Abstract
Corneal scarring following moderate to severe injury is inevitable. Despite significant advancements in the field, current treatments following these types of injuries are limited, and often, the visual recovery is poor. One of the problems and limitations is that corneal wound healing is a complex process, involving corneal cells, extracellular matrix components and growth factors. Therefore, further understanding is required, along with new treatments and techniques to reduce or prevent corneal scarring following injury. Two isoforms of transforming growth factor-beta (TGF-β), TGF-β1 and -β3 (T1 and T3, respectively), are associated with corneal wound healing. T1 has been shown to drive the corneal keratocytes to differentiate into myofibroblasts; whereas, T3 has been found to inhibit fibrotic markers. In the current study, we examined whether the fibrotic characteristics expressed by human corneal fibroblasts (HCF) in our 3-dimensional (3D) construct following T1 stimulation could be reversed by introducing T3 to the in vitro system. To do this, HCF were isolated and cultured in 10% serum, and when they reached confluence, the cells were stimulated with a stable Vitamin C (VitC) derivative for 4 weeks, which allowed them to secrete a self-assembled matrix. Three conditions were tested: (1) Control: 10% serum (S) only, (2) T1: 10%S+T1, or (3) Rescue: 10%S+T1 for two weeks and then switched to 10%S+T3 for another two weeks. At the end of 4 weeks, the constructs were processed for analysis by indirect-immunofluorescence (IF) and transmission electron microscopy (TEM). Different collagens that are normally present in healthy corneas in vivo, such as Type I and V, as well as Type III, which is a fibrotic indicator, were examined. In addition, we examined smooth muscle actin (SMA), a marker of myofibroblasts, and thrombospondin-1 (TSP-1), a multifunctional matrix protein known to activate the latent complex of TGF-β and appear upon wounding in vivo. Our data showed high expression of collagens type I and V under all conditions throughout the 3D constructs; however, type III and SMA expression were higher in the constructs that were stimulated with T1 and reduced to almost nothing in the Rescue samples. A similar pattern was seen with TSP-1, where TSP-1 expression following “rescue” was decreased considerably. Overall, this data is in agreement with our previous observations that T3 has a significant non-fibrotic effect on HCFs, and presents a novel model for the “rescue” of both cellular and matrix fibrotic components with a single growth factor.
Keywords: TGF-β3, Myofibroblast, Extracellular Matrix, Corneal fibrosis, Corneal stroma
1. Introduction
Corneal injury, or trauma, often leads to corneal fibrosis (scarring) resulting in the loss of corneal transparency and blindness (Anderson et al. 2004; Fullwood 2004; Whitcher et al. 2001). The concept of “curing” corneal opacity has been discussed in published form for over 200 years (Baradaran-Rafii et al. 2007; Chirila 2001; Coster et al. 2009; Guo et al. 2007; Niederkorn 2003); however, this discussion has mainly been focused on replacing the scarred cornea with a clear substitute, or treating the wound at the time of injury with various inhibitors of the scarring pathway. Although several studies have reported methods to prevent or lessen scarring, few if any have addressed treatments that might reverse the fibrotic pathway once initiated. This idea is the focus of our present study.
Fibrosis in the cornea occurs following an injury or some type of trauma. The mechanism by which the cornea scars is generally accepted and well understood. Briefly, upon wounding the resident keratocytes are activated (termed fibroblasts) and migrate to the wound site (Beales et al. 1999; Fini 1999; Funderburgh et al. 2003; Zieske et al. 2001). Once they reach the wound site, some of the fibroblasts undergo further differentiation into what is known today as myofibroblasts (Beales et al. 1999; Fini 1999; Funderburgh et al. 2003; Zieske et al. 2001). Main characteristics of these cells are the expression of α-smooth muscle actin (SMA), a marker of myofibroblasts, and the secretion of collagen extracellular matrix (ECM), mainly type III collagen (Col III).
Despite the generally accepted fact that development of fibroblasts and myofibroblasts is essential for connective tissue remodeling both during development and wound healing, the regulation of myofibroblast development remains an enigma. In the human cornea, myofibroblasts lead to fibrosis, which creates opacity and ultimately interferes with vision. In fact, myofibroblasts do not appear until there is an injury (Bernstein et al. 2007; Fini 1999; Garana et al. 1992). Although both fibroblasts and myofibroblasts contribute to normal wound repair in a fully healed wound, few if any myofibroblasts are found (Bernstein et al. 2007; Fini 1999; Garana et al. 1992). It is clear that our understanding of the myofibroblast’s origins and functions will be crucial to the future effectiveness of corneal tissue engineering and regenerative medicine.
One of the factors contributing to myofibroblasts existence and stimulation is transforming growth factor beta (TGF-β). Of the three isoforms (-β1, -β2 and -β3), TGF-β1, and perhaps -β2, are known to lead to corneal fibrosis. We have recently reported a variety of studies analyzing and characterizing these three TGF-β isoforms on a 3-dimensional (3D) in vitro model (Karamichos et al. 2010, 2011). Our artificial stromal constructs are comprised of human corneal fibroblasts (HCF) stimulated to secrete a matrix by a stable form of ascorbic acid (VitC). We have demonstrated that these constructs assemble a matrix that mimics the in vivo stroma with alternating layers of aligned collagen fibrils (Karamichos et al. 2010, 2011). When these constructs are stimulated with the TGF-β isoforms, there are significant differences, both in cellular and matrix regulation. TGF-β3 (T3) has been found to stimulate the generation of a matrix that mimics the normal adult or developing human cornea; whereas, TGF-β1 and -β2 (T1 and T2, respectively) drive the constructs toward a more fibrotic path (Karamichos et al. 2010, 2011).
The objective of the current study is to investigate the possibility of reversing, or “rescuing”, the fibrotic effect caused by T1 stimulation in vitro by using T3 as our “rescue” stimuli.
2. Materials and Methods
2.1. Human Corneal Fibroblasts culture
Primary human corneal fibroblasts (HCFs) were isolated and cultured as previously described in Guo et al. (2007) from human corneas that were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). All research adhered to the tenets of the Declaration of Helsinki. Briefly, corneal epithelium and endothelium were scrapped and removed from the donor cornea. The remaining stromal tissue was cut into small 2×2mm pieces and put into T25 flasks (4 or 5 pieces per well) and allowed to adhere. Explants were cultured with Eagle’s Minimum Essential Medium (EMEM: ATCC; Manassas, VA) containing 10% fetal bovine serum (FBS: ATCC). All cultures were allowed 1-2 weeks of cultivation, at which point cells were passaged into a 100mm cell culture plate. The cells were allowed to grow to 100% confluence before being used in the culture system. Passages up to number 6 were used throughout the experiments.
2.2. Construct Assembly
Constructs were assembled as previously described (Guo et al. 2007; Karamichos et al. 2010, 2011). The HCFs were plated on six-well plates containing polycarbonate membrane inserts with 0.4 m pores (Transwell; Corning Costar; Charlotte, NC) at a density of 106cells/ml. HCFs were cultured in EMEM with 10% FBS and stimulated with a stable VitC derivative (0.5mM 2-O-α-D-glucopyranosyl-L-ascorbic acid: Wako Chemicals USA., Richmond, VA). Three different groups were tested: (1) Control or C: cells were cultured with EMEM+FBS+VitC (construct medium); (2) T1: cells were grown in construct medium+T1 (0.1ng/ml) for the entire experiment; and (3) Rescue: cells were grown in construct medium+T1 (0.1ng/ml) for 2 weeks, then T1 was removed, and the cells were grown in construct medium+T3 (0.1ng/ml) for the remaining 2 weeks. All groups were repeated at least three times and each one was collected and processed for IF and TEM.
2.3. Transmission electron microscopy (TEM)
The constructs were collected after 4 weeks in culture, fixed in ½ strength Karnovsky’s fixative (2% paraformaldehyde, 2.5% gluteraldehyde in cacodylate buffer, pH 7.4) and processed for TEM using standard procedures, as described previously (Gipson et al. 1983). Briefly, constructs were rinsed in PBS, processed through post-fixation in 2% osmium tetroxide, en bloc stained in 0.5% uranyl oxide, dehydrated with alcohol to propylene oxide, and embedded (Embed 812: Electron Microscopy Sciences; Hatfield, PA). Thin sections were cut transverse to the plane of the construct using a diamond knife on an ultramicrotome (LKB; Bromma, Sweden). The sections were viewed and photographed with an electron microscope (Tecnai G2 Spirit: FEI Company; Hillsboro, OR).
2.4. Indirect-immunofluorescence (IF)
The constructs were collected, fixed, and stained for IF, as previously described (Guo et al. 2007; Karamichos et al. 2010, 2011). In brief, constructs were fixed in 4% paraformaldehyde at least 24 hours before processing. Constructs were incubated overnight at 4°C with primary antibodies against SMA (Dako North America,Carpinteria,CA), Col III (Southern Biotech, Birmingham, AL), Collagen I (Col I: Abcam, Cambridge, MA), Collagen V (Col V: Novus Biologicals, Littleton, CO), and Thrombospondin-1 (TSP-1: NeoMarkers, Fremont, CA) in 1% BSA+0.1% Triton-X. The next day, constructs were washed and incubated overnight at 4°C with the corresponding secondary antibody, anti-mouse IgG (SMA), anti-goat IgG (Col III), and anti-rabbit IgG (Col I, Col V and TSP-1) in 1% BSA+0.1% Triton-X. Iodide counterstain (TO-PRO-3; Life Technologies, Grand Island, NY) was used as a marker of all cell nuclei. Constructs were washed, mounted (Vectashield; Vector Laboratories, Burlingame, CA), observed, and photographed with a confocal microscope (TCS-SP2; Leica Microsystems, Bannockburn, IL). Negative controls, where the primary antibody was omitted, were run with all experiments.
In addition, construct thicknesses were also measured with the confocal microscope, beginning with the first cell visible at the top of the construct and the last cell visible at the bottom. Data was averaged and analyzed.
2.5. Statistical Analysis
All experiments were repeated at least 3 times, and data was analyzed for significant variations (p<0.05) using the Student’s t-test and Dunnett’s Multiple Comparison test (GraphPad Prism v.5.0b; La Jolla, CA).
3. Results
3.1. 3D Constructs Characterization
In our previous work (Karamichos et al. 2010, 2011, 2013), we have shown that HCFs have the ability to secrete and lay down a fibrous collagen ECM in the presence of VitC and that ability is enhanced upon stimulation with growth factors, such as T1 and T3. We have also demonstrated the effect of T1 and T3 in these cultures (Karamichos et al. 2011, 2013), and have shown that T3 did not stimulate the expression of fibrotic markers. In the current study, we examined the effect of T3 to reverse or “rescue” specific fibrotic markers following their upregulation in the presence of T1.
After 4 weeks in VitC only, the HCFs’ construct had a mean thickness of 25μm, whereas, the T1 stimulated HCFs synthesized a matrix of 44μm thick (Fig. 1). This is in agreement with previous observations (Karamichos et al. 2011, 2013). The “rescue” model constructs maintained a similar thickness as the T1 (43μm). This data suggests that switching between the two growth factors (T1 and T3) does not enhance the HCF’s ability to secrete more ECM components.
Figure 1.
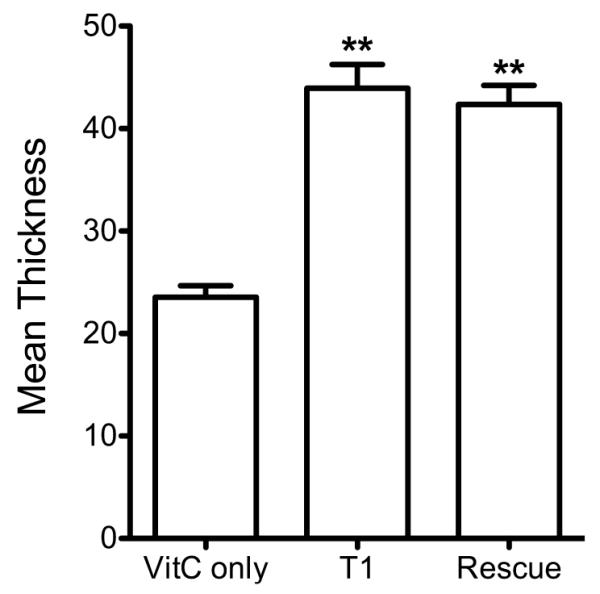
Graph of the mean thickness of the constructs treated with TGF-β isoforms and control. Three conditions were examined and analyzed: (1) Control: VitC only; (2) T1: VitC + TGF-β1; and (3) Rescue: VitC + TGF-β1 (2wks) then VitC + TGF-β3 (2wks). Both T1 and Rescue led to a significant increase in thickness as compared to the control (p<0.05).
3.2. Ultrastructural highlights
Cell-ECM interactions were examined using TEM for each of the HCF constructs at the end of 4 weeks. As seen in Figure 2 and in agreement with previous observations (Karamichos et al. 2010, 2011), Control and T1 showed areas of organized ECM, with collagen fibrils alternating directions (Fig. 2A and B, respectively). Their ECM alignment seemed very similar, although overall, T1 showed more fibril compaction than Controls. The “rescue” construct (Fig. 2C), based on our TEM observations, showed the highest collagen fibril density and ECM alignment out of all three conditions; however, the collagen fibrils seemed to be oriented in one direction rather than alternating, as seen in Controls and T1.
Figure 2.
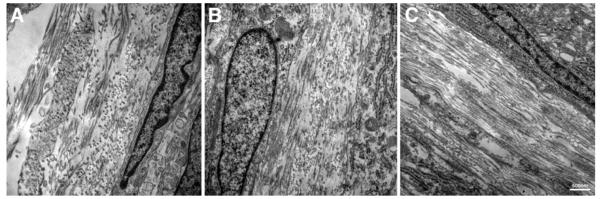
TEM (23,000×) showing cell-matrix interaction and matrix condition. A) Control, B) T1: TGF-β1 treated, and C) Rescue: TGF-β1 for 2 weeks then switched to TGF-β3 for 2 weeks. The fibril orientation alternated directions in Control and T1. Rescue construct showed fibrils oriented in one direction only. Rescue also showed higher density of collagen fibrils and overall ECM alignment compared to Control and T1. Bar = 500nm.
3.3. Specific fibrotic markers expression
3.3.1. Type I and Type V Collagen (Col I and Col V)
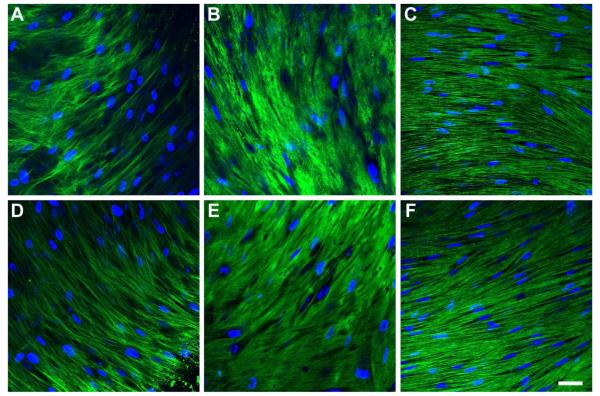
Col I and V are considered the major collagen types in the human corneal stroma and vital for the stroma’s integrity and strength. Both Col I (Fig. 3A-C) and V (Fig. 3D-F) were found to be present throughout all the constructs, and were not dependent upon the condition.
Figure 3.
Indirect immunofluorescent confocal images of collagen type I (A-C) and type V (D-F) on full-thickness constructs at 4 week. A and D) Control, B and E) T1: TGF-β1 treated, and C and F) Rescue: TGF-β1 for 2 weeks then switched to TGF-β3 for 2 weeks. Both collagens were found throughout the constructs under all conditions. Rescue constructs appeared to have more aligned collagen types I and V. Blue = TOPRO3 nuclear counterstain, Bar = 50 microns.
3.3.2. Type III Collagen (Col III)
One of the most common and widely accepted markers for corneal fibrosis and scarring is the appearance of Col III. Its expression is not normally present in healthy human corneas and is only seen following an injury. As seen in Figure 4, the HCFs expressed little Col III if left untreated (Fig. 4A); however, upon T1 treatment (Fig. 4B), Col III expression was massively upregulated, which is in agreement with our previous observations (Karamichos et al. 2010, 2011). Interestingly, Col III expression was diminished in the “rescue” model, indicating that Col III expression can be reversed with T3 (Fig. 4C).
Figure 4.
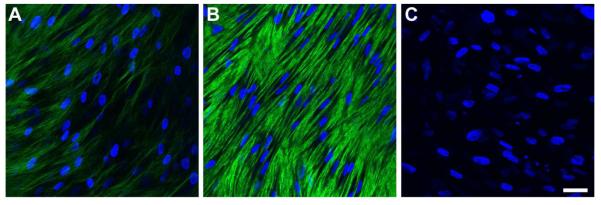
Indirect immunofluorescent confocal images of collagen type III on full-thickness constructs at 4 weeks. A) Control, B) T1: TGF-β1 treated, and C) Rescue: TGF-β1 for 2 weeks then switched to TGF-β3 for 2 weeks. Type III collagen was upregulated upon T1 stimulation, when compared to Control; however, it was diminished upon Rescue treatment. Blue = TOPRO3 nuclear counterstain, Bar = 50 microns.
3.3.3. Thrombospondin-1 (TSP-1)
A multifunctional matrix protein, TSP-1, does not appear to be expressed in the unwounded stroma (Matsuba et al. 2011; Sekiyama et al. 2006), but previous studies have shown that it is present after wounding (Cao et al. 2002; Matsuba et al. 2011; Uno et al. 2004). In our model, HCFs showed similar levels of TSP-1 expression both in Control and T1 constructs (Fig. 5A and B, respectively); however, TSP-1 was considerably downregulated with the “rescue” treatment (Fig. 5C).
Figure 5.
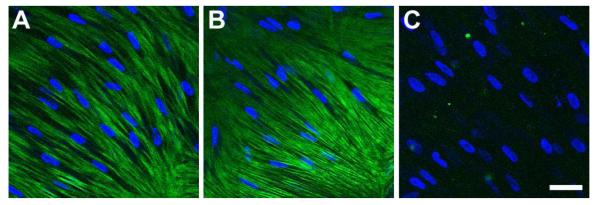
Indirect immunofluorescent confocal images of TSP-1 on full-thickness constructs at 4 weeks. A) Control, B) T1: TGF-β1 treated, and C) Rescue: TGF-β1 for 2 weeks then switched to TGF-β3 for 2 weeks. TSP-1 seemed unchanged between Control and T1 treated constructs; however, it was considerably downregulated with Rescue treatment. Blue = TOPRO3 nuclear counterstain, Bar = 50 microns.
3.3.4. Smooth Muscle Actin (SMA)
Differentiated fibroblasts into myofibroblasts are normally identified by SMA expression. As with Col III and TSP-1, SMA is not expressed by keratocytes in healthy human corneas. In agreement with previous reports (Karamichos et al. 2010, 2011), SMA expression in HCFs increased with T1 treatment (Fig. 6B) as compared with Control (Fig. 6A). Rescue treatment, however, resulted in a considerable decrease in cells expressing SMA (Fig. 6C), indicating that not only ECM components can be reversed in our “rescue” model, but cell-specific markers may be silenced.
Figure 6.
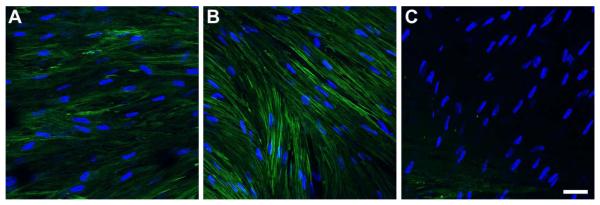
Indirect immunofluorescent confocal images of SMA on full-thickness constructs at 4 weeks. A) Control, B) T1: TGF-β1 treated, and C) Rescue: TGF-β1 for 2 weeks then switched to TGF-β3 for 2 weeks. SMA expression was upregulated upon T1 treatment and minimized upon Rescue. Blue = TOPRO3 nuclear counterstain, Bar = 50 microns.
4. Discussion
Over the past several years, we have developed an in vitro model of HCF to examine corneal fibrosis (HataSenoo 1989; Karamichos et al. 2010, 2011, 2013; Saika 1992). We have shown that HCFs stimulated by VitC stratify, secrete, and assemble ECM that mimics what is seen in vivo (Karamichos et al. 2010, 2011, 2013). Upon T1 treatment, ECM secretion and assembly was further enhanced and specific fibrotic markers, such as Col III and SMA, were up regulated (Karamichos et al. 2010, 2011). We also demonstrated that once the cultures were treated with T1, the fibrotic markers persisted for as long as 7 weeks after removal of T1 (Karamichos et al. 2010). Interestingly, T3 can prevent or minimize the expression of these markers, as well as TSP-1 (Karamichos et al. 2013). However, in those studies, T3 was present for the whole duration of the experiments (ie. 4 weeks). In order to investigate the possibility of “rescuing” constructs that have fibrotic characteristics and examining the possibility of turning the fibrotic markers on and off by simply switching growth factors, we developed a “rescue” model.
Although multiple studies have demonstrated that scarring can be blunted by inhibiting the fibrosis pathway at the time of wounding (Blalock et al. 2012; Buhren et al. 2009; Huxlin et al. 2013; Jester et al. 1997; Sriram et al. 2013; Talamo et al. 1991), the current studies, to the best of our knowledge, are the first to demonstrate that an established model of human fibrosis can be reversed. Our previous studies on TGF-β3 (Karamichos et al. 2011) have shown the prevention of scarring in vitro; however, in the present study, we attempted to reverse an established scar-like ECM. Previously published studies have demonstrated that fibrosis can be blocked at the time of wounding. For example, Jester and co-workers (1997) have demonstrated that TGF-β function-blocking antibodies blunt corneal fibrosis after corneal wounding in rabbits. Antibodies against TGF-β have also been tested following excimer laser ablation of the corneal surface, myofibroblast differentiation and migration was reduced in both rabbits and cats (Buhren et al. 2009; Huxlin et al. 2013). Other studies have suggested the role of connective tissue growth factor (CTGF) in mitigating TGF-β1-induced proliferation in corneal haze (Blalock et al. 2012). The same group has recently suggested treating corneal haze and scarring using a simultaneous targeting of TGF-β1, TGF-βRII, and CTGF genes using triple siRNA combination (Sriram et al. 2013). This was tested on rabbits with significant success. Also, numerous studies have demonstrated that decorin inhibits all three TGF-β isoforms (Border et al. 1992; Giri et al. 1997; Mohan et al. 2011). In HCFs, decorin overexpression has been shown to significantly prevent their transformation to myofibroblasts and reduces expression of profibrotic genes (Mohan et al. 2011). This suggests that it is possible for us to explore more treatments with our rescue model in combination with T3 or alone, in order to improve and optimize our model.
In terms of clinical approaches to control and reduce corneal scarring, almost all attempts involve the use of steroids or Mitomycin C (MMC). These have been successful in terms of decreasing myofibroblast differentiation and haze; however, side effects, such as toxicity and DNA damage, are of major concern (Arshinoff et al. 1996; Jester et al. 2012; Talamo et al. 1991). Another approach has been gene therapy, Mohan and co-authors (2011) demonstrated the delivery of tissue-selective targeted decorin gene with the promising results of significantly reducing SMA and fibronectin levels.
Overall, the data presented in this study raises several questions regarding the mechanisms involved in rescuing these fibrotic markers. One possibility is that Col III is been degraded by the resident cells following stimulation with T3. However, we have demonstrated that even though Col III is apparently being removed, the overall thickness of the construct is not being affected. Some studies have suggested collagen fibril degradation by corneal fibroblasts when seeded in collagen gels (Hao et al. 1999; Nagano et al. 2001). A second possibility is that the myofibroblasts undergo apoptosis, which contributes to rescuing the fibrosis.
In the current study, we made the provocative finding that it is possible to reverse a fibrotic human ECM following exposure to T1, by switching it to T3. This is potentially clinically important since it suggests that corneal stromal fibrosis may be reversed in vivo. Further studies are required to understand the mechanisms behind this intriguing phenomenon.
Highlights.
We examined whether we can “rescue” in vitro fibrosis by introducing TGF-β3.
Collagen type III and SMA expression were minimized in the “rescue” model.
The “rescue” constructs showed the highest collagen fibril density and ECM alignment.
Acknowledgements
The authors thank Patricia Pearson for technical expertise.
Funding Support This study was supported by the following grants: NIH/NEI R01EY020886 (DK and JDZ), R01EY005665 (JDZ), and P30EY03790 (Core-JDZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson K, El-Sheikh A, Newson T. Application of structural analysis to the mechanical behaviour of the cornea. J R Soc Interface. 2004;1(1):3–15. doi: 10.1098/rsif.2004.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshinoff SA, Mills MD, Haber S. Pharmacotherapy of photorefractive keratectomy. J Cataract Refract Surg. 1996;22(8):1037–1044. doi: 10.1016/s0886-3350(96)80116-7. [DOI] [PubMed] [Google Scholar]
- Baradaran-Rafii A, Karimian F, Javadi MA, Jafarinasab MR, Nowroozpour K, Hosseini M, Anisian A. Corneal Graft Rejection: Incidence and Risk Factors. IRANIAN JOURNAL OF OPHTHALMIC RESEARCH. 2007;2(1) [Google Scholar]
- Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40(8):1658–1663. [PubMed] [Google Scholar]
- Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell. 2007;18(7):2716–2727. doi: 10.1091/mbc.E06-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock TD, Gibson DJ, Duncan MR, Tuli SS, Grotendorst GR, Schultz GS. A Connective Tissue Growth Factor Signaling Receptor in Corneal Fibroblasts. Investigative Ophthalmology & Visual Science. 2012;53(7):3387–3394. doi: 10.1167/iovs.12-9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Buhren J, Nagy L, Swanton JN, Kenner S, MacRae S, Phipps RP, Huxlin KR. Optical effects of anti-TGFbeta treatment after photorefractive keratectomy in a cat model. Invest Ophthalmol Vis Sci. 2009;50(2):634–643. doi: 10.1167/iovs.08-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZY, Wu HK, Bruce A, Wollenberg K, Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Investigative Ophthalmology & Visual Science. 2002;43(9):2897–2904. [PubMed] [Google Scholar]
- Chirila TV. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials. 2001;22(24):3311–3317. doi: 10.1016/s0142-9612(01)00168-5. [DOI] [PubMed] [Google Scholar]
- Coster DJ, Jessup CF, Williams KA. Mechanisms of corneal allograft rejection and regional immunosuppression. Eye (Lond) 2009;23(10):1894–1897. doi: 10.1038/eye.2009.17. [DOI] [PubMed] [Google Scholar]
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18(4):529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Fullwood NJ. Collagen fibril orientation and corneal curvature. Structure. 2004;12(2):169–170. doi: 10.1016/j.str.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278(46):45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992;33(12):3271–3282. [PubMed] [Google Scholar]
- Gipson IK, Grill SM, Spurr SJ, Brennan SJ. Hemidesmosome formation in vitro. J Cell Biol. 1983;97(3):849–857. doi: 10.1083/jcb.97.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54(11):1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- Guo XQ, Hutcheon AEK, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Investigative Ophthalmology & Visual Science. 2007;48(9):4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Effect of galardin on collagen degradation by Pseudomonas aeruginosa. Exp Eye Res. 1999;69(6):595–601. doi: 10.1006/exer.1999.0755. [DOI] [PubMed] [Google Scholar]
- Hata RI, Senoo H. L-Ascorbic-Acid 2-Phosphate Stimulates Collagen Accumulation, Cell-Proliferation, and Formation of a 3-Dimensional Tissue-Like Substance by Skin Fibroblasts. Journal of Cellular Physiology. 1989;138(1):8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- Huxlin KR, Hindman HB, Jeon KI, Buhren J, MacRae S, DeMagistris M, Ciufo D, Sime PJ, Phipps RP. Topical Rosiglitazone Is an Effective Anti-Scarring Agent in the Cornea. Plos One. 2013;8(8) doi: 10.1371/journal.pone.0070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997;16(2):177–187. [PubMed] [Google Scholar]
- Jester JV, Nien CJ, Vasiliou V, Brown DJ. Quiescent keratocytes fail to repair MMC induced DNA damage leading to the long-term inhibition of myofibroblast differentiation and wound healing. Mol Vis. 2012;18:1828–1839. [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010;51(3):1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Hutcheon AE, Zieske JD. Transforming growth factor-beta3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med. 2011;5(8):e228–238. doi: 10.1002/term.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Rich CB, Zareian R, Hutcheon AE, Ruberti JW, Trinkaus-Randall V, Zieske JD. TGF-beta3 stimulates stromal matrix assembly by human corneal keratocyte-like cells. Invest Ophthalmol Vis Sci. 2013;54(10):6612–6619. doi: 10.1167/iovs.13-12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuba M, Hutcheon AE, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res. 2011;93(4):534–540. doi: 10.1016/j.exer.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med. 2011;11(2):110–128. doi: 10.2174/156652411794859241. [DOI] [PubMed] [Google Scholar]
- Nagano T, Hao JL, Nakamura M, Kumagai N, Abe M, Nakazawa T, Nishida T. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Invest Ophthalmol Vis Sci. 2001;42(6):1247–1253. [PubMed] [Google Scholar]
- Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74(2):167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- Saika S. Ultrastructural Effect of L-Ascorbic-Acid 2-Phosphate on Cultured Keratocytes. Cornea. 1992;11(5):439–445. doi: 10.1097/00003226-199209000-00014. [DOI] [PubMed] [Google Scholar]
- Sekiyama E, Nakamura T, Cooper LJ, Kawasaki S, Hamuro J, Fullwood NJ, Kinoshita S. Unique Distribution of Thrombospondin-1 in Human Ocular Surface Epithelium. Investigative Ophthalmology & Visual Science. 2006;47(4) doi: 10.1167/iovs.05-1305. [DOI] [PubMed] [Google Scholar]
- Sriram S, Robinson P, Pi L, Lewin AS, Schultz G. Triple combination of siRNAs targeting TGFbeta1, TGFbetaR2, and CTGF enhances reduction of collagen I and smooth muscle actin in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2013;54(13):8214–8223. doi: 10.1167/iovs.13-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo JH, Gollamudi S, Green WR, De La Cruz Z, Filatov V, Stark WJ. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch Ophthalmol. 1991;109(8):1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Kuroki M, Oshima K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004;315(4):928–934. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72(1):33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]