Abstract
Background
Selectins are adhesion molecules that are expressed by the vascular endothelium upon activation and may be an imaging target for detecting myocardial ischemia long after resolution. We hypothesized that molecular imaging of selectins with myocardial contrast echocardiography (MCE) molecular imaging could be used to detect recent brief ischemia in closed-chest non-human primates.
Methods
Myocardial ischemia was produced in anesthetized adult rhesus macaques (n=6) by percutaneous balloon catheter occlusion of the LAD or circumflex coronary artery for 5–10 minutes. Three separate macaques served as non-ischemic controls. MCE perfusion imaging was performed during coronary occlusion to measure risk area (RA) and at 100–110 min to exclude infarction. MCE molecular imaging was performed at 30 and 90 min after reperfusion using a lipid microbubble bearing dimeric recombinant human P-selectin glycoprotein ligand-1 (MB-YSPSL). Collection of blood for a safety data, electrocardiography, and echocardiography were performed at baseline, and before and 10 min after each MB-YSPSL injection.
Results
Vital signs, O2 saturation, ECG, ventricular systolic function, pulmonary vascular resistance, and serum safety markers were unchanged by intravenous injection of MB-YSPSL. On echocardiography, LV dysfunction in the RA had resolved by 30 min and there was no evidence for infarction on MCE perfusion imaging. On selectin-targeted MCE molecular imaging, signal enhancement was greater (p<0.05) in the RA than remote territory at 30 min (25±11 vs 11±4 IU) and 90 min (13±3 vs 3±2 IU) after ischemia. There was no enhancement (<1 IU) in control non-ischemic subjects.
Conclusion
In primates, MCE molecular imaging of selectins using a recombinant ligand appropriate for humans is both safe and effective for imaging recent myocardial ischemia. This technique may be useful for detecting recent ischemia in patients with chest pain even in the absence of necrosis.
There are well-recognized limitations of the diagnostic approaches currently used to evaluate patients with possible acute coronary syndrome (ACS). The initial ECG and cardiac enzymes are often non-diagnostic in those with ACS resulting in delayed initiation of appropriate therapy [1, 2]. These tests also lack positive predictive value and can be abnormal from conditions other than ACS. In response, new technologies are being developed to more accurately diagnose ACS, or to exclude ischemia in the close to six million individuals who present each year to emergency departments in the United States with chest pain, the majority of whom do not have ACS but nonetheless require significant resources in their diagnostic evaluation [3].
Molecular imaging of ischemia has emerged as a promising technology to address these challenges and relies on targeted imaging probes to non-invasively detect molecular events that occur with the onset of ischemia and persist for hours after resolution, a strategy termed ischemic memory imaging. Myocardial contrast echocardiography (MCE) molecular imaging of selectins has been used to detect recent ischemia in small animal models of disease [4–6]. Selectins are long adhesion molecules expressed upon vascular endothelial activation. Endothelial P- and E-selectin mediate leukocyte rolling by interacting with carbohydrate counterligands such as P-selectin glycoprotein ligand-1 (PSGL-1) found on the leukocyte membrane [7]. By using PSGL-1 as a targeting ligand for ultrasound contrast agents, it has been possible to detect both early (P-selectin) and late (P- and E-selectin) phases of myocardial ischemia-reperfusion injury [6].
The aim of this study was to evaluate both the safety and efficacy of an ultrasound microbubble contrast agent bearing a dimeric recombinant human PSGL-1 for ischemic memory imaging in non-human primates undergoing brief closed-chest myocardial ischemia produced by temporary coronary occlusion. These studies were performed as part of the development of a rapid and bedside method for rapidly diagnosing ACS in patients.
METHODS
Animal Preparation and Study Protocol
The study was approved by the Animal Care and Use Committees at Oregon Health & Science University and the Oregon National Primate Research Center. Nine adult male rhesus macaques (Macaca mulatta) 10–15 years of age were studied. Animals were randomized to ischemic (n=6) and non-ischemic control (n=3) groups. Animals were sedated with ketamine HCL (10mg/kg I.M.) followed by endotracheal intubation and maintenance of anesthesia with inhaled isofluorane (1.0–1.5%) during spontaneous respiration. Continuous electrocardiographic monitoring and O2 saturation was performed. The study protocols are schematically depicted in Figure 1. After an equilibration period of 20 min, a full baseline echocardiographic study and complete laboratory blood testing were performed. For the ischemic group, coronary ischemia was induced by percutaneous balloon catheter occlusion for 5–10 min. Myocardial perfusion imaging was performed during ischemia to define the risk area and 100–110 min after reflow to exclude infarction. Molecular imaging of P-selectin was performed 30 and 90 minutes after reflow or after similar post-induction anesthesia time in controls. Blood pressure, echocardiography and blood sampling was performed immediately before and 5–10 min after each targeted microbubble injection.
Figure 1.
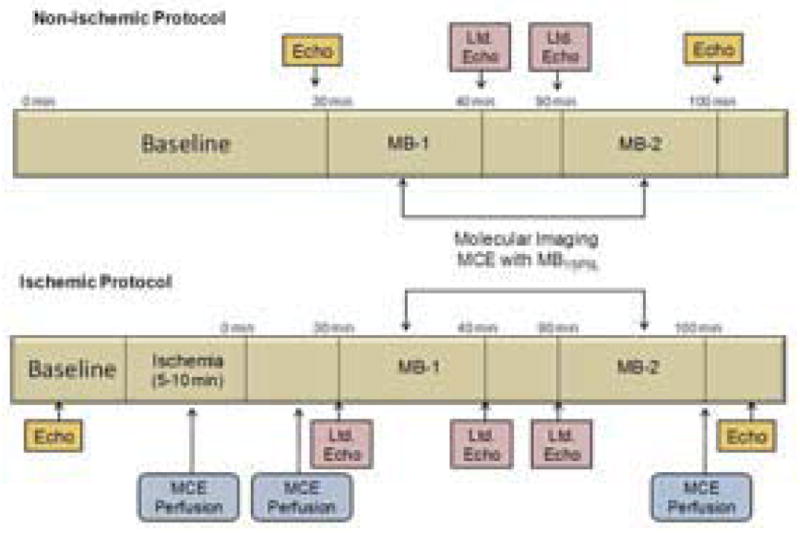
Schematic Depiction of the Study Protocol. Protocols are shown for non-ischemic control animals and animals undergoing ischemia-reperfusion injury. Ltd, limited; MB, targeted microbubble injection; MCE, myocardial contrast echocardiography.
Percutaneous Coronary Ischemia
A femoral artery sheath was placed after which animals received intravenous heparin (70 units/kg) and aspirin (100 mg) per rectum. A 5 F left guide catheter was then used to selectively engage the left main coronary artery. A 2 mm balloon catheter was advanced into the proximal portion of either the LAD or left circumflex coronary artery. The balloon catheter was inflated for 5 (n=4) or 10 min (n=2) depending on whether hemodynamic instability necessitated early deflation. Ischemia was confirmed by ST-elevation on ECG monitoring and a new regional wall motion abnormality on 2D echocardiography, and the ischemic risk area was spatially defined during occlusion by MCE perfusion imaging.
Perfusion Imaging
Myocardial contrast echocardiography (Sonos 5500, Philips Ultrasound; S5-1 transducer) was performed at baseline, during coronary occlusion and upon completion of the final targeted imaging protocol (100 to 110 min post-reperfusion). Harmonic power Doppler imaging was performed at a transmission frequency of 1.3 MHz and a mechanic index of 1.2. Lipid-shelled octofluoropropane microbubbles (Definity, Lantheus Medical Imaging) were diluted with saline and infused intravenously at 4×108 min−1. End-systolic images were acquired in apical 2-, 3-, and 4-chamber views with incremental pulsing intervals from every 1 to 5 cardiac cycles. The spatial extent of the perfusion defect (risk area) was defined as the region lacking opacification defined by a reader blinded to treatment assignment and stage, and was expressed as a ratio to total myocardial end-systolic area. The final MCE study was used to exclude infarction or residual hypoperfusion. For quantitative analysis, regions-of-interest were placed over the risk area and a remote myocardial territory. Acoustic intensity within the regions were fit to the function y=A(1−e−βt) where y is acoustic intensity at time t, A is the plateau intensity reflecting relative blood volume, and the rate constant β is the microvascular flux rate. The myocardial blood flow was calculated as the product of A and β [8].
MCE Molecular Imaging
For selectin targeting, lipid-shelled decafluorobutane microbubbles containing 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyerthylene glycol)2000] (5% molar concentration) were prepared and PSGL-1 synthesized as a dimeric fusion protein with human IgG1 (YSPSL, Y’s Therapeutics) was conjugated to the surface of microbubbles by maleimide coupling to form a stable thioether bond with the IgG portion of the YSPSL (synthesized in a good manufacturing process laboratory and kindly provided by Thierry Bettinger, Bracco Research, Geneva). Molecular imaging was performed at 30 and 90 minutes after coronary reflow, or at corresponding times in non-ischemic animals by intravenous injection of 2×108 targeted microbubbles (MB-YSPSL). Imaging was performed 8 minutes after injection to allow for microbubble attachment and clearance of freely circulating agent [9–11]. End-systolic images were obtained using harmonic power Doppler imaging (1.3 MHz, mechanical index 1.2) in the apical 2, 3 and 4 chamber views. Several frames were obtained at a PI of 1 then every 5 cardiac cycles. Signal enhancement from retained targeted contrast agent alone was determined by signal from the initial frame acquired after digitally subtracting the signal from several averaged frames obtained at a PI of 5 cycles to remove signal from any freely circulating contrast as previously described [12]. Signal was measured from regions-of-interest placed over the risk area and the remote non-ischemic territory in post-ischemic subjects, and over the anterior wall in non-ischemic controls. The spatial extent of enhancement on molecular imaging was measured independently by a reader blinded to animal identity and stage.
Echocardiography
Comprehensive 2-D and Doppler transthoracic echocardiographic studies were performed at baseline and at completion of the study protocol. Two-dimensional imaging was performed with tissue harmonic settings at a transmission frequency of 1.6 MHz. Left ventricular ejection fraction and LV end-systolic and end-diastolic volumes were measured using a modified biplane method. Stroke volume was calculated using the product of the left ventricular outflow tract area and the time-velocity integral measured by pulsed-wave spectral Doppler. Cardiac output (CO) was determined by the product of heart rate and stroke volume. Radial wall thickening in the ischemic group was measured in the center of the risk area and in a remote territory defined by perfusion data during coronary occlusion; or averaged for the anterior and lateral wall for the non-ischemic group. Pulmonary artery systolic pressure (PASP) was estimated from the peak pressure gradient between the right ventricle calculated using the tricuspid regurgitant velocity and the modified Bernoulli’s equation, and right atrial pressure estimated from inferior vena cava dimensions. An index of pulmonary vascular resistance (PVRI-1) was calculated by dividing PA systolic pressure by CO. Mitral valve inflow velocities in early (E) and late (A) diastole were evaluated using pulsed wave Doppler at the mitral leaflet tips. The peak systolic (S′) and early diastolic (E′) longitudinal velocities were be measured by tissue Doppler at the medial and lateral mitral annulus from the apical 4-chamber view and averaged. The ratio of E to E′ was used as an index of left atrial pressure and a second index of PVR (PVRI-2) was calculated by: (PASP-[E/E′])/CO.
A focused echocardiographic exam was performed immediately before and 5–10 min after each of the two targeted imaging acquisitions. For these exams, only LVEF, regional fractional thickening, stroke volume, CO, RV systolic pressure, PVR, mitral inflow and mitral annular longitudinal velocities were measured.
Laboratory Blood Testing
Peripheral venous blood samples were obtained for laboratory safety testing. The frequency and type of blood laboratory testing was limited by Animal Care and Use Committee guidelines that limit daily phlebotomy volume in non-human primates. In the non-ischemic group samples were obtained prior to and after each of the two targeted imaging acquisitions. In the ischemic group where there was additional procedure-related blood loss, samples were obtained at baseline and at the completion of the protocol. Blood tests included serum chemistries, a complete liver panel, blood coagulation studies (PTT, INR), a complete blood count (CBC), C3 complement, lactate dehydrogenous (LDH), and Troponin I.
Statistical Analysis
Data are expressed as mean ±SD unless otherwise noted. Comparison of echocardiographic data, vital signs, ECG measurements, and laboratory blood testing data between time periods was performed using a non-paired t test. Radial wall thickening and coronary flow data was compared with the non-paired t test. For multiple comparisons of the molecular imaging data, a Kruskal-Wallis test with Dunn’s post-hoc test was used. Differences were considered significant at p<0.05.
RESULTS
Electrocardiographic and Safety Studies
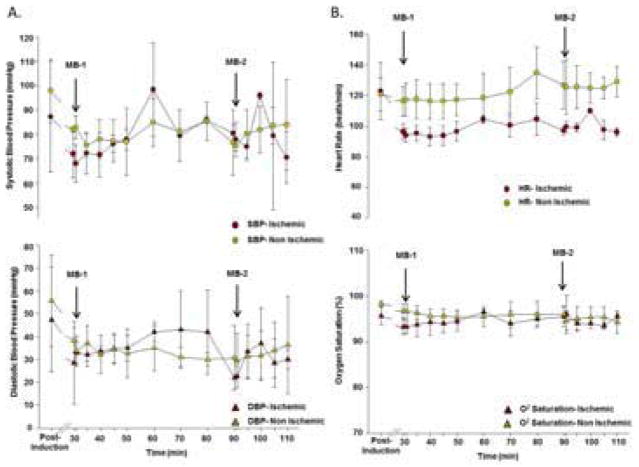
Data could not be obtained in two macaques in the ischemia group because of severe global LV dysfunction and arrhythmias after reperfusion or because of technical issues relating to vascular access. Results therefore represent ischemic (n=4) and non-ischemic control (n=3) subjects that completed the entire protocol. In the ischemic group, HR decreased during ischemia and then stabilized after reperfusion (Figure 2). In both the ischemic and non-ischemic control groups, HR was unaffected by injection of MB-YSPSL at 30 min and 90 min. In non-ischemic animals, BP decreased between baseline and the 30 min time interval, likely as a result of deeper anesthesia, and remained constant thereafter. In ischemic animals, BP was low during ischemia and was lower than the non-ischemic animals only during the early post-reflow period. In both groups, injection of MB-YSPSL did not alter systolic or diastolic BP. Oxygen saturation and body temperature were constant for both groups and were not altered by MB-YSPSL injection. On ECG monitoring, there were frequent ventricular extrasystoles during ischemia and in the first 10 min of reperfusion in several animals. However, administration of MB-YSPSL did not produce any arrhythmias, extrasystoles, ST-segment changes, or changes in electrocardiographic intervals including QTc (Supplemental table).
Figure 2.
Vital Signs During Microbubble Injection. Mean (±SEM) values for (A) systolic (SBP) and diastolic (DBP) blood pressure, and (B) heart rate (HR) and O2 saturation are shown for animals undergoing ischemia and for non-ischemic controls. The time points for injection of MB-YSPSL (MB-1 and MB-2) are displayed.
Laboratory Safety Data
In the non-ischemic group, there were no significant changes during the course of the study for any of the laboratory values (Table 1). In the ischemic group, hemoglobin and hematocrit decreased slightly at the conclusion of the study in the ischemic group, probably from procedure-related blood loss; and PTT increased due administration of heparin. Serum troponin did not increase at study completion even in the ischemic group. There were no detectable changes in serum C3 complement.
Table 1.
Laboratory Safety Data
| Non-Ischemic (n=3) | Ischemic (n=4) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-MB 1 (30min) | Post-MB 1 (40min) | Pre-MB 2 (90min) | Post-MB 2 (100min) | Baseline | Post MB 2 (100min) | |
|
|
|
|||||
| WBC (103/mm3) | 7.1±0.4 | 5.9±1.6 | 5.9±1.8 | 5.8±1.1 | 5.6±2.5 | 5.8±2.0 |
| HBG (g/dl) | 13.3±0.1 | 12.8±0.6 | 12.5±0.3 | 12±0.2 | 12.6±0.6 | 11.4±1.0* |
| HCT (%) | 40±1 | 39±2 | 38±1 | 36±1 | 38±2 | 34±2* |
| PLT (103/mm3) | 340±65 | 364±10 | 331±7 | 354±36 | 271±42 | 263±59 |
| INR | 1.6±0.2 | 1.4±0.3 | 1.3±0.2 | 1.4±0.2 | 1.1±0.1 | 1.4±0.0 |
| PTT (sec) | 33±2 | 30±3 | 28±1 | 29±2 | 26±2 | 36±9* |
| C3 (mg/dl) | 104±4 | 93±2 | 88±5 | 89±4 | 130±20 | 94±11 |
| Troponin I (ng/ml) | 0.04±0.01 | 0.05±0.02 | 0.05±0.02 | 0.05±0.01 | 0.04±0 | 0.05±0.01 |
| Na (mmol/L) | 146±1 | 146±1 | ||||
| Calcium (mg/dl) | 9±9 | 9±9 | ||||
| Glucose (mg/dl) | 73±5 | 65±7 | ||||
| BUN (mg/dl) | 18±2 | 19±2 | ||||
| Creatinine (mg/dl) | 0.8±0.0 | 0.8±0.1 | ||||
| AST (IU/L) | 27±14 | 30±9 | ||||
| ALT (IU/L) | 38±21 | 34±17 | ||||
| Alk Phos (IU/L) | 120±83 | 110±76 | ||||
| T. Bili (mg/dl) | 0.3±0.1 | 0.6±0.5 | ||||
| Albumin (g/dl) | 3.8±0.1 | 3.3±0.2† | ||||
| LDH (IU/L) | 160±32 | 135±22 | ||||
| LDH (IU/L) | 160±32 | 135±22 | ||||
p<0.05 vs. baseline;
p<0.05 vs pre-MB 1
Ventricular Function and Pulmonary Vascular Hemodynamics
Echocardiographic measurements of ventricular function and pulmonary hemodynamics were unchanged after administration of MB-YSPSL in both treatment groups (Table 2). In the ischemic group, cardiac output was reduced at all post-ischemic intervals compared to baseline which was attributable more to a reduction in heart rate than stroke volume. In the ischemic group, peak systolic and early diastolic mitral annular longitudinal velocities on tissue Doppler (S′ and E′) were reduced at the 90 min post-ischemic interval compared to baseline but were unaffected by MB-YSPSL administration.
Table 2.
Safety and Hemodynamic Data from Echocardiography
| Non-Ischemic (n=3) | Ischemic (n=4) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pre MB 1 (30min) | Post MB 1 (40min) | Pre MB 2 (90min) | Post MB 2 (100min) | Baseline | Pre MB 1 (30min) | Post MB 1 (40min) | Pre MB 2 (90min) | Post MB 2 (100min) | |
|
|
|
||||||||
| CO (L/min) | 1.2±0.3 | 1.3±0.2 | 1.4±0.2 | 1.1±0.2 | 1.3±0.1 | 0.9± 0.2* | 0.9±0.2* | 0.9±0.1* | 0.8±0.1* |
| SV (ml) | 9.9± 1.5 | 10.5±1.1 | 10.5±0.9 | 9.3±2.5 | 11.4±1.7 | 9.5±2.3 | 10.1±2.3 | 8.6±0.2 | 8.4±0.6* |
| LVEF (%) | 55±2.5 | 56±4.9 | 59±5.3 | 60±1.6 | 68±5.5 | 63±5.7 | 61±1.9 | 64±0.3 | 65±1.3 |
| LVIDs (ml) | 5.1±1.2 | 5.2±1.8 | 4.4±0.9 | 4.5±0.4 | 3.8±1.1 | 5.0±1.4 | 5.2±0.9 | 3.4±0.5 | 3.5±0.3 |
| LVIDd (ml) | 11.5±2.1 | 11.6±3.1 | 10.8±1.6 | 11.4±1.1 | 11.8±2.0 | 13.3±2.8 | 13.3±2.2 | 9.2±1.5 | 10.2±1.4 |
| PASP (mm Hg) | 12.6±2.3 | 12.8±1.2 | 13.6±2.3 | 12.2±3.8 | 17.6±2.4 | 18.9±0.8 | 19.1±0.1 | NM | 17.5±0.8 |
| PVRI-1 | 10.7±2.4 | 10.5±1.9 | 9.0±0.7 | 10.5±1.3 | 13.3±1.9 | 16.2±0.2 | 18.2±1.2£ | NM | 21±0.1£ |
| PVRI-2 | 6.6±2.2 | 6.1±2.3 | 5.8±0.9 | 6.2±2.9 | 7.2±1.8 | 9.8±5.1 | 12.4±5.9 | NM | 9.1±1.3 |
| Mitral E/A | 1.9±0.3 | 2.0±0.6 | 1.4±0.2 | 1.8±0.7 | 2.0±0.5 | 1.9±0.8 | 2.0±0.5 | 2.0±0.5 | 2.0±1.1 |
| Mitral E′ (cm/s) | 12.5±2.9 | 11.5±2.7 | 10.8±0.4 | 11.7±1.6 | 11.2±1.5 | 8.8±2.1 | 9.7±2.0 | 7.4±3.0* | 7.0±1.8* |
| Mitral S′ (cm/s) | 10.4±1.6 | 9.1±1.6 | 11.6±3.5 | 9.7±1.4 | 10.7±1.5 | 6.2±3.4* | 7.0±1.4* | 5.4±1.5* | 5.7±0.4* |
| Mitral E/E′ | 4.8±1.2 | 5.6±2.5 | 5.5±1.4 | 5.2±2.5 | 7.9±2.3 | 8.8±2.3 | 7.5±1.8 | 7.0±1.8 | 9.9±0.6 |
| TAPSE (cm) | 0.58±0.03 | 0.60±0.05 | 0.64±0.09 | 0.62±0.06 | 1.03±0.11 | 1.02±0.11 | 1.02±0.11 | 0.99±0.08 | 0.94±0.05 |
| Tricuspid S′ (cm/s) | 10.8±2.9 | 10.4±3.3 | 12.5±3.8 | 7.9±1.0 | 8.1±1.7 | 5.8±2.1 | 6.7±1.3 | 5.2±1.4 | 7.3±5.3 |
CO, cardiac output; E′, peak early diastolic annular velocity; LVIDd, left ventricular internal diameter at end-diastole LVIDs, left ventricular internal diameter at end-systole; MB, microbubble; NM, not measuared; PASP, pulmonary artery systolic pressure; PVRI, pulmonary vascular resistance index; S′, peak systolic annular velocity; SV, stroke volume; TAPSE, tricuspid annular plane systolic excursion;
p<0.05 vs. baseline
In animals undergoing ischemia, fractional radial wall thickening in the risk area during coronary occlusion was reduced compared to the remote region and to baseline values (Figure 3A). Wall thickening in the risk area recovered and was similar to remote regions at both 30 and 90 minutes after reperfusion, indicating resolution of post-ischemic stunning. Wall thickening in both groups was unaffected by administration of MB-YSPSL (Figure 3B).
Figure 3.
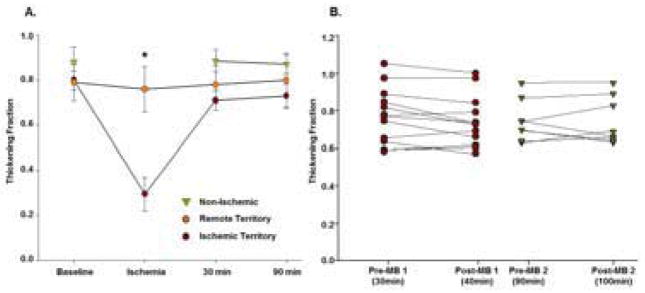
Regional Left Ventricular Wall Thickening. (A) Mean (±SEM) radial wall thickening fraction for the center of the risk area and remote territories for the ischemic group; and averaged for the anterior and lateral myocardium for the non-ischemic group. *p<0.01 vs baseline and remote territory. (B) Radial wall thickening in the risk area and remote territory immediately before and 10 min after injection of MB-YSPSL at the 30 and 90 min post-reflow time intervals.
Myocardial Perfusion
During coronary occlusion, myocardial blood flow within the reader-defined risk area on MCE was severely reduced compared to the remote territory (Figure 4). The median size of the risk area was 51% (range 32% to 65%) of the left ventricular area. Risk area blood flow was similar to that in the remote territory during early reperfusion and at the completion of the study (Figure 4). Myocardial blood flow decreased slightly between early reperfusion and the completion of the study even after normalizing flow to double product (Figure 4). At the completion of the study, there were no perfusion defects identified by the reader blinded to identity and stage.
Figure 4.
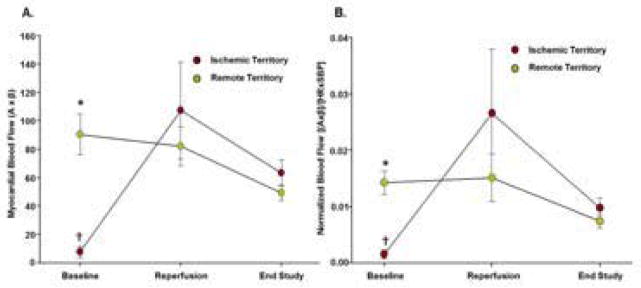
Myocardial Perfusion Imaging. Relative myocardial blood flow measured by MCE expressed as either (A) the product of relative microvascular blood volume and microvascular flux rate (A×β); or (B) flow normalized to the double product ([(A×β]/[HR×SBP]) where SBP is systolic blood pressure. *p<0.01 vs remote territory; †p<0.05 by paired analysis vs reperfusion.
Ischemic Memory Imaging
On MCE molecular imaging of selectins with MB-YSPSL, there was no significant signal enhancement in the non-ischemic control animals (Figure 5). Signal enhancement in the post-ischemic risk area was strong and significantly greater than both the remote territory and to signal from non-ischemic animals at both the 30 (240 ± 85% increase vs remote) and 90 min (280 ± 80% increase vs remote) intervals. Signal enhancement in the remote territory was significantly greater than in non-ischemic controls at the 30 min time interval.
Figure 5.
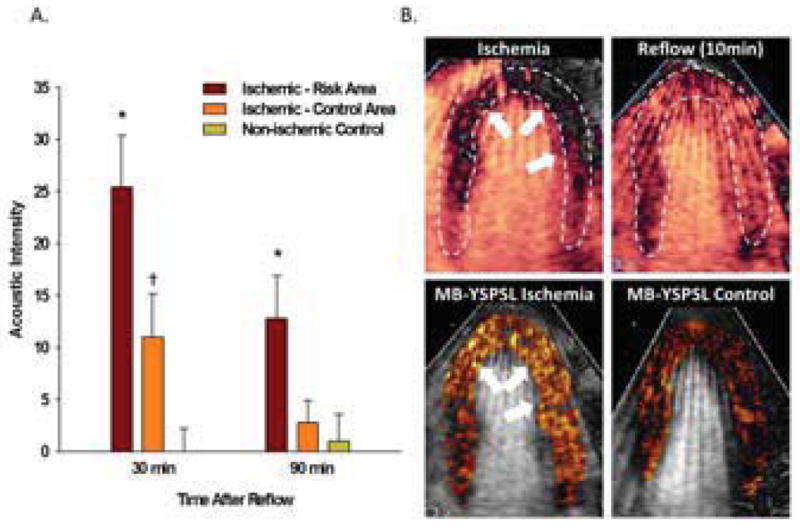
Myocardial Ischemic Memory Imaging. (A) Mean (±SEM) background-subtracted acoustic intensity from MB-YSPSL in ischemic and remote territories in animals undergoing ischemia-reperfusion; and in the anterior wall in non-ischemic control animals. *p<0.05 vs remote territory and non-ischemic control; †p<0.05 vs non-ischemic control. (B) Examples of MCE images from an apical 4-chamber view from a single animal illustrate: an anterior and lateral wall perfusion defect (arrows) during LAD occlusion (top left); complete resolution of hypoperfusion 10 min after reflow (top right); color-coded images demonstrating signal enhancement from MB-YSPSL 30 min after reflow in ischemic (bottom left) and corresponding time point in non-ischemic (bottom right) primates after digital subtraction of the signal from freely circulating agent.
DISCUSSION
Development of an echocardiographic approach for ischemic memory imaging in humans requires pre-clinical testing of molecular imaging probes in large animal models of ischemia. The aim of this study was to test the safety and efficacy of a selectin-targeted ultrasound contrast agent in a primate model of closed-chest brief myocardial ischemia produced by temporary coronary occlusion. We demonstrated that microbubbles targeted to human P-selectin were well tolerated and produced signal enhancement in the post-ischemic risk area at a time when both myocardial blood flow and wall motion had returned to normal.
Imaging of Ischemia in Patients with Chest Pain
Echocardiographic evaluation of resting wall motion has been shown to provide useful information for early identification of ACS in patients presenting to the emergency department with chest pain or other symptoms [13]. It is of particular use in those who have non-diagnostic ECGs or low-to-medium pretest probability of ischemia. In this setting, wall motion abnormalities can result from ongoing ischemia, post-ischemic stunning, or prior ischemic events. The addition of perfusion imaging with MCE can provide additional information on these different possibilities and, hence, adds prognostic information [13]. This approach does not necessarily address the need to detect and spatially quantify myocardial ischemia in those whose symptoms have resolved before being evaluated, particularly if ischemia was not severe enough to produce long-term stunning.
Molecular imaging of alterations that persist even after brief hypoperfusion have being developed to rapidly diagnose ACS or to exclude ischemia in the majority of patients that present with chest pain who do not have ACS. Ischemic memory imaging would also be expected to provide useful prognostic information regarding the spatial extent of recent ischemia and could potentially be used to diagnose ischemia in those who have a pre-existing wall motion abnormalities from prior ischemic damage. A radionuclide approach for ischemic memory imaging has been developed using tracers that detect an abnormal shift away from myocardial fatty acid metabolism and has been shown to identify ACS in patients in the emergency department [14]. Interest in an echocardiographic approach to ischemic memory imaging is based on practical considerations such as speed, portability, availability, cost and lack of exposure to radiation.
Selectins as an Ischemia Imaging Target
The first description of ultrasound imaging of selectin expression was with P-selectin-targeted microbubbles in a murine model of moderate renal ischemia-reperfusion injury [12]. In these studies, intravital microscopy confirmed direct microbubble attachment to the venular endothelium, the primary site of selectin expression and selectin-dependent leukocyte rolling. Since then, MCE with selectin-targeted microbubbles have successfully been used in mice and rats to detect the territory of recent but resolved ischemia without infarction, even after resolution of post-ischemic stunning [4–6].
The use of dimeric recombinant human PSGL-1 fusion protein (YSPSL) as a targeting ligand in this study was chosen with the intent of investigating an approach that may be feasible to use in humans. The safety of non-conjugated YSPSL in humans has been established in Phase 2 and 3 clinical trials where high doses were used as a possible treatment for renal or liver allograft rejection [15]. In this study it was conjugated to microbubbles to detect selectin expression which is one of several inflammatory responses to ischemia. Because tissue injury or ischemia produces an immediate venular endothelial P-selectin and a later E-selectin response, and because both selectins bind PSGL-1, YSPSL may be particularly effective targeting ligand for detecting the antecedent ischemia early or late after resolution. Recent studies in mice have demonstrated that MB-YSPSL binds to both the early P-selectin and late E-selectin response [6], although the true time window that ischemic memory imaging would be possible in humans is currently unknown.
A model of myocardial ischemia in Rhesus macaques was chosen to evaluate feasibility of MCE ischemic memory imaging in primates for the first time. With regard to the closed-chest approach, in previous small animal studies an open-chest method for producing coronary occlusion has resulted in P-selectin expression even in non-ischemic myocardium [4]. In our current study, selectin-targeted MCE signal was much greater in the previously ischemic risk area than in remote territories. However, a lower level of signal enhancement was detected in the remote territory at 30 min, suggesting regional ischemia produces a mild but detectable global P-selectin mobilization. In agreement with our findings, histopathology of primate hearts subjected to closed-chest ischemia-reperfusion injury has detected upregulation of venular P-selectin in the risk area, although it was also noted in remote regions [16].
Safety Considerations
A major focus of our study was to produce some preliminary information on the safety of MB-YSPSL in primates. Recent studies have definitively established the safety of commercially-produced non-targeted ultrasound contrast agents in approved doses [17]. It should be noted, however, that lipid-shelled microbubble agents have been shown to activate human complement at their surface [18]. Our studies failed to detect any changes in vital signs, ventricular function, hemodynamics, surface ECG, or serum safety markers including serum complement after bolus injection of MB-YSPSL in non-ischemic and post-ischemic animals.
Limitations
A major limitation of this study was that sample size was small because of both cost and institutional availability of primate test subjects. Although there were no hemodynamic or immunologic changes with MB-YSPSL, larger pre-clinical safety studies will be needed. With regard to study design, because we did not vary the duration of ischemia or image beyond 90 min, we do not know whether the degree of ischemia influences molecular signal intensity or duration of the effective time window for ischemic memory imaging with MB-YSPSL. We instead used only a very brief period of ischemia due to the low number of test subjects. Results from mice indicate that when ischemia is brief, the spatial extent of selectin signal may gradually decrease with time. We also could not compare contrast enhanced ultrasound molecular and perfusion imaging to other cardiac imaging techniques such as cardiac MR or SPECT as these imaging modalities are not currently available at the Oregon National Primate Research Center. Finally we lack a comparative gold standard for confirming endothelial P-selectin expression. Our protocols were designed to avoid termination of life since previous studies in primates studies have already documented an increase in venular endothelial P-selectin after brief ischemia and reperfusion [16]. More importantly, the degree of P-selectin staining on immunohistochemistry may not reliably reflect how much has transported from its pre-formed storage sites within the Weibel-Palade bodies to the luminal surface where it participates in leukocyte rolling and can be detected by microbubble contrast agents.
Conclusion
We conclude that molecular ischemic memory imaging is possible in non-human primates with MCE and a selectin-targeted microbubble contrast agent bearing a recombinant human PSGL-1. The comprehensive hemodynamic, ventricular function and laboratory evaluation for safety in this small group of subjects did not raise concerns. This technique may provide a method for the bedside detection of recent or ongoing ischemia in patients with chest pain; although further pre-clinical evaluation of safety and efficacy will be required.
Supplementary Material
Acknowledgments
Dr. Lindner is supported by grants R01-HL-078610, R01-DK-063508, R01HL111969, and RC1-HL-100659; and Dr Davidson is supported by grant T32-HL-094294-01 from the National Institutes of Health, Bethesda, MD. Dr. Chadderdon is supported by a Fellow-to-Faculty Transition Award from the American Heart Association, Dallas, TX.
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndrome
- CO
cardiac output
- MB
microbubble
- MCE
myocardial contrast echocardiography
- PASP
pulmonary artery systolic pressure
- PI
pulsing interval
- PSGL-1
P-selectin glycoprotein ligand-1
- PVR
pulmonary vascular resistance
- YSPSL
dimeric recombinant P-selectin glycoprotein ligand-1
Footnotes
Disclosures: MB-YSPSL was manufactured and provided for the study by Bracco Imaging SA. There was no input from Bracco Imaging on study design, data analysis or manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 2.Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126:461–9. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28:2011–7. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva FS, Lu E, Bowry S, Kilic S, Tom E, Wang J, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115:345–52. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson BP, Kaufmann BA, Belcik JT, Xie A, Qi Y, Lindner JR. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. Journal of the American College of Cardiology. 2012;60:1690–7. doi: 10.1016/j.jacc.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–8. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 8.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: bolus or continuous infusion? J Am Coll Cardiol. 1998;32:252–60. doi: 10.1016/s0735-1097(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 9.Chadderdon SM, Belcik JT, Bader L, Kirigiti MA, Peters DM, Kievit P, et al. Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation. 2014;129:471–8. doi: 10.1161/CIRCULATIONAHA.113.003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba Y, Davidson BP, Kim S, Liu YN, Packwood W, Belcik JT, et al. Echocardiographic evaluation of the effects of stem cell therapy on perfusion and function in ischemic cardiomyopathy. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2014;27:192–9. doi: 10.1016/j.echo.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111:3248–54. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 12.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107–12. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 13.Tong KL, Kaul S, Wang XQ, Rinkevich D, Kalvaitis S, Belcik T, et al. Myocardial contrast echocardiography versus Thrombolysis In Myocardial Infarction score in patients presenting to the emergency department with chest pain and a nondiagnostic electrocardiogram. J Am Coll Cardiol. 2005;46:920–7. doi: 10.1016/j.jacc.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 14.Kontos MC, Dilsizian V, Weiland F, DePuey G, Mahmarian JJ, Iskandrian AE, et al. Iodofiltic acid I 123 (BMIPP) fatty acid imaging improves initial diagnosis in emergency department patients with suspected acute coronary syndromes: a multicenter trial. J Am Coll Cardiol. 2010;56:290–9. doi: 10.1016/j.jacc.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 15.Gaber AO, Mulgaonkar S, Kahan BD, Woodle ES, Alloway R, Bajjoka I, et al. YSPSL (rPSGL-Ig) for improvement of early renal allograft function: a double-blind, placebo-controlled, multi-center Phase IIa study. Clin Transplant. 2011;25:523–33. doi: 10.1111/j.1399-0012.2010.01295.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomas R, Cheng Y, Yan J, Bettinger T, Broillet A, Rioufol G, et al. Upregulation of coronary endothelial P-selectin in a monkey heart ischemia reperfusion model. J Mol Histol. 2010;41:277–87. doi: 10.1007/s10735-010-9289-z. [DOI] [PubMed] [Google Scholar]
- 17.Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, et al. The safety of Definity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21:1202–6. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Fisher NG, Christiansen JP, Klibanov A, Taylor RP, Kaul S, Lindner JR. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J Am Coll Cardiol. 2002;40:811–9. doi: 10.1016/s0735-1097(02)02038-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.