Abstract
It has been suggested that the hippocampus and the prefrontal cortex (PFC) play key roles in representing contextual memory and utilizing contextual information for flexible response selection. During response selection, a correct response should be facilitated and an incorrect response should be inhibited flexibly in association with a cueing stimulus. However, it is poorly understood how the hippocampal and PFC networks behave during such flexible control of facilitation and inhibition of behavioral responses. To find neural correlates of context-cued flexible response selection, the current study employed an object-place paired-associate (OPPA) task in which object A is only rewarded in place 1 and object B is associated with reward in place 2 while recording single units simultaneously from the hippocampus and PFC. During the task, response inhibition in front of a contextually wrong object is required for successful performance and such inhibitory responses were observed before the rat learned the task. A significant proportion of neurons that fired differentially depending on the existence of inhibitory behavior in the PFC was observed during the pre-learning stage. By contrast, the proportion of such neurons in the hippocampus was significantly greater than chance during post-learning stage. The results suggest that the development of inhibitory behavior is a critical behavioral marker that foretells an upcoming acquisition of the task and the hippocampus and PFC are involved in learning contextual response selection by learning how to control the inhibition of behavior as learning progresses.
Keywords: hippocampus, prefrontal cortex, electrophysiology, object, context, memory, learning
INTRODUCTION
A behavioral response toward an object may or may not be considered appropriate depending on the context in which an event takes place. This is more likely so when the same or similar objects are encountered across different contextual settings. That is, contextual information constrains and guides our behaviors. Deficits in flexible contextual response selection lead to neuropsychiatric disorders such as ADHD (attention deficit hyperactivity disorder) and frontotemporal dementia [1, 2, 3]. Originally proposed by Hirsh [4], the contextual memory theory suggests that the hippocampus plays critical roles in deploying flexible and conditional responses according to a surrounding context. It is also suggested in the literature that the prefrontal cortex (PFC) plays also key roles in flexibly controlling behavioral responses, using contextual stimuli [5, 6, 7, 8].
We previously showed that the PFC and hippocampus are both necessary in choosing an object associated with a specific spatial context in the object-place paired-associate (OPPA) task [9, 10]. Specifically, when rats were required to choose one of two objects positioned in one of the arms of a radial maze, but choose the other object when the same objects were encountered in a different arm, lesioning the hippocampus or inactivating the PFC resulted in profound performance deficits [10]. By recording single units and local field potentials in the task, we also showed that the neurons in the hippocampus and PFC fired critically in association with the object-in-place task demand of the OPPA task and there was a significant synchronization of local field potentials at theta rhythm between the two regions before the rat made object choices [9]. We later found that the dentate gyrus within the hippocampus plays key roles in the task [11].
In the OPPA task, the rat typically started the training with a response bias toward an object on a particular side within a choice platform at the end of an arm. For example, the rat chose either object (within the pair of objects) on its left side in arm 3. Then, as learning progressed, the rat learned to inhibit this response bias when the wrong object for that arm was encountered on that side and to target its response toward the object on the other side (e.g., right side). We previously described this as transition from response bias to object-in-place strategy, which occurred critically on day 7 or so on average [9, 10]. This pattern of choice behavior persisted throughout the task even after the rat reached asymptotic performance. It seems that the inhibitory response of the rat shown before the animal reaches performance criterion is a critical behavioral marker that foretells the occurrence of learning (or strategy shift) in the OPPA task.
In the current study, we explored the possibility that such inhibitory behavior might have some physiological correlates in the hippocampus and PFC. Impulsive action or deficits in inhibitory response are indeed observed in animals when frontostriatal neural circuits are damaged [12], glutamatergic transmissions are blocked in PFC [13], or when PFC was inactivated by muscimol, a GABAA receptor agonist [14]. Hippocampal lesioned animals also typically show perseverative behavior that may stem from the lack of inhibition of improper behavior [15]. Therefore, we have investigated whether the neural correlates of inhibitory behavior are found in the OPPA task and whether the neural correlates, if exist, change across learning.
MATERIALS AND METHODS
Subjects
Three male Long-Evans rats (300~400 g) were used in the study. All animals were maintained on a 12 h/12 h light/dark cycle and all the behavioral testing and recordings were conducted during the light phase of the cycle. The rats were maintained at 85% of their free-feeding weights (with water provided ad libitum) to facilitate motivation for the behavioral task. All the protocols for animal care and surgery followed the guidelines of the National Institute of Health and the Institutional Animal Care and Use Committee.
Behavioral apparatus
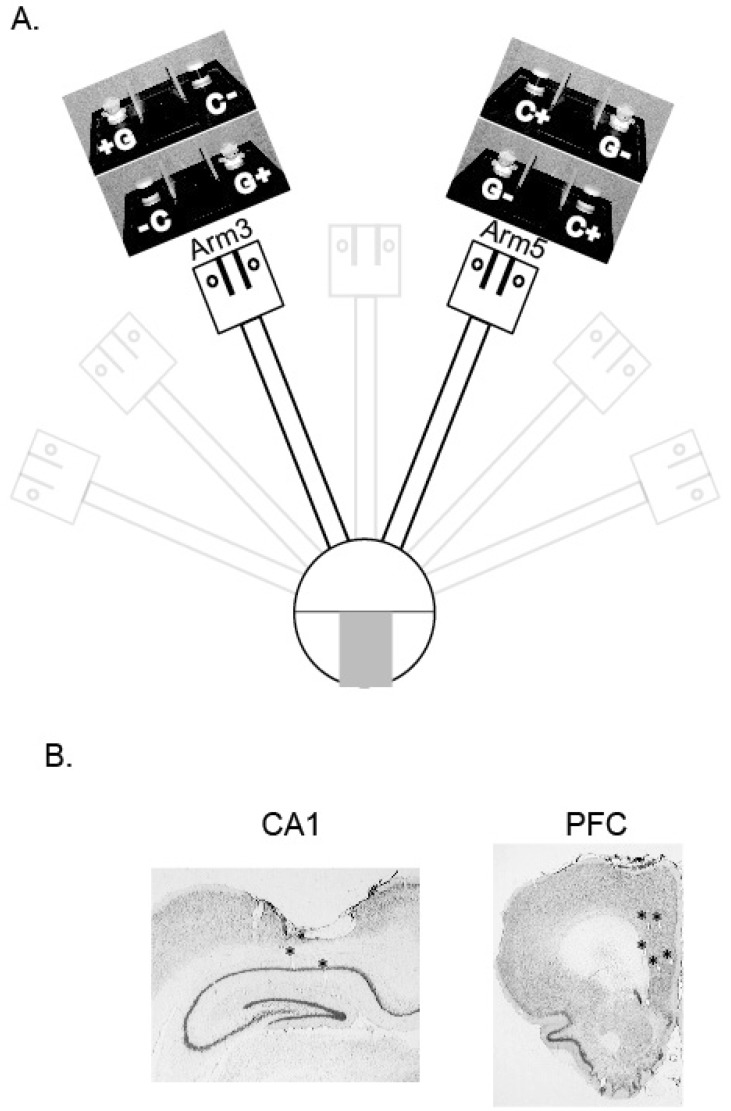
Detailed information of the apparatus can be found in our previous studies [9, 10, 16] and will only be described briefly here. A modified radial-arm maze was used throughout the experiment (Fig. 1A). The maze was placed in the center of a testing room and the walls and ceilings of the room were decorated with distinctive visual cues. A start box with an opaque guillotine door was located in a circular center stage, from which seven arms (each 8×80 cm and separated by 25.7° from each other) radiated outward. The distal end of each arm was connected to a rectangular platform (23×30 cm; choice platform as shown in Fig. 1A) in which objects were presented. A set of infrared emitter and detector was installed in the center of each food well for detecting the moment of displacement of an object. Food wells were separated from each other by transparent vertical dividers made of Plexiglas. The dividers were to encourage a more explicit and targeted response to a discrete object. A transparent guillotine door was available at the entrance of each arm to allow access to the arm. A digital CCD camera on the ceiling recorded behavioral sessions and white noise was provided through a loud speaker under the center platform of the maze.
Fig. 1.
Behavior paradigm and verification of recording sites. (A) The object-place paired-association (OPPA) task. Two objects (toy girl and dummy cylindrical object denoted by G and C, respectively) were presented in a choice platform at the end of either arm 3 or 5. Only one of the objects was rewarded in arm 3 (Girl, denoted by G+) and arm 5 (Cylinder, denoted by C+) irrespective of its local position in the choice platform. All possible configurations of objects associated with both arms are shown above each arm's choice platform. On each trial, only one arm was open in the maze and the rat was required to push one of the objects to obtain reward. (B) Histological verification of recording sites. Representative examples of the positions of electrodes in CA1 and mPFC within the same animal are shown (marked by asterisks).
Pre-surgical training
All rats were tamed and handled by an experimenter for 1~2 weeks. Once the animal showed a sign of being tamed (e.g., no defecation or urination in the presence of the experimenter), a shaping procedure began to train the rats to displace an object. For this purpose, the rat was first placed in the start box and, when the guillotine door was opened, it entered an opened arm (arm 3 or arm 5) that had already been chosen randomly by the experimenter. A sugar-coated cereal was placed in the center food well (which was not used in the main task) and a black junk object (not used for the OPPA task) was placed over the center food well. Once the rat learned to displace the object to obtain food reward, they were given surgery for being implanted with recording devices.
Surgical implantation of hyperdrive
A custom-made recording drive (hyperdrive) with eighteen tetrodes was used for the electrophysiological recording of single units. Tetrodes were made by twisting four nichrome wires (12 µm in diameter; Kanthal). The final impedance of each wire was adjusted to 150~300 kΩ (measured in gold solution at 1 kHz with an impedance tester; IMP-1, BAK electronics) before implantation. Sixteen tetrodes were used for recording and two other tetrodes were used as reference electrodes. The hyperdrive was composed of two stainless steel cannulae (each cannula carrying 8 recording tetrodes and 1 reference electrode), one targeting the hippocampal CA1 region (3.0 mm posterior to bregma and 1.7 mm lateral to midline) and the other targeting the medial PFC (i.e., prelimbic and infralimbic PFC, mPFC henceforth; 3.0 mm anterior to bregma and 1.0 mm lateral to midline) for simultaneously recording different brain regions (Fig. 1B). For surgery, the animal was initially anesthetized with the injection of ketamine (55 mg/kg) and xylazine (6 mg/kg) before being placed in a stereotaxic frame and anesthesia was maintained throughout surgery by isoflurane (1~2% isoflurane with 100% O2). One week was given for recovery afterwards.
Recording setup
After recovery from surgery, tetrodes were lowered individually to the target regions over several days while the rat slept in a custom-built recording booth located outside the experimental room. Spiking signals from each tetrode was amplified (1000~10000 times) and digitized (sampled at 32 kHz and filtered at 300~6000 Hz) using a Digital Lynx data acquisition system (Neuralynx). In the behavioral recording room, neural signals were transferred to the data acquisition system through a slip-ring commutator (Neuralynx). For tracking the position of the animal, an array of red and green LEDs was attached to a preamplifier connected to the hyperdrive. The LED signal was captured by a digital ceiling camera and was fed to the acquisition system simultaneously via a frame grabber (30Hz sampling rate). Spiking data from single units and position information were time-stamped and stored by the data acquisition machine for offline analyses. The entire maze area was mapped to a 640×480 pixel space with a single pixel representing 0.31 cm2.
Acquisition of the OPPA task
Once the majority of tetrodes were lowered to the target regions, the acquisition of an object-place paired-associate task (OPPA task) began. The rat was placed in the start box with the guillotine door closed while the experimenter opened either arm 3 or arm 5 and placed the two objects (a toy girl and a cylinder) in the choice platform (Fig. 1A). The objects were only available in the arm to be visited in a given trial but not in the closed arm. The configuration of the object positions in the choice platform and the arm information changed pseudorandomly in a counterbalanced manner throughout the session. In the OPPA task, a particular object was always rewarded in association with a certain arm and whether the object occupied the left or right food well (i.e., object's position) in the choice platform did not matter. A trial started as the experimenter opened the guillotine door of the start box. When the rat made a correct choice, it was allowed to retrieve the cereal reward and the experimenter gently guided the animal to the start box so that the food reward was consumed in the start box. In contrast, if the rat pushed a wrong object, a further attempt to displace the other object was blocked by the experimenter using a small plastic panel and the rat was gently guided back to the start box without being rewarded. A single trial ended when the animal returned to the start box and the guillotine door was closed. A typical intertrial interval was approximately 20 sec. Sixty-four trials were given typically in a behavioral session in a day.
Histological verification of electrode positions
Tetrode locations were verified after the completion of experiments. Positions of individual tetrodes were marked by electrolytic lesions (10 µA current for 10 sec). The rat was then sacrificed and the brain was perfused transcardially with physiological saline followed by 10% formalin. The frozen brain was sectioned at 30 µm thickness using a sliding microtome. Cut sections were stained with thionin and photomicrographs were taken under a digital microscope. The series of photomicrographs of tissues were used along with the physiological recording profile in order to reconstruct tetrode tracks and recording sites in the hippocampus and mPFC.
Unit isolation and criteria
Single units were isolated offline using a Windows-based custom software as previously described elsewhere [16]. Single units were isolated by comparing the signals recorded from four wires of a tetrode and multiple parameters such as peak (the main parameter), width, height, and energy associated with spike waveforms were used during the isolation process. Single units recorded from the tetrodes whose tips were located in the mPFC and CA1 of the hippocampus (Fig. 1B) were only included. Inter-spike interval histograms were also examined for ensuring single unit activity. Only neurons showing complex-spike bursts (average spike width=260.6 µs) were used for hippocampal analysis. Fast spiking and regular spiking neurons were not distinguished from each other in mPFC in this study and the neurons recorded from the prelimbic and infralimbic cortices (average spike width=295.7 µs) were all considered as mPFC units. For each behavioral recording session, units showing poor stability between the pre- and post-sleep recording sessions were not included in the analysis. The isolated units were used in final analyses only if the number of spikes exceeded 50 in the critical region of the maze (i.e., the upper 1/3 of the arm and the choice platform) for the task during outbound journeys within a recording session. An outbound journey started when the rat entered the arm and ended when the rat displaced one of the objects. The inbound journey after the object choice was made was excluded from the analysis because of frequent interventions by the experimenter for guiding the rat to the start box especially after wrong choices were made. The data used in the current study were used in our prior studies for reporting related, but different phenomena [9, 16].
RESULTS
Parsing behavioral response to an object
During the earlier phase of learning in the OPPA task, the rat tended to turn to one side (e.g., toward left food well) in a given arm as it entered the choice platform. We called this "response bias" in prior studies [9, 10, 16]. After a few days as the rat almost reached the moment of acquisition of the task, however, it started to show an inhibitory behavior in front of the object encountered at the end of the movement trajectory by giving a brief pause or hesitation. The rat sometimes pushed the first encountered object anyway (categorized as "direct push response" or DPR in this study; Fig. 2A left), or the animal inhibited the initial pushing response and turned to the other object and pushed it other times (categorized as "inhibition-and-push response" or IPR; Fig. 2A right). In order to categorize behavioral responses into DPR and IPR, position traces were used as follows. First, an average trajectory from entering the choice platform to displacing an object (based on all the trials in which the object was approached as the first response target) was obtained and the position points that fell outside the confidence bounds (i.e., mean±2 standard deviations) were filtered out. This procedure removed messy and jagged variations in the position data mostly attributable to the animal's irregular head movements especially during earlier days of learning. Afterwards, the DPR-IPR categorization was conducted by the following algorithm implemented in custom written software (Matlab): If rat first approached an object (detected by a position point entering a circular zone with 25-pixel radius from the center of the object position) but moved to the other object, such movement trajectory was categorized as IPR. In contrast, if the position trace ended within the circular zone associated with the first object, it was categorized as DPR.
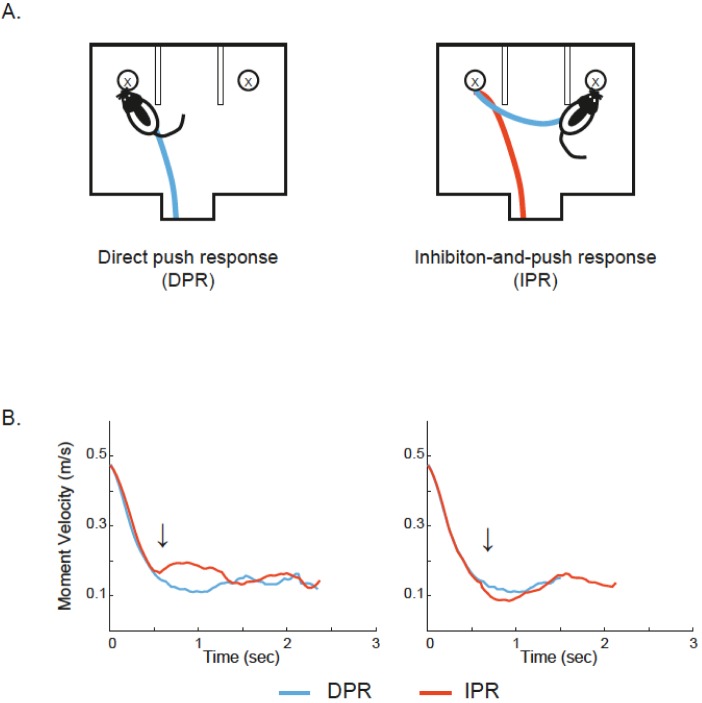
Fig. 2.
Examples of two response types and their speed profiles. (A) Schematic illustration of direct push response (DPR) and inhibition-and-push response (IPR). The rat pushed first object encountered, which was categorized as DPR (left, shown in blue trajectory). In contrast, the rat inhibited pushing it to displace the object on the other side instead, which was categorized as IPR (right, shown in red and blue trajectory). (B) Speed profiles. Abrupt increase in average moment velocity of IPR (marked by red) was observed due to the behavior of approaching the object on the other side (left, indicated by the arrow). The moment velocity of IPR remained relatively low compared to the moment velocity of DPR sometimes because of the hesitation or pause upon encountering the first encountered object (right, indicated by the arrow). Note that the time points (arrows) showing differences in the moment velocities between IPR and DPR were used for dividing IPR trajectories into two categories (the inhibit segment of IPR and the push response of IPR).
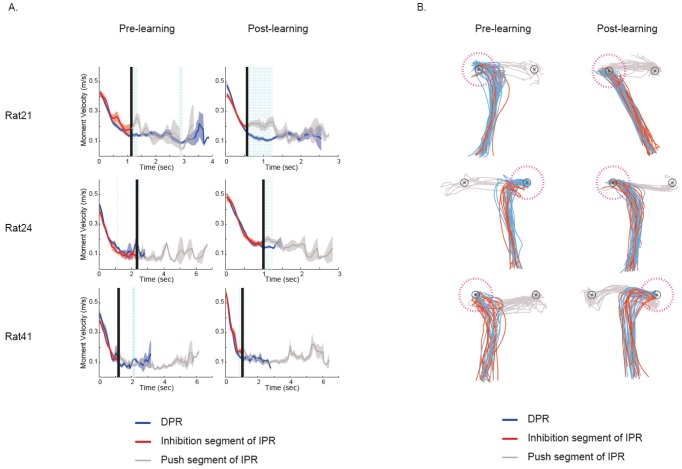
To conduct more detailed neural analysis, IPR trajectories were further segmented into two categories: (a) the trajectory associated with inhibitory responses to the first encountered object (response inhibition segment of IPR; red line in Fig. 2A right) and (b) the one associated with the push response that came after the inhibition for the first object (push segment of IPR; blue line in Fig. 2A right) on the basis of speed profiles (Fig. 2B). Specifically, once the animal entered the choice platform, the moment velocity was calculated every 33 ms until the object was displaced. The moment velocity of IPR was measured until the rat left the first object (detected by measuring the exit time for a position point from a circular zone with 25-pixel radius) to confine end points of trajectories of DPR and IPR in the same spatial zone. On IPR, abrupt changes of the moment velocity were typically observed in front of the first object due to the acceleration of the speed associated with the rat's movement for approaching the other object (Fig. 2B, left). The difference between the DPR and IPR might occur in the opposite direction. That is, when the rat was still learning to inhibit the first response to an object, the animal tended to pause or slow down in front of the object, which caused the moment velocity associated with the IPR remained at a lower level compared to DPR's (Fig. 2B, right). The time points showing differences in the moment velocities between DPR and IPR were considered as the parsing points for dividing behavioral responses into the inhibition segment and the pushing segment within IPR. Significant differences in the moment velocities between DPR and IPR were detected by independent samples t-test with a moving window paradigm. A 5-bin size window (165 ms) was shifted by one bin (33 ms). If three consecutive bins containing the moment velocities showed significant differences between DPR and IPR, the parsing points were chosen to divide IPR trajectories into the inhibit segment and push segment (Fig. 3A). The DPR trajectories were also parsed by the parsing point, so that the push of DPR excludes the trajectories that might have different speed profiles from the inhibit response of IPR (Fig. 3B). Therefore, the push response of DPR and the inhibitory response of IPR were not different from each other for both spatial and temporal domains (Fig. 3).
Fig. 3.
Parsing IPR into inhibition and push segments based on trajectories. (A) Representative examples of movement speed profiles across pre-learning and post-learning sessions. Changes in average moment velocity (until object push) associated with DPRs (blue, Mean ± S.E.M.) and IPRs (red) across time within a recording session are shown. The moment of entering the choice platform is aligned at time zero on the x-axis. The dotted vertical lines in cyan indicate the time points at which significant differences in the moment velocities were observed between DPRs and IPRs (p < 0.05; independent samples t-test with a moving window paradigm). The black vertical line indicates the time point that divides the IPR trajectories into two segments: (a) trajectories associated with inhibitory responses to the first encountered object and (b) the ones associated with push responses to the object on the opposite side. (B) Representative examples of the result of parsing IPR shown in A. The push response of DPR and inhibit response of IPR were marked in blue and red lines, respectively. The behavior traces colored in gray indicate the push segment of IPR resulted from parsing IPR. The magenta dotted circle indicates the first encountered object (radius=20 pixels). Note that the end points of both the push response of DPR and the inhibit response of IPR were restricted within magenta dotted circle.
Response bias and the development of IPR across learning
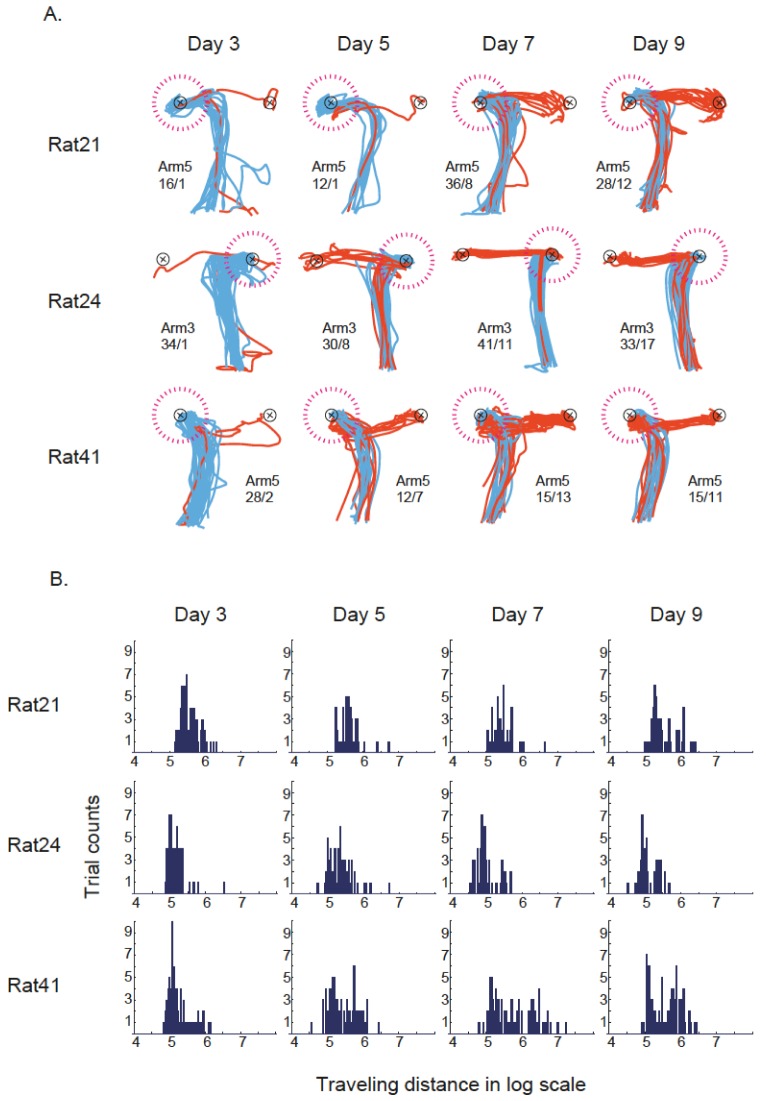
When the rat entered the choice platform, it tended to approach one side in a given arm. This response bias did not disappear throughout learning, but the rat started to show an inhibitory behavior immediately in front of the wrong object after a few days of acquisition (Fig. 4A). The development of both IPR and more stereotyped movement trajectories were observed as rats learned the task across learning sessions. That is, in addition to the increase of IPR, jagged movement patterns disappeared as the rat became more efficient in solving the task. The development of smooth movement pattern was quantified by using the distribution of traveling distance as shown in Figure 4B. The traveling distance of IPR was longer than DPR's, because the IPR contained additional trajectory toward the object located on the opposite side of the firstly headed object. Therefore, a bimodal distribution of traveling distance indicated an increase of IPR (Fig. 4B), and there was a development of bimodal distributions toward the later learning session. A two-sample Kolmogorov-Smirnov test confirmed that the distributions of traveling distance were significantly different from each other across days in each rat except for day7 and day9 of rat 21 (all p's<0.05).
Fig. 4.
Analysis of movement patterns near object-choice moments across learning stages. (A) The movement pattern for each rat across learning sessions. For a day's recording session, the rat's trajectories associated with object choices in either arm 3 or 5 were overlaid for illustration purposes. For each day's illustration, there are two black circles (each with x mark in the center) and they represent the locations of the two objects (corresponding to the food well locations) in the choice platform. Between the two black circles, the one associated with a dotted circle in magenta color indicates the object that was approached initially as the rat entered the choice platform (on the basis of trajectory analysis). The blue trajectories (direct push response, DPR) indicate that the rat actually pushed the object at the end of those movements (thus the first object encountered was pushed). The red trajectories (inhibition-and-push response, IPR) indicate that the rat did not push the first object encountered (thus inhibition of push behavior), but pushed the object on the opposite side instead. The numbers beneath the arm information indicate the ratio between the incidents of DPR and IPR. Note the increase in the proportion of IPR (with the decrease in DPRs) and the development of more stereotyped movement trajectories as the rats learned the task across days. (B) Histograms showing the distribution of traveling distances measured (in log scale) for the trajectories shown in A in the choice platform. Note the development of bimodal distributions toward the later learning session, indicating the increase in IPR.
Acquisition of the OPPA task and the development of IPR across learning
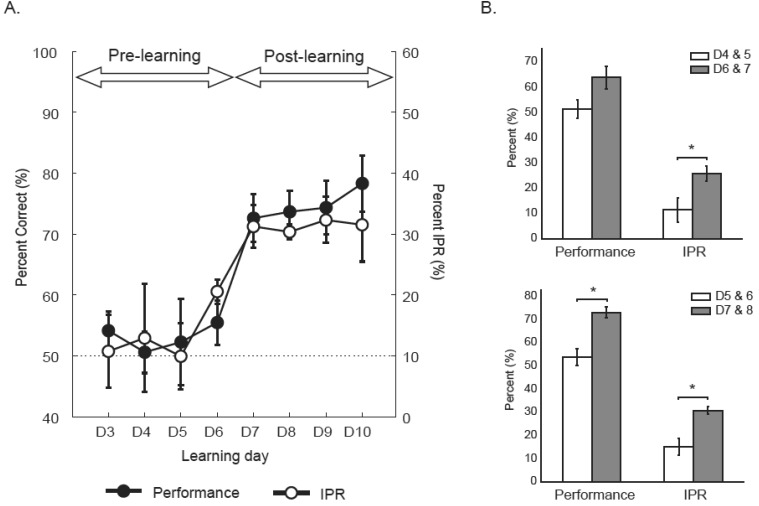
As reported previously [9, 10, 11, 15, 16], rats typically showed almost 50% correct responses (i.e., chance level) for several days (pre-learning stage), but suddenly exhibited a sharp transition to a learned stage (>70% correct; post-learning stage) on the 7th day of acquisition on average (D7 in Fig. 5A). The performance levels were significantly different between the pre- and post-learning stages (t(22)=7.82, p<0.0001; independent samples t-test). Importantly, the proportion of IPR on the first encountered object in the choice platform jumped (from approximately 10%) abruptly between day 5 and 6 even before the performance increase was observed (Fig. 5B). In order to detect the increase in the proportion of IPR across learning sessions, two consecutive learning sessions were grouped into one block and an independent samples t-test was conducted. When the boundary was set between day 5 and day 6, the proportion of IPR was significantly different between the block of D4-5 and the block of D6-7 (t(10)=2.59, p<0.05), but there was no significant difference in performance between the same blocks (t(10)=2.20, p>0.05) (Fig. 5B). By contrast, if the boundary for the day block was set between D6 and D7, there were significant differences between the block of D5-6 and the block of D7-8 for both performance level and the proportion of IPR (performance, t(10)=4.46, p<0.01; IPR, t(10)=3.99, p<0.01) (Fig. 5B). That is, the block design analysis showed that the proportion of IPR increased before the performance jumped.
Fig. 5.
Simultaneous developments of IPR and the acquisition of the OPPA task. (A) The dotted line indicates the chance level performance. There was an abrupt increase in performance between day 6 and day 7 and the learning period was divided into pre-learning and post-learning using the performance boundary. Note that the proportion of IPR (open circles) started to jump (from approximately 10%) abruptly from day 6 just before the performance jumped likewise from day 7. (B) Two consecutive learning sessions were grouped into one block and an independent samples t-test was conducted. Note that IPR significantly increased between day 5 and day 6, but performance did not. All graphs show Mean±S.E.M.
Proportions of neurons selective to different task-related factors
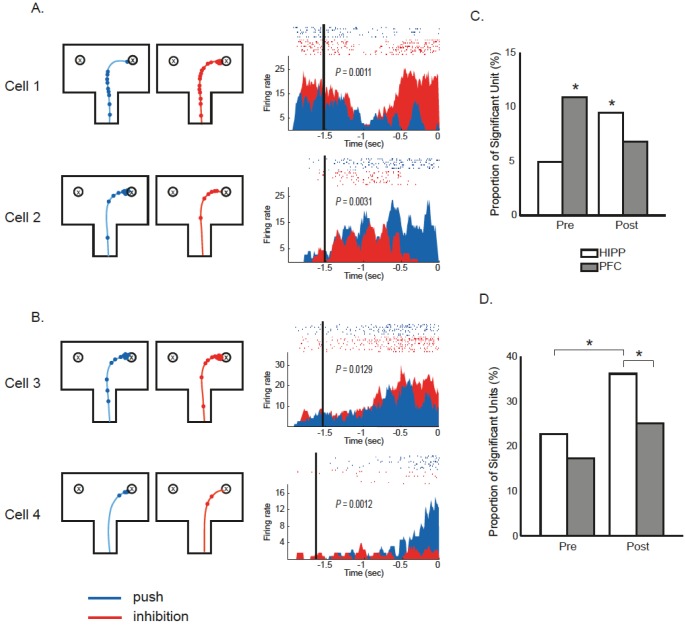
We then compared the proportion of neurons encoding key components of the OPPA task (such as push-inhibit response, object, and correctness) using an ANOVA. The push-inhibit response-selective unit was defined as the single unit that showed a significant firing-rate modulation in association with the push-inhibit response type on the first encountered object (i.e., push response of DPR and inhibit segment of IPR; Fig. 2A). Briefly, on each trial, the spikes that occurred in the critical region of the maze (i.e., the upper 1/3 of the arm and the choice platform) before the response execution (either push or inhibition) were counted and the number of spikes was divided by the elapsed time to calculate the firing rate. Afterwards, an ANOVA was performed to determine the difference in firing rate associated with the response type (i.e., push response vs. inhibitory response). The single unit that showed a firing rate modulation depending on the response type was referred to as response-selective units (alpha level=0.05; Fig. 6A and 6B for CA1 and mPFC, respectively). The proportion of response-selective units in the hippocampus was 4.9% (n=11/224) during the pre-learning stage and it increased to 9.5% (n=24/254) during the post-learning stage (Fig. 6C). The proportion of response-selective units in mPFC was 10.9% (n=18/165) in the pre-learning stage and 6.8% (n=18/265) in the post-stage (Fig. 6C). Pre-learning and post-learning stages included session in D3 to D6, and D7 to D10, respectively. By using a bootstrap analysis, we were able to calculate the level (~4%) at which the response-selective units might be observed by chance. When the proportions of response-selective units were compared against the chance level, we found signification proportion of response-selective neurons in mPFC during the pre-learning stage and the significant fraction of units in the hippocampus during the post-learning stage (mPFC, chi-square=4.23, df=1, p<0.05; hippocampus, chi-square=4.27, df=1, p<0.05). It indicates that the hippocampus and mPFC were involved in push-inhibition-related response selection at different learning stages.
Fig. 6.
Response selective units in CA1 and mPFC. A. Single trajectory and spikes associated with push response (blue line and circle, respectively) and inhibit response (red) are separately shown with maze boundary (left panel). Raster plots and PETHs are shown in right panel following the same color-coding scheme. Each PETH (bin size=10 ms) was generated with spikes that occurred in critical region of the maze (i.e., the upper 1/3 of the arm and the choice platform). The moment of response execution (either push response or inhibit response) is aligned at time zero on the x-axis. The moment in which rat entered the choice platform is marked by the vertical line with black color. Average firing rates associated with push response and inhibit response were compared using ANOVA and p values are shown in each PETH. Note that single units that showed selective firing-rate modulation in association with response types were defined as response-selective units. (A) Response selective units from CA1 of hippocampus. (B) Response selective units from mPFC. (C) Response selection (push or inhibition) was used as a factor for ANOVA. Pre-learning and post-learning stage included D3-6 and D7-10 sessions, respectively. A significant proportion of units in the mPFC (n=18/165 or 10.9%) was observed in the pre-learning stage (chi-square=4.23, df=1, p<0.05), and in the hippocampus (n=24/254 or 9.5%) during the post-learning stage (chi-square=4.27, df=1, p<0.05). (D) Percentage of selective units that showed firing-rate modulations for either push IPR or push DPR. The proportion of units which were selectively firing on either the push response of DPR (pushing the first targeted object) or the push response of IPR (pushing the other object after inhibition on first encountered object). All of the proportions were significantly different from chance.
We further examined whether the neurons in the hippocampus and mPFC differentially responded in between the push response in DPR and the push segment of IPR (i.e., after the initial inhibitory segment). The proportions of units that showed significant firing-rate modulations between the push response of DPR and the push segment of IPR were compared with each other (Fig. 6D). All the proportions for both regions during both the pre- and post-learning stages were significantly greater than what was expected by chance (chi-square test, all p's<0.005). Importantly, the proportion of significant units in the hippocampus was significantly different between the pre-learning and post-learning stages (pre-learning, n=37/163 or 22.7%; post-learning, n=82/227 or 36.1%; chi-square=7.44, df=1, p<0.01). Also, compared to the neurons in the mPFC, neurons in the hippocampus responded differently to the push responses associated with DPR and IPR after learning occurred (hippocampus, n=82/227 or 36.1%; mPFC, n=66/263 or 25.1%; chi-square=6.51, df=1, p<0.05). The results suggest that the same response (i.e., push) was encoded differently depending on the sequence of behavior (i.e., direct push versus push after inhibitory behavior) or behavioral episodes associated with the response in the hippocampus, but not in the mPFC.
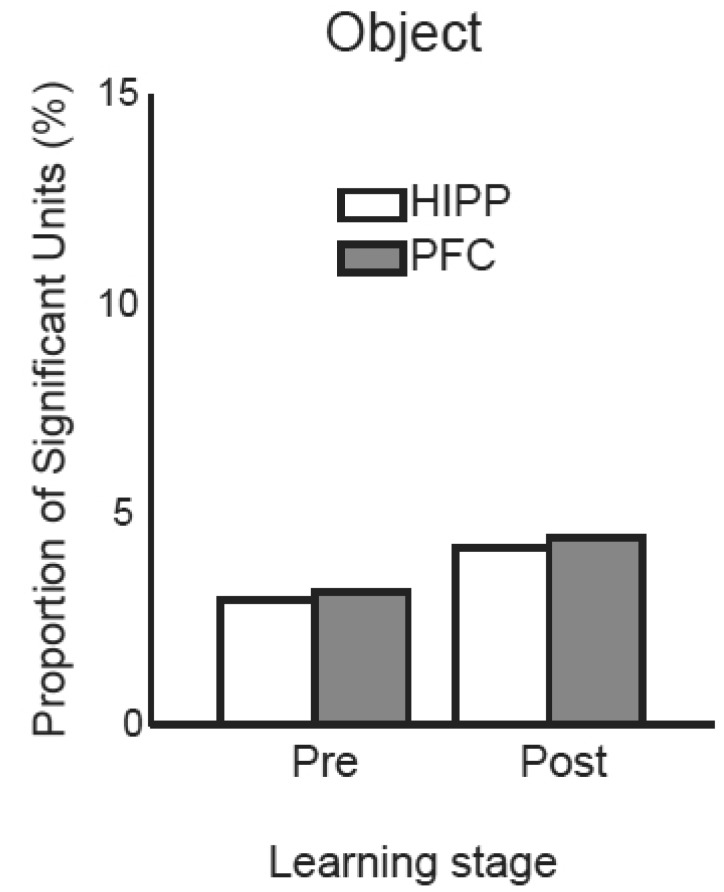
We next examined object-selective units in the hippocampus and mPFC. In the OPPA task, objects should be identified correctly for correct performance. It has been implicated that the hippocampus receives non-spatial information as well as spatial information. Therefore, object information might be represented in the hippocampus for the task. To test this hypothesis, an ANOVA was run for the object factor to find object-selective units (Fig. 7A). All proportions obtained for object-selective units in the hippocampus and mPFC were below 5% (mPFC in pre-learning, n=7/222 or 3.2%; mPFC in post-learning, n=12/270 or 4.4%; hippocampus in pre-learning, n=8/270 or 3%; hippocampus in post-learning, n=11/261 or 4.2%). When the proportions of object-selective units were compared against the chance level estimated by the bootstrap analysis, none of the proportion was turned out to be significant. It appears that the hippocampus and mPFC were not involved in representing object information by itself in the current task.
Fig. 7.
Object identity-selective neurons in the hippocampus and mPFC across the learning stages.
DISCUSSION
In the current study, we examined the neural correlates of flexible response selection in the OPPA task by simultaneously recording single units from the hippocampus and mPFC. All the rats showed, without exception, innate response bias throughout the OPPA task. That is, once entering the choice platform, the animal typically turned to an object on a certain side. It appears that, during learning, this dominance of response bias did not decrease, but the rat started to show inhibitory behavior immediately in front of a wrong object after a few days of acquisition. The development of IPR suggests that the response inhibition was a sure sign that predicted an upcoming performance surge in the task. We were able to find push-inhibition response-selective units in the hippocampus and mPFC at different learning stages. That is, the mPFC was more involved in response selection during the pre-learning stage and the hippocampus played important roles in response selection during the post-learning stage. There was no significant proportion of object-selective units in either region. It is still possible, however, that the response-selective units might be responding to other task-related variables such as the arm, object, and object location information.
As a functionally unified network, the mPFC and hippocampus play various cognitive functions in a goal-directed task [9, 10, 17, 18]. Most of all, it is implicated that the PFC and hippocampus are involved in response inhibition. Deficits in response inhibition may lead to impulsive or perseverative behavior [19]. Impulsive behavior refers to action that occurs without foresight and perseverative behavior refers to compulsive repetition of responding. The hippocampal perturbations induce a state of behavioral rigidity characterized by abnormally high response rates [20, 21] and the persistence of the previously rewarded response [22, 23]. That is, the malfunctioning hippocampal network might underlie flexible control of inhibition and facilitation of a given behavior depending on the context. It has been suggested that the PFC is also involved in controlling impulsivity in response. The perturbation studies on the PFC show that the animal's impulsive actions might stem from the PFC not exerting enough control over the inappropriate actions [12, 13, 14].
We were able to observe a significant proportion of push-inhibition response-selective units in the mPFC in the pre-learning stage but not in the post-learning stage. In the hippocampus, the significant proportion of these response-selective units was observed in the post-learning stage but not in the pre-learning stage. This might be explained as follows. When the rat was trained to push an object to obtain reward during the shaping stage, the rat learns the association between the object and push response. However, once the acquisition of the OPPA task started, this association (object-push association) did not guide successful performance any more, so that it should be inhibited in a conditional fashion. The significant proportion of push-inhibition response-selective units in the mPFC in the earlier phases of learning might be because the mPFC was initially recruited just to learn that sometimes the push response should be inhibited in this OPPA task (i.e., learning of inhibitory behavior). Early recruitment of PFC for executive control was reported in monkeys [24]. It is possible that discrete event memories including appropriate actions (push or inhibition) associated with different arms and objects might be represented in the hippocampus after several days of mPFC learning of inhibition behavior. The mPFC control over push-inhibition behavior during the learning process might be very critical for the hippocampal learning of different events and their associated values. That is, once certain responses are repeatedly associated with different values and outcomes and as these experiences are represented as discrete memories in the hippocampus, mPFC would be no longer involved in response selection as there is no need to learn new rules of new ways of controlling behaviors.
In our study, we found no significant proportion of neurons that selectively responded to object alone. This might be due to the fact that objects are contextually represented in the hippocampus with the background information and spatial information. Specifically, the hippocampus receives both spatial and non-spatial information. Spatial information arrives at the hippocampus via the postrhinal cortex and the medial entorhinal cortex. By contrast, non-spatial information is conveyed to the hippocampus through the perirhinal cortex and lateral entorhinal cortex [25]. Based on such anatomical connections, the hippocampus may combine object information with contextual information for representing contextual object memory [26].
The push responses of DPR and IPR are seemingly identical from purely motoric perspective because both involve pushing behavior directed toward an object. However, the push responses associated with DPR and IPR might be different from the cognitive perspective. For example, the push response of IPR might not be related to the retrieval of a discrete event memory representation. Instead, it could merely follow the first inhibitory behavior in IPR (because there was only one choice left once the rat decided to inhibit the first push response in IPR). Also, the push responses of DPR and IPR were different in terms of head direction, body turn and movement speed. It is known that the firing patterns of neurons in the hippocampus are modulated by those movement-related factors as well. Alternatively, it is possible that the same pushing behavior might be interpreted differently by the neural network depending on the behavioral history or event history [9, 27, 28].
ACKNOWLEDGEMENTS
The current study was supported by the WCU program (R32-10142), the Brain Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2013M3C7A1044017 and 2013R1A1A2062882), the BK21+ Program through the National Research Foundation of Korea, and NIH RO1 grant (MH079971
References
- 1.Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: Characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G, Albert M, Grossman M, Miller B, Dickson D, Trojanowski JQ Work Group on Frontotemporal Dementia and Pick's Disease. Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick's disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Ind Diff. 2006;40:305–315. [Google Scholar]
- 4.Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;444:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- 5.Haddon JE, Killcross S. Prefrontal cortex lesions disrupt the contextual control of response conflict. J Neurosci. 2006;26:2933–2940. doi: 10.1523/JNEUROSCI.3243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson BS. Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. J Neurosci. 2011;31:16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kesner RP, Ragozzino ME. The role of the prefrontal cortex in object-place learning: a test of the attribute specificity model. Behav Brain Res. 2003;146:159–165. doi: 10.1016/j.bbr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Lee I, Shin JY. Medial prefrontal cortex is selectively involved in response selection using visual context in the background. Learn Mem. 2012;19:247–250. doi: 10.1101/lm.025890.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Delcasso S, Lee I. Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J Neurosci. 2011;31:16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn Mem. 2008;15:357–367. doi: 10.1101/lm.902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee I, Solivan F. Dentate gyrus is necessary for disambiguating similar object-place representations. Learn Mem. 2010;17:252–258. doi: 10.1101/lm.1678210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neill D. Frontal-striatal control of behavioral inhibition in the rat. Brain Res. 1976;10:89–103. doi: 10.1016/0006-8993(76)90925-2. [DOI] [PubMed] [Google Scholar]
- 13.Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee I, Kim J. The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus. Learn Mem. 2010;17:381–393. doi: 10.1101/lm.1829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whishaw IQ, Tomie J. Perseveration on place reversals in spatial swimming in pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Jo YS, Lee I. Disconnection of the hippocampal-perirhinal cortical circuits severely disrupts object-place paired associative memory. J Neurosci. 2010;30:9850–9858. doi: 10.1523/JNEUROSCI.1580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins T. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Clark CV, Isaacson RL. Effect of bilateral hippocampal ablation on DRL performance. J Comp Physiol Psychol. 1965;59:137–140. doi: 10.1037/h0021599. [DOI] [PubMed] [Google Scholar]
- 21.Devenport LD. Superstitious bar pressing in hippocampal and septal rats. Science. 1979;205:721–723. doi: 10.1126/science.462183. [DOI] [PubMed] [Google Scholar]
- 22.Kimble DP, Kimble RJ. Hippocampectomy and response perseveration in the rat. J Comp Physiol Psychol. 1965;60:474–476. doi: 10.1037/h0022550. [DOI] [PubMed] [Google Scholar]
- 23.Stevens R, Cowey A. Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Res. 1973;52:203–224. doi: 10.1016/0006-8993(73)90659-8. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin SJ, Blackman RK, Sakellaridi S, Chafee MV. Executive control over cognition: stronger and earlier rule-based modulation of spatial category signals in prefrontal cortex relative to parietal cortex. J Neurosci. 2012;32:3499–3515. doi: 10.1523/JNEUROSCI.3585-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 26.Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- 28.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]