Abstract
Isoliquiritigenin (ILTG) is a chalcone compound and shows various pharmacological properties, including antioxidant and anti-inflammatory activities. In recent study, we have reported a novel role of ILTG in sleep through a positive allosteric modulation of gamma-aminobutyric acid type A (GABAA)-benzodiazepine (BZD) receptors. However, the effect of ILTG in GABAAR-mediated synaptic response in brain has not been tested yet. Here we report that ILTG significantly prolonged the decay of spontaneous inhibitory postsynaptic currents (sIPSCs) mediated by GABAAR in mouse hippocampal CA1 pyramidal neurons without affecting amplitude and frequency of sIPSCs. This enhancement was fully inhibited by flumazenil (FLU), a specific GABAA-BZD receptor antagonist. These results suggest a potential role of ILTG as a modulator of GABAergic synaptic transmission.
Keywords: Isoliquiritigenin, GABAA-BZD receptor, sIPSC
INTRODUCTION
Isoliquiritigenin (ILTG, 2',4',4'-trihydroxychalcone) is a chalcone compound and found in various flavonoids such as Glycyrrhiza uralensis (licorice), Allium ascalonicum, Sinofranchetia chinensis, Dalbergia odorifera, and Glycine max L. [1, 2, 3, 4, 5]. It has been reported that ILTG has various pharmacological properties including anti-inflammatory, antioxidant, anticancer, anti-angiogenic, and anti-allergic [5, 6, 7, 8, 9]. In addition to these properties, it has been demonstrated that ILTG has some neurological functions such as inhibition of cocaine-induced dopamine release and hypnotic effect by modulating gamma-aminobutyric acid receptors (GABARs) [10, 11]. However, the exact effect of ILTG in inhibitory synaptic activity and type of GABA receptor have not been demonstrated yet.
In the present study, we investigated the modulation of GABAergic synaptic response by ILTG in mouse hippocampal CA1 pyramidal cell using whole-cell patch clamp technique. We found that ILTG specifically enhanced the decay tau of sIPSC by modulating GABAA-BZD receptor. However, the amplitude and frequency of sIPSC were not affected by ILTG. Therefore, our results suggest a potential role of ILTG as a modulator of GABAergic synaptic transmission.
MATERIALS AND METHODS
Slice preparation
Adult mice (7~9 weeks) were deeply anaesthetized until cessation of breathing and subsequently decapitated. The brain was rapidly removed and submerged in an ice-cold oxygenated artificial cerebrospinal fluid (ACSF) composed of (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1 CaCl2, 3 MgCl2, 10 glucose at pH 7.4, and was bubbled with 5% CO2 / 95% O2. Transverse mouse brain slices (350~400 µm) containing hippocampus were acutely prepared with a Leica vibratome (Leica VT1000S), and incubated in a chamber with oxygenated ACSF at room temperature for 1 hr before use.
Recording of sIPSCs
The standard ACSF recording solution was composed of (mM): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.5 CaCl2, 1.5 MgCl2 and 10 glucose saturated with 95% O2~5% CO2, at pH 7.4. The internal solution was composed of (mM): 140 CsCl, 10 EGTA, 10 HEPES, 4 Mg-ATP, 2 QX-314. To block the spontaneous EPSC, APV (50 µM; Tocris) and CNQX (20 µM; Tocris) were added into ACSF. Recordings were obtained using Axopatch 200A (Axon instruments, Union City, CA, USA) and filtered at 2 kHz. In case of sIPSC recording, recordings were digitized at 10 kHz, and analyzed using pCLAMP 9 (Molecular devices) and Mini Analysis Program (Synaptosoft) as previously described [12]. The sEPSCs were automatically detected and grouped as fast (1~5 ms) and slow rise time (5~10 ms). All experimental procedures described were performed in accordance with the institutional guidelines of Korea Institute of Science and Technology (KIST, Seoul, Korea).
Statistical analyses
Statistical comparisons were performed using independent t-tests for two groups and one-way ANOVA test for three groups. Data were expressed as the mean±S.E.M. Differences were considered significant at **p<0.01.
RESULTS
ILTG does not affect amplitude and frequency of sIPSCs
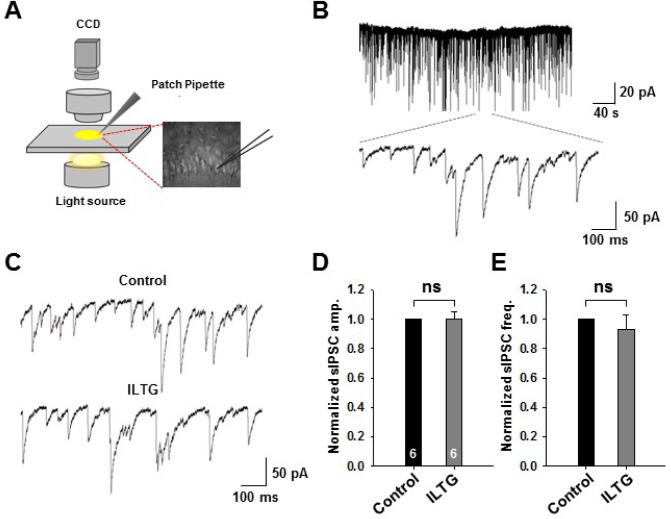
To examine the role of ILTG in the inhibitory synaptic response, we performed the whole-cell patch clamp in hippocampal CA1 pyramidal neurons (Fig. 1A) and measured the sIPSCs at -60mV in the presence of APV and CNQX to block the EPSCs (Fig. 1B). However, the amplitude and frequency of sIPSC were not changed before and after treatment of ILTG (1 µM) by bath application (Fig. 1C~E). This indicates that ILTG does not affect the presynaptic release of GABA and postsynaptic receptor number.
Fig. 1.
ILTG does not affect amplitude and frequency of sIPSCs. (A) Schematic showing slice patch clamp. (B) Representative whole trace (upper) and magnified trace (lower) of sIPSC. (C) Representative trace of sIPSC before and after treatment of ILTG. (D and E) Summary bar graphs of the amplitude (D) and frequency (E) of sIPSC after normalization by control response (Students' tailed t-test). Data are represented as mean +/- SEM. ns indicates nonsignificant difference p>0.05, n=6.
ILTG significantly prolonged the sIPSC decay
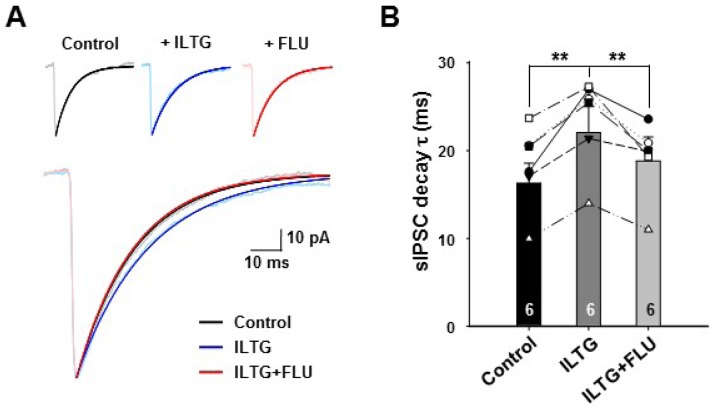
Although ILTG had little effect on sIPSC amplitude and frequency, we analyzed the sIPSC in more detail by measuring the decay tau value after single-exponential decay fitting. Interestingly, ILTG significantly increased the decay time (Fig. 2 control: 18.3±1.9 ms; ILTG: 23.5±2.1 ms). In a recent study, it has been reported that ILTG enhanced the GABA-induced current in dorsal raphe neurons by modulating GABAAR [11]. To test this, we applied flumazenil (FLU, 5 µM), a specific GABAAR antagonist, into ILTG-treated slice. The enhancement of decay time of sIPSC by ILTG was fully restored to control (Fig. 2B, control: 18.3±1.9 ms; ILTG: 23.5±2.1 ms; ILTG+FLU: 19.1±1.7 ms).
Fig. 2.
ILTG significantly prolonged the sIPSC decay. (A) Averaged sIPSCs after normalization by peak. Decay was fitted to one-exponential functions. Upper: individual traces of each conditions. Lower: Superimposed traces. (B) Summary bar graph of sIPSC decay time. Symbols represent individual neurons. Data are represented as mean +/- SEM. **p<0.01, one-way ANOVA, n=6.
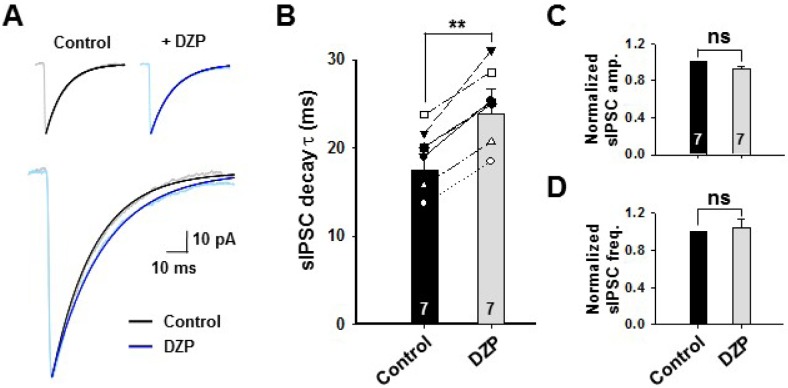
We have reported that ILTG functions as a positive allosteric modulator of GABAA-BZP receptor [11]. To confirm this, we measured the decay time of sIPSC using well known modulator for GABAA-BZP receptor, diazepam (DZP, 1 µM). Just as ILTG, DZP significantly enhanced the decay time of sIPSC (Fig. 3A and B, control: 19.2±1.3 ms; ILTG: 24.9±2.1.6 ms), but did not affect the amplitude and frequency of sIPSC (Fig. 3C and D). These results suggest that ILTG prolonged the sIPSC decay effectively by modulating the GABAA-BZP receptor via a mechanism similar to that of DZP.
Fig. 3.
DZP significantly prolonged the sIPSC decay but not amplitude and frequency of sIPSC. (A) Averaged sIPSCs after normalization by peak. Decay was fitted to one-exponential functions. Upper: individual traces of each conditions. (B) Summary bar graph of sIPSC decay time. Symbols represent individual neurons. (C and D) Summary bar graphs of the amplitude (C) and frequency (D) of sIPSC after normalization by control response. **p<0.01, Students' tailed t-test. ns indicates nonsignificant difference p>0.05, n=7.
DISCUSSION
Here we reported that ILTG significantly enhanced the decay time of sIPSC but had little effect on sIPSC amplitude and frequency. These results is consistent with other modulator for GABAA-BZP receptor, flunitrazepam [13] and DZP (Fig. 3). These results suggest that a similar number of GABA channels are activated initially during the brief synaptic release of GABA generating IPSC. However, the channel opening frequency is increased by modulators for GABAA-BZP receptor producing longer decays after channel activation [14, 15]. In a previous study, we showed that ILTG enhanced the GABA-induced current in a dose-dependent manner in dorsal raphe neurons [11]. The enhancement was 266% by DZP and 151% by ILTG at 1 µM concentration. We prediced that ILTG can enhance the decay time of sIPSCs in a concentration dependent manner, however, in the present study we used 1 µM ILTG to obtain maximal enhancement. Contrary to the GABA-induced current, the enhancement of sIPSC decay time was similar between DZP and ILTG at 1 µM concentration.
GABAARs activity can be modulated by various drugs including benzodiazepines, barbiturates, ethanol, neurosteroids, anesthetics, and ionic zinc with separate binding sites [16, 17, 18]. This modulation was measured in various brain regions such as thalamus, hippocampus, suprachiasmatic nucleus [13, 19, 20] and had many critical role in brain activity not only in physiological condition but in pathophysiological condition including various diseases such as depression, seizure, schizophrenia, and etc [21, 22, 23].
Here we have investigated that ILTG modulates the sIPSC by enhancing response time as a positive allosteric modulator of GABAA-BZP receptor in hippocampal CA1 pyramidal neurons. It has been reported that ILTG inhibits the mitogen-activated protein kinase (MAPK) pathway and chronic benzodiazepine administration reduced the NMDAR activation in hippocampus [8, 19]. Both MAPK pathway and NMDAR are critically involved in hippocampal synaptic plasticity and spatial memory [24, 25, 26, 27]. We can postulate that ILTG could be a negative regulator for NMDAR-dependent synaptic plasticity and relating behavior, which has not been studied yet. Future studies are needed to study the function of ILTG in hippocampal brain function such as synaptic plasticity and memory.
DZP is widely used to treat various diseases including anxiety, seizures and insomnia by modulating GABAA Rs through the binding to the GABAA-BZP receptor. However, DZP has some adverse effects including anterograde amnesia, sedation and depression [28, 29, 30]. ILTG shows hypnotic effects, having 65 fold higher binding affinity than that of DZP [11], suggesting that ILTG could be a potential drug for the treatment of sleep as a natural compound from flavonoids. Therefore, this study suggests that ILTG is a potential drug to treat various diseases of sleep and seizures related to GABAergic synaptic transmission.
ACKNOWLEDGMENTS
This work was supported by the World Class Institute (WCI 2009-003) programs of the National Research Foundation (NRF) funded by the Korean Ministry of Science, Education and Technology (MEST), and also supported by National Agenda Project (NAP) of the Korea Research Council of Fundamental Science and Technology (NAP-09-04).
References
- 1.Cao Y, Wang Y, Ji C, Ye J. Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection. J Chromatogr A. 2004;1042:203–209. doi: 10.1016/j.chroma.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Hsu YL, Chia CC, Chen PJ, Huang SE, Huang SC, Kuo PL. Shallot and licorice constituent isoliquiritigenin arrests cell cycle progression and induces apoptosis through the induction of ATM/p53 and initiation of the mitochondrial system in human cervical carcinoma HeLa cells. Mol Nutr Food Res. 2009;53:826–835. doi: 10.1002/mnfr.200800288. [DOI] [PubMed] [Google Scholar]
- 3.Kong LD, Zhang Y, Pan X, Tan RX, Cheng CH. Inhibition of xanthine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Cell Mol Life Sci. 2000;57:500–505. doi: 10.1007/PL00000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Kim JY, Seo GS, Kim YC, Sohn DH. Isoliquiritigenin, from Dalbergia odorifera, up-regulates anti-inflammatory heme oxygenase-1 expression in RAW264.7 macrophages. Inflamm Res. 2009;58:257–262. doi: 10.1007/s00011-008-8183-6. [DOI] [PubMed] [Google Scholar]
- 5.Kape R, Parniske M, Brandt S, Werner D. Isoliquiritigenin, a strong nod gene- and glyceollin resistance-inducing flavonoid from soybean root exudate. Appl Environ Microbiol. 1992;58:1705–1710. doi: 10.1128/aem.58.5.1705-1710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin YW, Jung HA, Liu Y, Su BN, Castoro JA, Keller WJ, Pereira MA, Kinghorn AD. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra) J Agric Food Chem. 2007;55:4691–4697. doi: 10.1021/jf0703553. [DOI] [PubMed] [Google Scholar]
- 7.Ii T, Satomi Y, Katoh D, Shimada J, Baba M, Okuyama T, Nishino H, Kitamura N. Induction of cell cycle arrest and p21(CIP1/WAF1) expression in human lung cancer cells by isoliquiritigenin. Cancer Lett. 2004;207:27–35. doi: 10.1016/j.canlet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Kang SW, Choi JS, Choi YJ, Bae JY, Li J, Kim DS, Kim JL, Shin SY, Lee YJ, Kwun IS, Kang YH. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J Nutr Biochem. 2010;21:55–65. doi: 10.1016/j.jnutbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Kakegawa H, Matsumoto H, Satoh T. Inhibitory effects of some natural products on the activation of hyaluronidase and their anti-allergic actions. Chem Pharm Bull (Tokyo) 1992;40:1439–1442. doi: 10.1248/cpb.40.1439. [DOI] [PubMed] [Google Scholar]
- 10.Jang EY, Choe ES, Hwang M, Kim SC, Lee JR, Kim SG, Jeon JP, Buono RJ, Yang CH. Isoliquiritigenin suppresses cocaine-induced extracellular dopamine release in rat brain through GABA(B) receptor. Eur J Pharmacol. 2008;587:124–128. doi: 10.1016/j.ejphar.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Cho S, Kim S, Jin Z, Yang H, Han D, Baek NI, Jo J, Cho CW, Park JH, Shimizu M, Jin YH. Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. Biochem Biophys Res Commun. 2011;413:637–642. doi: 10.1016/j.bbrc.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol. 2007;581:1057–1081. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strecker GJ, Park WK, Dudek FE. Zinc and flunitrazepam modulation of GABA-mediated currents in rat suprachiasmatic neurons. J Neurophysiol. 1999;81:184–191. doi: 10.1152/jn.1999.81.1.184. [DOI] [PubMed] [Google Scholar]
- 14.Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 16.Davies M, Bateson AN, Dunn SM. Molecular biology of the GABA(A) receptor: functional domains implicated by mutational analysis. Front Biosci. 1996;1:d214–d233. doi: 10.2741/a127. [DOI] [PubMed] [Google Scholar]
- 17.Johnston GA. GABAA receptor pharmacology. Pharmacol Ther. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 18.Dunn SM, Bateson AN, Martin IL. Molecular neurobiology of the GABAA receptor. Int Rev Neurobiol. 1994;36:51–96. doi: 10.1016/s0074-7742(08)60303-7. [DOI] [PubMed] [Google Scholar]
- 19.Van Sickle BJ, Cox AS, Schak K, Greenfield LJ, Jr, Tietz EI. Chronic benzodiazepine administration alters hippocampal CA1 neuron excitability: NMDA receptor function and expression(1) Neuropharmacology. 2002;43:595–606. doi: 10.1016/s0028-3908(02)00152-1. [DOI] [PubMed] [Google Scholar]
- 20.Christian CA, Herbert AG, Holt RL, Peng K, Sherwood KD, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Endogenous positive allosteric modulation of GABA(A) receptors by diazepam binding inhibitor. Neuron. 2013;78:1063–1074. doi: 10.1016/j.neuron.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallager DW, Mallorga P, Thomas JW, Tallman JF. GABA-benzodiazepine interactions: physiological, pharmacological and developmental aspects. Fed Proc. 1980;39:3043–3049. [PubMed] [Google Scholar]
- 22.Moura D, Soares-da-Silva P. Drug activation of GABAergic transmission in the central nervous system: benzodiazepines and GABAergic agonists. Acta Med Port. 1985;6:57–64. [PubMed] [Google Scholar]
- 23.Fritschy JM, Brünig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 24.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 25.Wu SP, Lu KT, Chang WC, Gean PW. Involvement of mitogen-activated protein kinase in hippocampal long-term potentiation. J Biomed Sci. 1999;6:409–417. doi: 10.1007/BF02253672. [DOI] [PubMed] [Google Scholar]
- 26.English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 28.Möhler H. The rise of a new GABA pharmacology. Neuropharmacology. 2011;60:1042–1049. doi: 10.1016/j.neuropharm.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 30.Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]