541. [DOI] [PubMed] [Google Scholar]
- 6.Brenton JD, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23(29):7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 7.Caldeira JR, et al. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer. 2006;6:48. doi: 10.1186/1471-2407-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes EC, et al. Kaiso contributes to DNA methylation-dependent silencing of tumor suppressor genes in colon cancer cell lines. Cancer research. 2008;68(18):7258–7263. doi: 10.1158/0008-5472.CAN-08-0344. [DOI] [PubMed] [Google Scholar]
- 10.Dalla-Favera R, et al. BCL-6 and the molecular pathogenesis of B-cell lymphoma. Cold Spring Harbor symposia on quantitative biology. 1994;59:117–123. doi: 10.1101/sqb.1994.059.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Ye BH, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science (New York, NY. 1993;262(5134):747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 12.Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19(5):3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cofre J, et al. Knock-down of Kaiso induces proliferation and blocks granulocytic differentiation in blast crisis of chronic myeloid leukemia. Cancer cell international. 12(1):28. doi: 10.1186/1475-2867-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai SD, et al. Cytoplasmic Kaiso is associated with poor prognosis in non-small cell lung cancer. BMC Cancer. 2009;9:178. doi: 10.1186/1471-2407-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prokhortchouk A, et al. Kaiso-deficient mice show resistance to intestinal cancer. Molecular and cellular biology. 2006;26(1):199–208. doi: 10.1128/MCB.26.1.199-208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soubry A, et al. Expression and nuclear location of the transcriptional repressor Kaiso is regulated by the tumor microenvironment. Cancer Res. 2005;65(6):2224–2233. doi: 10.1158/0008-5472.CAN-04-2020. [DOI] [PubMed] [Google Scholar]
- 17.Jones J, et al. Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am J Pathol. 2012;181(5):1836–1846. doi: 10.1016/j.ajpath.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prokhortchouk A, et al. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes & development. 2001;15(13):1613–1618. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SW, et al. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nature cell biology. 2004;6(12):1212–1220. doi: 10.1038/ncb1191. [DOI] [PubMed] [Google Scholar]
- 20.Spring CM, et al. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Experimental cell research. 2005;305(2):253–265. doi: 10.1016/j.yexcr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson NS, et al. Kaiso represses the cell cycle gene cyclin D1 via sequence-specific and methyl-CpG-dependent mechanisms. PloS one. 7(11):e50398. doi: 10.1371/journal.pone.0050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeulen JF, et al. Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer. PLoS One. 7(5):e37864. doi: 10.1371/journal.pone.0037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang X, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. 2012;19(6):699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel JM, Ireton RC, Reynolds AB. Monoclonal antibodies to Kaiso: a novel transcription factor and p120ctn-binding protein. Hybridoma. 2001;20(3):159–166. doi: 10.1089/027245701750293484. [DOI] [PubMed] [Google Scholar]
- 25.Manne U, et al. Re: loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. Journal of the National Cancer Institute. 1997;89(8):585–586. doi: 10.1093/jnci/89.8.585. [DOI] [PubMed] [Google Scholar]
- 26.WE G, et al. Immunohistochemical Evaluation of Biomarker Expression in Neoplasia. Totowa, NJ: Humana Press Inc; 1998. [Google Scholar]
- 27.Daniel JM, et al. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002;30(13):2911–2919. doi: 10.1093/nar/gkf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermeulen JF, et al. Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer. PLoS One. 2012;7(5):e37864. doi: 10.1371/journal.pone.0037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata T, et al. Cytoplasmic p120ctn regulates the invasive phenotypes of E-cadherin-deficient breast cancer. The American journal of pathology. 2004;164(6):2269–2278. doi: 10.1016/S0002-9440(10)63783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Hengel J, et al. Nuclear localization of the p120(ctn) Armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):7980–7985. doi: 10.1073/pnas.96.14.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10(1):1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 32.Newman LA, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95(1):21–27. doi: 10.1002/cncr.10639. [DOI] [PubMed] [Google Scholar]
- 33.McBride R, et al. Within-stage racial differences in tumor size and number of positive lymph nodes in women with breast cancer. Cancer. 2007;110(6):1201–1208. doi: 10.1002/cncr.22884. [DOI] [PubMed] [Google Scholar]
- 34.Moll R, et al. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. The American journal of pathology. 1993;143(6):1731–1742. [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi HS, et al. E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am J Clin Pathol. 2006;125(3):377–385. [PubMed] [Google Scholar]
- 36.Brooks SA, Hall DM. Investigations into the potential role of aberrant N-acetylgalactosamine glycans in tumour cell interactions with basement membrane components. Clin Exp Metastasis. 2002;19(6):487–493. doi: 10.1023/a:1020399516305. [DOI] [PubMed] [Google Scholar]
- 37.Graff JR, et al. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 1995;55(22):5195–5199. [PubMed] [Google Scholar]
- 38.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. Journal of cell science. 2005;118(Pt 5):873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 39.Rodova M, et al. Regulation of the rapsyn promoter by kaiso and delta-catenin. Molecular and cellular biology. 2004;24(16):7188–7196. doi: 10.1128/MCB.24.16.7188-7196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruzov A, et al. Kaiso is a genome-wide repressor of transcription that is essential for amphibian development. Development (Cambridge, England) 2004;131(24):6185–6194. doi: 10.1242/dev.01549. [DOI] [PubMed] [Google Scholar]
- 41.Yoon HG, et al. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell. 2003;12(3):723–734. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Donaldson NS, et al. Kaiso represses the cell cycle gene cyclin D1 via sequence-specific and methyl-CpG-dependent mechanisms. PLoS One. 2012;7(11):e50398. doi: 10.1371/journal.pone.0050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhold WC, et al. Detailed DNA methylation profiles of the E-cadherin promoter in the NCI-60 cancer cells. Molecular cancer therapeutics. 2007;6(2):391–403. doi: 10.1158/1535-7163.MCT-06-0609. [DOI] [PubMed] [Google Scholar]
- 44.Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem. 1998;273(15):9078–9084. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]
- 45.Lo HW, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickerson NK, et al. Decreased autocrine EGFR signaling in metastatic breast cancer cells inhibits tumor growth in bone and mammary fat pad. PLoS One. 2012;7(1):e30255. doi: 10.1371/journal.pone.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297(1):42–48. doi: 10.1016/j.canlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, et al. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol Cancer. 2009;(8):81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang T, et al. Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Res Treat. 2012;135(2):445–458. doi: 10.1007/s10549-012-2175-5. [DOI] [PubMed] [Google Scholar]
- 50.Wu JM, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14(7):1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asgeirsson KS, et al. Altered expression of E-cadherin in breast cancer. patterns, mechanisms and clinical significance. Eur J Cancer. 2000;36(9):1098–1106. doi: 10.1016/s0959-8049(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 52.Kashiwagi S, et al. Significance of E-cadherin expression in triple-negative breast cancer. British journal of cancer. 2010;103(2):249–255. doi: 10.1038/sj.bjc.6605735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.