Abstract
IgA nephropathy is characterized by mesangial cell proliferation and extracellular matrix expansion associated with immune deposits consisting of galactose-deficient polymeric IgA1 and C3. We have previously shown that IgA-binding regions of streptococcal M proteins co-localize with IgA in mesangial immune deposits in patients with IgA nephropathy. In the current study, the IgA-binding M4 protein from group A streptococcus was found to bind to galactose-deficient polymeric IgA1 with higher affinity than to other forms of IgA1, as shown by surface plasmon resonance and solid-phase immunoassay. The M4 protein was demonstrated to bind to mesangial cells not via the IgA-binding region but rather via the C-terminal region, as demonstrated by flow cytometry. IgA1 enhanced binding of M4 to mesangial cells, but not vice versa. Co-stimulation of human mesangial cells with M4 and galactose-deficient polymeric IgA1 resulted in a significant increase in IL-6 secretion compared to each stimulant alone. Galactose-deficient polymeric IgA1 alone, but not M4, induced C3 secretion from the cells and co-stimulation enhanced this effect. In addition, co-stimulation enhanced mesangial cell proliferation compared to each stimulant alone. These results indicate that IgA-binding M4 protein binds preferentially to galactose-deficient polymeric IgA1 and that these proteins together induce excessive pro-inflammatory responses and proliferation of human mesangial cells. Thus, tissue deposition of streptococcal IgA-binding M proteins may contribute to the pathogenesis of IgA nephropathy.
Introduction
IgA nephropathy (IgAN), the most common form of primary glomerulonephritis worldwide, is characterized by a proliferation of mesangial cells and matrix and deposits containing predominantly IgA1 and C3 (1). The pathogenesis of IgAN has so far not been completely elucidated but much research has focused on the importance of galactose-deficient IgA1 (2). IgA1 differs from IgA2 mainly by the presence of the hinge region, an 18 amino-acid sequence between the Cα1 and Cα2 part of the heavy chains of IgA1, with three to six attached O-glycans (3). The O-glycans consist of N-acetylgalactosamine (GalNAc), with the possible addition of galactose and up to two sialic acid residues. IgA1 is considered galactose-deficient when some of the O-glycans lack terminal galactose (4).
In sera from patients with IgAN, a higher proportion of polymeric IgA1 was found to be galactose-deficient when compared to healthy controls, and galactose-deficient polymeric IgA1 constitutes the major part of the IgA found in renal tissue deposits in IgAN (reviewed in (5)). Galactose-deficient polymeric IgA1 was found to have a higher affinity for human mesangial cells than normoglycosylated polymeric IgA1 (6) and induced an inflammatory response as measured by elevated secretion of interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, and other inflammatory mediators, as well as proliferation of these cells in vitro (7, 8). This cell activation may be further enhanced by antibodies to galactose-deficient IgA1 that form immune complexes, which activate mesangial cells (reviewed in (3, 5)). However, as galactose-deficient IgA1 is also found in healthy relatives of patients with IgAN and unrelated controls (9-11) and deposits of IgA are also found in kidneys examined at autopsies of individuals without known kidney disease (12), other factors presumably contribute to the pathogenesis of IgAN.
The onset and exacerbations of IgAN are commonly preceded by infections, often affecting the upper respiratory tract, and various infectious agents have been investigated as possible triggers of IgAN (13-19). In particular, interest has focused on group A streptococcus (GAS; Streptococcus pyogenes), a common cause of respiratory-tract infections, which expresses surface-located M proteins. Many GAS strains express an M protein that contains a region that binds to the Fc-region of human IgA (20, 21). We have previously shown that children with IgAN are more likely than controls to have been infected with GAS expressing an IgA-binding M protein (22). Furthermore, we detected M protein-derived IgA-binding regions co-localizing with IgA in kidney samples of children with IgAN (16). These findings suggest a possible role of GAS strains expressing IgA-binding M proteins in the etiology and pathogenesis of IgAN.
Several observations indicate that the pro-inflammatory cytokine IL-6 is of importance for the development of IgAN. The synthesis and secretion of IL-6 is up-regulated in renal tissues of patients with IgAN during active disease (23). The amount of IL-6 detectable in urine correlated with disease activity during IgAN (24). In vitro experiments have shown that IL-6 induces mesangial cell proliferation and matrix expansion, which are typical features of IgAN kidney pathology (25). In addition, IL-6 synthesis by human mesangial cells is up-regulated by exposure to IgA1-containing immune complexes (6, 26).
Complement activation in the kidney has been proposed to promote renal damage during IgA nephropathy (27). Deposited C3 is found in the mesangium in IgAN patients (1) and may result from activation of the alternative (28) or lectin pathway of complement (29). Deposition of C3 on human mesangial cells may promote tissue inflammation by release of C3a and C5a, which have chemotactic and anaphylactic properties, as well as cell injury by assembly of the terminal complement pathway. Human mesangial cells have been shown to synthesize and secrete C3 in response to pro-inflammatory cytokines and immune complexes (30, 31) and mesangial C3 synthesis has been shown to be up-regulated in situ in patients with IgAN (32) .
Our previous studies demonstrated mesangial deposits of IgA-binding regions of GAS M proteins in the kidneys of IgAN patients. In the present study, we tested the hypothesis that IgA-binding M proteins contribute to IL-6 and C3 release from human mesangial cells as inflammatory mechanisms contributing to IgA nephropathy. We investigated binding of the IgA-binding M4 protein to galactosylated and galactose-deficient IgA1 as well as to mesangial cells, and the capacity of M4 protein to induce IL-6 and C3 secretion from mesangial cells, and their proliferation, alone and in combination with galactose-deficient IgA1.
Materials and Methods
Streptococcal M proteins
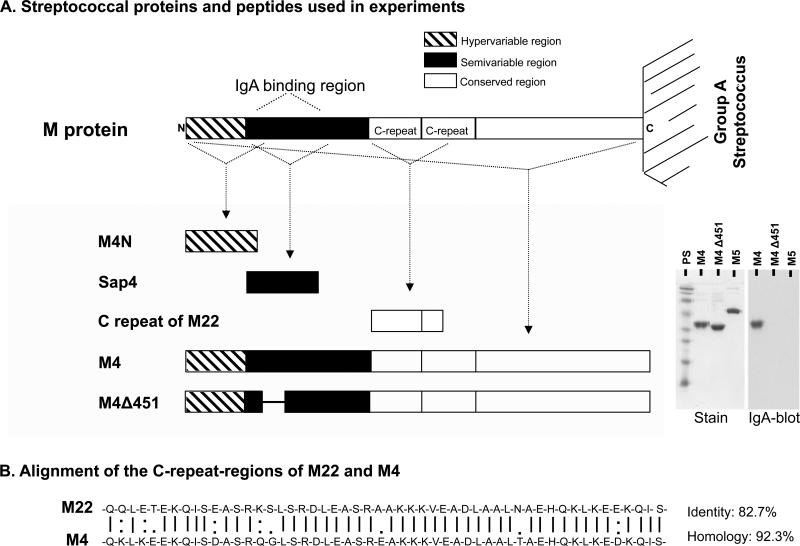
M proteins and streptococcal peptides used in this study are described in Table I and Figure 1A. M proteins from group A streptococcus serotype 4 (M4, also known as Arp4) and from serotype 5 (M5) have been previously described and characterized (20, 33, 34). The M4 protein binds to human IgA-Fc due to the presence of an IgA-binding region in its semi-variable region. The M4Δ451 mutant protein lacks this property due to a deletion of 18 amino acids within the IgA-binding domain (33) and the M5 protein does not contain an IgA-binding region and, thus, does not bind human IgA (21). Recombinant M4 and M5 proteins were produced in Escherichia coli and purified as described (34). LPS contamination was ruled out by the Limulus amoebocyte lysate assay (Coatex AB, Gothenburg, Sweden). Synthetic peptides corresponding to various regions of the M protein were also used. These peptides have been fully characterized and included the N-terminal hypervariable region of the M4 protein (M4N), the IgA-binding region of M4 (Sap4) and part of the C-repeat domain in the conserved region of the M22 protein (16, 34-36). The latter peptide has a 92.3% amino acid homology with the C-repeat domain of the conserved region of M4 (Figure 1B). Rabbit anti-sera to M4 protein, Sap4 and the C-repeat domain of the M22 protein were raised as described (34) and the IgG fraction was further purified using a Protein A-Sepharose column (Amersham Bioscience, Uppsala, Sweden).
Table I.
IgA1 and M proteins used in this study
| IgA | Abbreviation | Source | Use | Ref |
|---|---|---|---|---|
| Galactose-deficient polymeric IgA1-Ale | gdpIgA1-Ale | Purified from plasma of a patient with multiple myeloma | 1, 2 | (37-39) |
| Galactose-deficient polymeric IgA1-IgAN | gdpIgA1-IgAN | Purified from sera of two patients with IgAN* | 1, 3 | |
| Galactosylated polymeric IgA1 | gpIgA1 | Purified from pooled normal sera | 1, 2 | |
| Galactose-deficient monomeric IgA1 | gdmIgA1 | 1, 2 | ||
| Galactosylated monomeric IgA1 | gmIgA1 | 1, 2 | ||
| Galactose-deficient polymeric IgA1-ps | gdpIgA1-ps | 3,4 | ||
| M proteins | Characterization | |||
| M4 protein | IgA-binding M protein from GAS M-type 4. Binds to the Fc region of IgA. | Recombinantly produced in Escherichia coli | 1-5 | (20, 33, 34) |
| M4A451 mutant protein | Non-IgA-binding analogue to M4, deletion of 18 amino acids within the IgA-binding domain | 1, 3-5 | ||
| M5 protein | Non-IgA-binding M protein from GAS M-type 5 | 5 | ||
| M4-N | N-terminal 45 amino acids of M4 (hypervariable region) | Synthetic peptides | 4 | (16, 34, 35) |
| Sap4 | Amino acids 35-83 of M4 (IgA-binding region) | |||
| C-repeat of M22 protein | 92.3% amino acid homology with the C-repeat domain of M4 | (36) |
1: surface plasmon resonance; 2: M4/IgA-binding ELISA, 3: mesangial cell stimulation, 4: M4 and IgA binding to mesangial cells analyzed by flow cytometry; 5: Stain and IgA-blot in Figure 1.
For surface plasmon resonance IgA from one patient was used, for mesangial stimulation samples from two patients were pooled.
PS: pooled sera from healthy donors.
Figure 1. Characterization of streptococcal proteins and peptides.
A) The structure of streptococcal M proteins and other peptides and proteins used in this study. The lower-right panel shows on the left (Stain) protein bands of M4, M4Δ451 and the IgA non-binding M protein serotype 5 (M5) as a negative control (SDS-PAGE under non-reducing conditions, Coomassie stain as described (67)), and on the right (IgA-blot) IgA-binding (M4) or lack thereof (M4Δ451, M5) (SDS-PAGE under non-reducing conditions probed with 125I-labeled monoclonal IgA and detected by autoradiography as described (67)). PS: protein standard. B) Alignment of the C-repeat domain of M protein of group A streptococcus serotype 22 (M22) and serotype 4 (M4) depicting a high degree of homology.
Purification of galactosylated and galactose-deficient serum IgA1
IgA1 forms used in this study are described in Table I. Galactose-deficient polymeric IgA1 (Ale) was isolated from the plasma of a patient with multiple myeloma using salt precipitation, jacalin precipitation, IgG-depletion on an anti-IgG column and size-exclusion chromatography (37, 38). Heterogeneity of O-glycans of this IgA1 was fully characterized by high-resolution mass spectrometry (39, 40).
Venous blood was obtained from five healthy adults (median age 45 years) and from two boys (16 and 17 years old) with biopsy-proven IgAN. Samples were obtained in 4-mL vacutainer Hemogard SST tubes (Becton Dickinson, Plymouth, UK) and centrifuged, after blood-clotting, at 2000 g for 10 min. IgA1 was purified from the sera (pooled sera of the five healthy controls, termed IgA1-ps, or from the two patients with IgAN, termed IgA1-IgAN) by ammonium sulphate precipitation (Merck, Darmstadt, Germany) followed by affinity chromatography using Jacalin immobilized on agarose (Vector Labs, Burlingame, CA) (41). Separation of monomeric and polymeric forms of IgA1 was carried out by two methods, either by size-exclusion chromatography on a Sephacryl S300-HR-column (GE Healthcare, Uppsala Sweden) or by a centrifugal device with a spin-filter (Nanosep 300K Omega, Pall Norden AB, Lund, Sweden) followed by further purification by native gel electrophoresis under non-reducing conditions. After excision of gel bands corresponding to different molecular sizes, IgA fractions were eluted from the excised sections, kept one day at room temperature in PBS (Medicago AB, Uppsala, Sweden) and concentrated using ultrafiltration (10 kDa molecular mass cut-off ultrafilters; Millipore Corp., Billerica, MA). The latter separation was only carried out for galactose-deficient IgA1 used in cell stimulation experiments (described below).
The galactosylated and galactose-deficient glycoforms of IgA1 were separated using N-acetyl galactosamine (GalNAc)-specific lectin from Helix aspersa immobilized on agarose (HAA, Vector Labs, San Mateo, CA) (42). The two fractions of IgA1 (monomeric and polymeric) were separately processed overnight at 4°C. The unbound fraction (flow-through) contained galactosylated IgA1. Galactose-deficient glycoforms of IgA1 were eluted by addition of 0.05 M GalNAc (Vector Labs).
Purifications and size-differentiation were confirmed by silver-stained gels (Pierce Biotechnology, Rockford, IL) and immunoblotting under non-reducing conditions using rabbit anti-human IgA:horseradish peroxidase (HRP, Dako Cytomation, Glostrup, Denmark) detected by chemiluminescence (ECL-Plus, GE Healthcare, Little Chalfont, UK). The degree of galactosylation of the different purifications of IgA1 was confirmed using jacalin- and HAA-binding assays as previously described (37, 43). For further information see supplementary Figure 1. In polymeric purifications of IgA1 traces of monomeric IgA1 were detected. The presence of IgA-IgG immune complexes was ruled out by immunoblotting under non-reducing conditions using rabbit anti-human IgG:HRP (Dako Cytomation) detected by chemiluminescence.
Sera were used with written informed consent from the patients and their parents and from controls. The study was approved by the Ethics Committee of the Medical Faculty of Lund University.
Binding of the M4 proteins to IgA1 detected by surface plasmon resonance
The binding affinity of both M4 proteins to IgA1 was determined by surface plasmon resonance using BIAcore technology. The IgA-binding M4 and non-IgA-binding M4Δ451 were diluted in 10 mM sodium acetate (pH 4) (Merck, Darmstadt, Germany) and immobilized via amine coupling on separate CM5 sensor chip chambers (GE Healthcare, Uppsala, Sweden) at moderate response levels (1500 response units) as previously described (44). A flow chamber subjected to the immobilization conditions but without addition of protein was used as a control (blank) for each experiment. Five IgA1 fractions (galactose-deficient polymeric IgA1-Ale and IgA1-IgAN, galactose-deficient monomeric IgA1, galactosylated polymeric and monomeric IgA1, Table I) were diluted in five dilution steps (500 nM, 250 nM, 125 nM, 62 nM, 31 nM, based on the relative molar mass of monomeric and dimeric IgA, respectively) in running buffer (10 mM HEPES, 150 mM NaCl, 0.005% Surfactant P20, pH 7.5, BIAcore, Uppsala, Sweden) and injected separately over the surfaces. In between experiments the immobilized proteins were regenerated and washed as previously described (44). Experiments were performed on a BIAcore 2000 instrument (BIAcore). Binding curves were displayed and affinities (KD) calculated using BIAevaluation 4.1 software (BIAcore) as previously described (44).
Binding of the M4 protein to IgA1 detected by ELISA
Microtiter wells (Maxisorp, Nunc, Roskilde, Denmark) were coated with M4 at 5 μg/mL in 0.1 M NaHCO3 pH 9.6 (Merck, Darmstadt, Germany) overnight at room temperature. Wells were washed three times with phosphate buffered saline-Tween 0.05% (PBS-T, Medicago, Uppsala, Sweden) and nonspecific binding sites were blocked with 3% bovine serum albumin (BSA, MP Biomedicals, Irvine, CA) in PBS for 2 h at room temperature. Wells were washed and incubated with different fractions of IgA1 (galactose-deficient polymeric IgA1-Ale, galactosylated polymeric IgA1, galactose-deficient and galactosylated monomeric IgA1, Table I) at 20 μg/mL in 1% BSA-PBS for various times. The wells were then washed and rabbit anti-human IgA: HRP (Dako, Glostrup, Denmark) 1:2000 (v/v) in 1% BSA-PBS was added and incubated for 1 h at room temperature. After an additional wash binding was detected by incubation with O-phenylendiamine dihydrochloride tablets (OPD, Dako) for 15 min at room temperature. The reaction was terminated by addition of 0.5 M H2SO4 (Scharlau Chemie, Barcelona, Spain) and optical density measured at 490 nm.
Culture and stimulation of human mesangial cells
Primary human mesangial cells at passage 5-7 were grown to near confluence in CS-C complete serum-free medium (cells and media from Cell Systems Corp, Kirkland, WA) in 96-well culture plates (NUNC, Roskilde, Denmark) at 21% O2, 5% CO2 and 37°C. The cells were identified as mesangial cells by their typical stellate morphology and by immunofluorescence as previously described (45) showing positive staining for smooth muscle actin (monoclonal mouse anti-α SMA, Sigma, St. Louis, MO) and negative results for cytokeratin (monoclonal mouse anti-human cytokeratin, Dako) and for von Willebrand factor (polyclonal rabbit anti-human VWF, Dako) excluding epithelial and endothelial cells, respectively.
Mesangial cells were washed twice with Dulbecco's PBS (D-PBS, PAA Laboratory, Pasching, Austria). Cells were kept in resting condition for 36 h in CS-C serum-free maintenance medium (Cell Systems Corp) and then exposed to galactose-deficient polymeric IgA1 (IgA1-ps or IgA1-IgAN) or galactosylated polymeric IgA1 (both at 100 μg/mL), M4 or M4Δ451 (both at 10 μg/mL) in CS-C serum-free maintenance medium, as well as M4 and M4Δ451 pre-incubated with galactose-deficient polymeric IgA1-ps for 1 h at room temperature. Cells were incubated with each of the reagents for 4 h, 24 h and 48 h with the exception of IgA1-IgAN, which was incubated with the cells for 24 h. After the stimulations, supernatants were removed, Complete-Mini protease inhibitor (Roche Diagnostics, Mannheim, Germany) was added and supernatants were centrifuged at 200 × g for 5 min and stored at −80 °C until analyzed.
Viability of the mesangial cells was tested at the beginning of incubation, as well as after 4 h, 24 h, and 48 h using Trypan blue (Sigma). More than 95% of the cells were viable at all time points.
IL-6 in culture supernatants detected by ELISA
Supernatants from mesangial cell experiments, diluted in PBS, were analyzed for IL-6 using an ELISA kit (R&D systems, Minneapolis, MN) according to the manufacturer's instructions.
C3 in culture supernatants detected by ELISA
Supernatants from mesangial cell experiments were analyzed for C3 protein using an ELISA as previously described (46), with certain modifications. Briefly, microtiter wells (Maxisorp, Nunc) were coated with polyclonal rabbit anti-human C3c (1:2000 v/v, Dako) in 0.1 M NaHCO3, pH 9.6 (Merck) and blocked. Cell culture supernatants (diluted in PBS) or purified C3 as standard (Complement Technology, Tyler, TX) were added, incubated for 1 h at room temperature, and the bound C3 was detected with goat anti-human C3c (1:1000 v/v, Sigma). The secondary antibody was rabbit anti-goat immunoglobulin:horse radish-peroxidase (HRP, 1:1000 v/v, Dako).
Human mesangial cell proliferation assays
Primary human mesangial cells were grown to near confluence in 96-well culture plates (Nunc) using CS-C complete serum free medium and kept in resting condition as described above. Cells were left unstimulated or incubated with stimulants suspended in CS-C serum-free-maintenance medium containing 5-bromo-2′-deoxyuridine (BrdU)-labeling reagent (100 μM, Roche Diagnostics) for 24 h. Stimulants included galactose-deficient polymeric IgA1-IgAN (100 μg/mL), M4 or M4Δ451 (both at 10 μg/mL) as well as M4 and M4Δ451 pre-incubated for 1 h with galactose-deficient polymeric IgA1-IgAN. The positive control was murine platelet-derived growth factor (PDGF)-BB (50 ng/ml, PeproTech Inc, Rocky Hill, NJ). Proliferation was detected using a calorimetric BrdU cell proliferation ELISA-kit (Roche Diagnostics) according to manufacturer's instructions.
Binding of M4 protein and IgA to human mesangial cells detected by flow cytometry
Flow cytometry experiments were performed using the human mesangial cells at passage 6-10, detached from culture flasks using TrypLE (GIBCO, Grand Island, NY), washed and resuspended in CS-C complete serum-free medium, and a BD FACSCanto II cytometer with FACSDiva software (Becton Dickinson Immunocytometry Systems, San Jose, CA) as previously described (47). Preliminary experiments showed enhanced binding of M4 and galactose-deficient polymeric IgA1-ps to mesangial cells in the presence of TNF-α. Thus, mesangial cells were first stimulated with TNF-α (20 ng/mL, Sigma Aldrich, St Louis, MO) for 120 min at 37°C followed by M4 proteins or IgA1. Although TNF-α was used here it was not added in cell stimulation experiments, as it may, in itself, affect IL-6 secretion in these cells (48). M4 at 10 μg/mL was incubated with the cells for 60 min at 37°C. Binding of M4 was detected by rabbit-anti M4-IgG or rabbit-anti Sap4 (10 μg/mL). Galactose-deficient polymeric IgA1-ps (50 μg/mL) was incubated with the cells for 60 min at 37°C and detected with mouse anti-human IgA (1 μg/mL, Dako). As control antibodies the IgG-fractions of rabbit pre-immune sera or mouse IgG1 (Dako) were used. The secondary antibodies were swine anti-rabbit-FITC (1/250, Dako) or goat anti-mouse-FITC (1/1500, Dako). Results are presented as mean fluorescence intensity (MFI) with subtracted auto-fluorescence.
To investigate if the IgA-binding region of M4 was involved in binding to mesangial cells, the cells were incubated with M4Δ451, as described above for M4. The contribution of the N terminal or C-repeat domain (C terminal) of M protein to binding to mesangial cells was studied by preincubating the cells with M4-N or the C-repeat peptide of M22 (100 μg/mL), respectively, for 1 h at 37°C followed by incubation with M4 protein. Alternatively, the M4 protein was preincubated with rabbit anti-C-repeat peptide of M22 (50μg/mL) and then added to mesangial cells. In the latter experiments, M4 protein binding to mesangial cells was detected by rabbit-anti Sap4.
The effect of M4 or M4Δ451 on binding of IgA1 to mesangial cells, or vice versa, the effect of IgA1 on binding of M proteins to cells, was investigated by preincubating the cells with either M4 or M4Δ451 (both at 10 μg/mL) or preincubation with galactose-deficient polymeric IgA1-ps (50 μg/mL) for 1 h at 37°C. M4 or M4Δ451 preincubated cells were then incubated with galactose-deficient polymeric IgA1-ps and vice versa, IgA1 preincubated cells were incubated with M4 protein for an additional hour and binding detected by flow cytometry as described above.
Statistics
Differences in IgA binding to M4, IL-6 and C3 secretion, mesangial cell proliferation, as well as M4 and IgA binding to mesangial cells were calculated by the Mann-Whitney U-test using SPSS version 20.0 (Chicago, IL). P values <0.05 were considered significant.
Results
Streptococcal M4 protein preferentially binds to galactose-deficient polymeric IgA1
Binding of various forms of IgA1, monomeric and polymeric as well as galactosylated and galactose-deficient (Table I), to M4 was investigated by two methods, surface plasmon resonance (SPR) and ELISA.
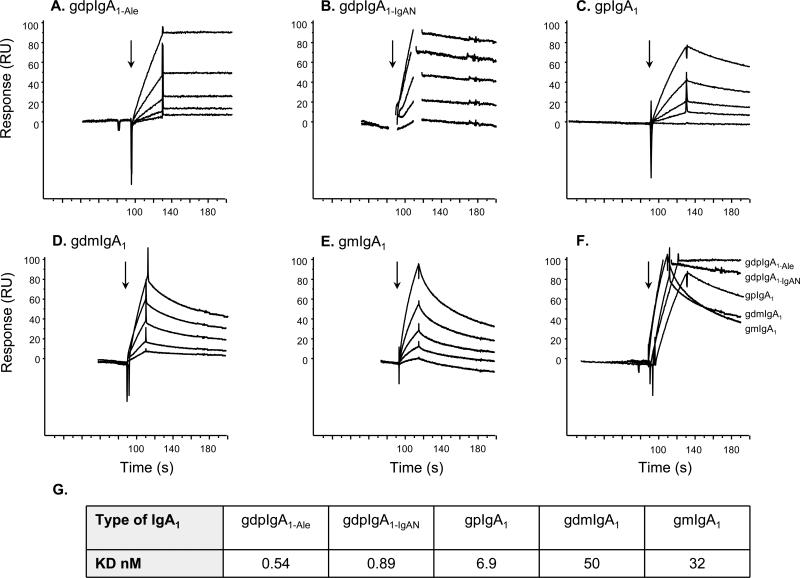
Results of the SPR analysis are presented in Figure 2A-F. Galactose-deficient polymeric IgA1 (IgA1-Ale and IgA1-IgAN) exhibited better binding to M4 than the other forms of IgA1. Binding affinities were calculated by injecting five concentrations of each form of IgA1. The results showed that galactose-deficient polymeric IgA1-Ale and IgA1-IgAN had a considerably lower KD and, thus, higher affinity for M4 (Figure 2G). None of the IgA preparations bound to a non-IgA-binding mutant protein derived from M4, the M4Δ451protein (described in Figure 1), as expected (data not shown).
Figure 2. Binding of IgA to M4 detected by surface plasmon resonance.
Five different forms of IgA1 were used: A: galactose-deficient polymeric IgA1 (IgA1-Ale), B: galactose-deficient polymeric IgA1 from an IgAN patient (IgA1-IgAN), C: galactosylated polymeric IgA1, D: galactose-deficient monomeric IgA1, E: galactosylated monomeric IgA1, and injected at five different concentrations (31 – 500 nM) onto M4 immobilized on CM5 sensor chip chambers. Panel F shows the comparative effect of the highest concentration (500 nM) of each IgA1 form on association and dissociation. Arrows indicate time point of injection of IgA. Due to high response buffer bulk refraction spikes, resonance data in Panel B were cut for a short time-frame at the injection start and stop, respectively. G: the binding affinities of the different IgA1 fractions for M4. gdpIgA1: galactose-deficient polymeric IgA1; gpIgA1: galactosylated polymeric IgA1; gdmIgA1: galactose-deficient monomeric IgA1; gmIgA1: galactosylated monomeric IgA1.
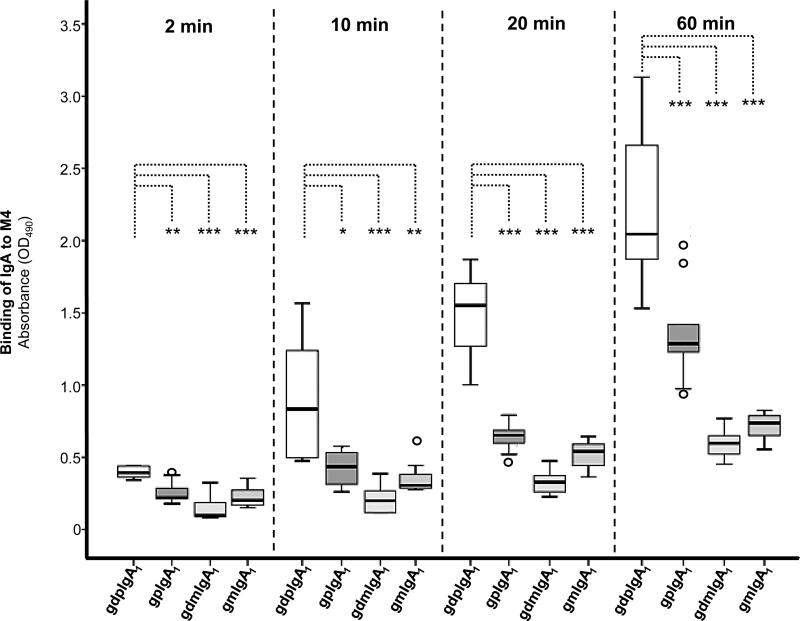
Binding results were confirmed using ELISA showing that galactose-deficient polymeric IgA1 (IgA1-Ale) bound significantly more to M4 than galactosylated polymeric IgA1 (Figure 3). Both of the polymeric fractions of IgA1 bound significantly more to M4 than monomeric IgA1 (p<0.001).
Figure 3. Binding of IgA to M4 measured by ELISA.
M4 immobilized onto ELISA wells was incubated with either of four different IgA1 preparations: gdpIgA1: galactose-deficient polymeric IgA1 myeloma protein (IgA1-Ale), gpIgA1: galactosylated polymeric IgA1, gdmIgA1: galactose-deficient monomeric IgA1, gmIgA1: galactosylated monomeric IgA1. Results represent optical density levels measured in five independent experiments with duplicate wells (box-plots representing medians, interquartal ranges and ranges, outliers are shown). P-values comparing binding of gdpIgA1 to binding of other forms of IgA1. * p <0.05, ** p < 0.01; *** p<0.001.
Galactose-deficient polymeric IgA1 and M4 exert an enhanced effect on IL-6 secretion from human mesangial cells
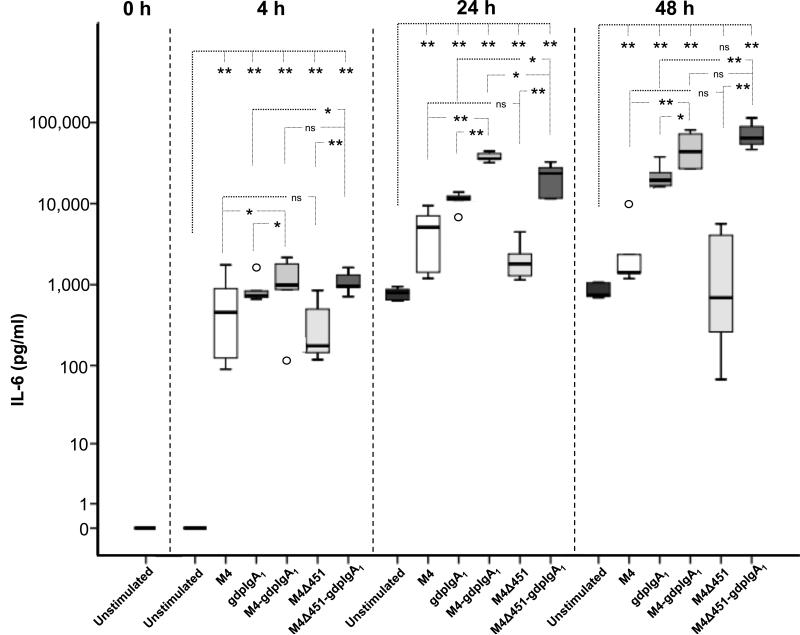
IL-6 was measured in supernatants from human mesangial cells stimulated with M4, the non-IgA-binding mutant protein M4Δ451, galactose-deficient polymeric IgA1-ps, or a combination of either of the M proteins with this IgA1 (Figure 4). After 4 h or 24 h stimulation, all tested proteins induced a significant increase in IL-6 secretion compared to unstimulated mesangial cells. Even after 48 h, a significant increase in IL-6 was detected for all proteins compared with unstimulated cells, with the exception of cells stimulated with M4Δ451. Co-stimulation with galactose-deficient polymeric IgA1 and M4 or M4Δ451 resulted in significantly higher IL-6 secretion than stimulation with either of the stimulants alone at all three time points. This indicates an enhanced effect of co-stimulation.
Figure 4. IL-6 secretion from human mesangial cells stimulated with M4 and galactose-deficient polymeric IgA1.
IL-6 levels in human mesangial cell culture supernatants from three independent experiments with duplicate wells per stimulant are shown. Box plots represent medians, interquartal ranges and ranges, outliers are shown. * p <0.05, ** p < 0.01, ns: not significant. gdpIgA1: galactose-deficient polymeric IgA1-ps. The Y-axis is displayed in logarithmic scale.
The similar stimulatory effect of M4 and M4Δ451 indicated that the IgA-binding region of the M protein was not involved in the induction of mesangial cell IL-6 secretion. There was no difference in IL-6 secretion between cells co-stimulated with galactose-deficient polymeric IgA1 and either of the two M proteins, with the exception of stimulation for 24 h, when M4-IgA1 induced a higher IL-6 secretion than M4Δ451-IgA1.
Galactose-deficient polymeric IgA1-IgAN (isolated from serum of IgAN patients) induced a 3-fold increase in IL-6 secretion compared to galactose-deficient polymeric IgA1-ps (isolated from serum of controls) after 24 h stimulation of the cells (P=0.021, data not shown). Comparison of these preparations at other time-points was precluded due to limited amounts of galactose-deficient polymeric IgA1-IgAN.
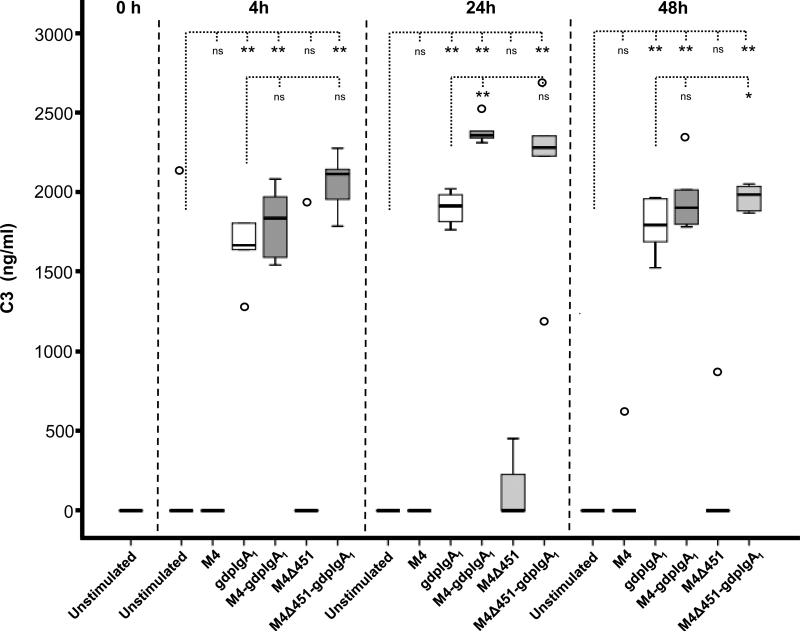
Polymeric IgA1 induced C3 secretion from human mesangial cells
Stimulation of mesangial cells with galactose-deficient polymeric IgA1-ps induced C3 secretion from mesangial cells within 4 h whereas M4 and M4Δ451 did not do so even after 48 h (Figure 5). However, co-stimulation with IgA1-ps and M4 or M4Δ451 caused some increase in the effect of IgA1-ps on mesangial C3 secretion, particularly the combined effect of IgA1-ps and M4 after 24 h stimulation and of IgA1-ps and M4Δ451 after 48 h stimulation. Stimulation of mesangial cells with galactose-deficient polymeric IgA1- ps did not lead to more secretion of C3 in comparison to stimulation with galactosylated polymeric IgA1 (data not shown).
Figure 5. C3 secretion from human mesangial cells stimulated with M4 and galactose-deficient polymeric IgA1.
C3 levels in human mesangial cell culture supernatants from three independent experiments with duplicate wells per stimulant are shown. Box plots represent medians, interquartal ranges and ranges, outliers are shown. * p <0.05, ** p < 0.01, ns: not significant. gdpIgA1: galactose-deficient polymeric IgA1-ps.
Galactose-deficient polymeric IgA1-IgAN did not induce more C3 secretion than galactose-deficient polymeric IgA1-ps (isolated from serum of controls) after 24 h stimulation (data not shown).
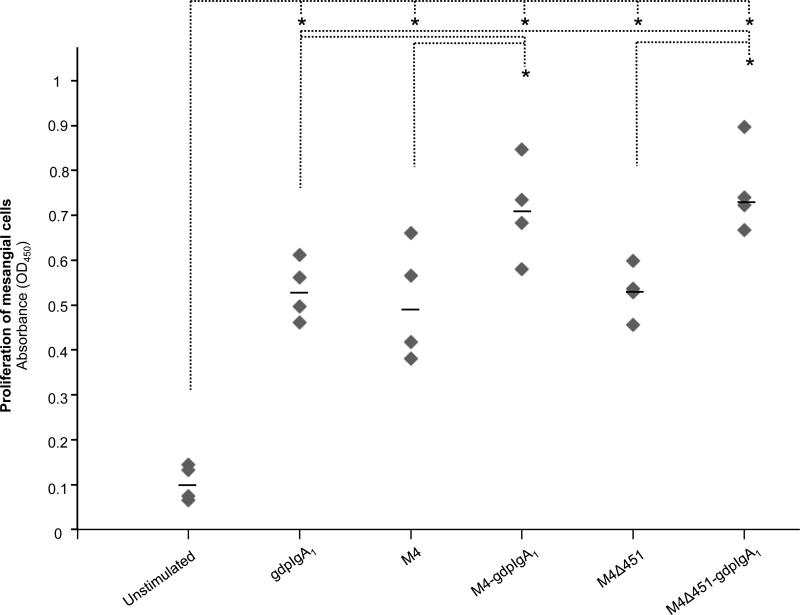
Galactose-deficient polymeric IgA1 and M4 induced mesangial cell proliferation
Stimulation of mesangial cells with galactose-deficient polymeric IgA1-IgAN, M4 or M4Δ451 induced significantly more proliferation than unstimulated cells (Figure 6). Co-stimulation with galactose-deficient polymeric IgA1-IgAN and either of the M proteins induced significantly more proliferation than either of the stimulants alone. The similar effect of M4 and M4Δ451 on mesangial cell proliferation indicated that the IgA-binding region of the M4 protein was not involved in the induction of mesangial cell proliferation.
Figure 6. Proliferation of human mesangial cells stimulated with M4 and galactose-deficient polymeric IgA1.
Proliferation of human mesangial cells measured with BrdU-assay is displayed as ELISA-absorbance values. Each measurement (grey square) represents proliferation of mesangial cells in one experimental setting. The median value for each stimulant is indicated as a black line. * p <0.05. gdpIgA1: galactose-deficient polymeric IgA1-IgAN.
Binding of M4 protein to human mesangial cells
Binding of the M4 protein to human mesangial cells was investigated by flow cytometry as binding would be a prerequisite for the induction of a cellular response. M4 was detected on mesangial cells (Table II). Experiments were designed in order to investigate which region of the M protein was involved in binding. There was no major difference in the degree of binding of M4 or M4Δ451 to mesangial cells indicating that the IgA-binding region of M4 does not mediate cell binding. Likewise, preincubation of M4 with a peptide corresponding to the N-terminal of M4 (M4N, Table I, Figure 1) did not block M4 binding to mesangial cells. Preincubation of mesangial cells with a peptide corresponding to the C-repeat region did, however, block binding of M4 to mesangial cells. For technical reasons this experiment was performed using a peptide analogous to the C-repeat region of the M protein of GAS emm-type 22, bearing 92 % homology to M4 within the same region (described in Table I, Figure 1). Furthermore, preincubation of M4 with antibodies to the C-repeat region blocked M4 binding to mesangial cells (Table II). These results suggest that the M4 protein binds to mesangial cells via its C-repeat conserved region.
Table II.
Binding of M4 or IgA to human mesangial cells
| Proteins used for incubation | Detection antibody | MFI* | % of M4-bindinga | % of IgA-bindingb | P value |
|---|---|---|---|---|---|
| M4 n=3 | αM4 | 4151 (3976-4406) | |||
| M4Δ451 n=3 | 3759 (2185-5373) | 85 % (53-135 %) | NS | ||
| M4 n=8 | αSap4 | 2288 (1451-3628) | |||
| M4N/M4c n=3 | 2856 (2010-4324) | 92 % (87-138 %) | NS | ||
| C-repeat/M4d n=4 | 175 (0-823) | 6 % (0-29 %) | 0.004 | ||
| M4 + anti-C-repeate n=3 | 598 (396-1507) | 25% (12-53 %) | 0.024 | ||
| IgA/M4f,g n=3 | 3670 (3480-4458) | 221% (123-253%) | 0.024 | ||
| IgA n=3 | αIgA | 3114 (1250-3185) | |||
| M4/IgAh n=3 | 3598 (3153-4305) | 135% (101-288 %) | NS | ||
| M4Δ451/IgAi n=3 | 3087 (2778-5131) | 161% (99-222%) | NS |
MFI: mean fluorescent intensity. IgA, galactose-deficient polymeric IgA1-ps.
MFI displayed as median and range.
Percent related to M4 in the same experiment.
Percent related to IgA in the same experiment.
Detection of M4 binding after preincubation of mesangial cells with the M4N peptide (N terminal of M4).
Detection of M4 binding after preincubation of the cells with a peptide corresponding to the C-repeat domain of M22 (92% homologous to that of M4).
Detection of M4 binding to mesangial cells using M4 preincubated with anti-C-repeat antibodies.
Detection of M4 binding after preincubation of the cells with IgA.
Binding of anti-Sap4 to IgA-preincubated mesangial cells was tested as a control for IgA/M4 and was comparable to that of isotype-controls.
Detection of IgA binding after preincubation of the cells with M4.
Detection of IgA binding after preincubation of the cells with M4Δ451.
P values are in comparison with M4-MFI using the same detection antibody or IgA-MFI using αIgA. P<0.05 was considered significant. NS: not significant.
Pre-incubation of mesangial cells with galactose-deficient polymeric IgA1-ps significantly increased binding of M4. In contrast, pre-incubation of cells with either of the M proteins (M4 or M4Δ451) did not affect mesangial binding of galactose-deficient polymeric IgA1 (Table II).
Discussion
IgAN is a chronic progressive form of glomerulonephritis characterized by mesangial deposits of galactose-deficient IgA1 and C3 with variable co-deposits of IgG and/or IgM (1, 49). In a previous study, we demonstrated that mesangial IgA deposits co-localized with streptococcal IgA-binding regions of M proteins and suggested that M protein association with IgA1 may contribute to the pathogenesis of disease (16). Here we show that the IgA-binding M protein from GAS serotype 4, one of the most common serotypes among clinical GAS isolates (50), bound preferentially to galactose-deficient polymeric IgA1. IgA1 enhanced binding of M protein to mesangial cells and co-stimulation of human mesangial cells with both galactose-deficient polymeric IgA1 and M4 induced excessive IL-6 secretion from the cells. Polymeric IgA1 induced C3 secretion from mesangial cells, a novel finding in itself, and an effect enhanced by co-stimulation with M4 protein although the M4 protein alone did not have this effect. In addition, co-stimulation of cells with galactose-deficient polymeric IgA1 and M4 protein induced excessive mesangial cell proliferation thus contributing to a mesangioproliferative process. IgA-binding M proteins could bind to galactose-deficient polymeric IgA1 in the circulation and deposit together in the mesangium promoting the secretion of IL-6 and C3, as well as cell proliferation, and thus contribute to the inflammatory process occurring during IgAN.
During GAS infection surface-localized M proteins, or parts thereof, may detach from the bacterial cell wall due to the effect of streptococcal or neutrophil granulocyte-derived proteases (51, 52). These detached M proteins (or M protein fragments) gain access to the circulation and consequently reach the kidney (16). The results presented here indicate that M4 binds with higher affinity to polymeric than to monomeric IgA1 and preferentially binds to galactose-deficient polymeric IgA1 and thus M4-IgA1 can reach the renal circulation as a complex. Galactose-deficient IgA1 has indeed been detected in the circulation (3, 4, 53, 54) and kidneys of patients with IgAN and shown to bind preferentially to human mesangial cells (6, 55). The M protein-IgA1 complex will consequently reach the mesangium (16) enabling it to exert an inflammatory response.
The increased binding of M4 to galactose-deficient compared to galactosylated polymeric IgA1 may have several explanations related to tertiary structure or charge. Given the relative distance between the hinge-region and the binding-site for streptococcal M proteins on IgA at the Cα2-Cα3 interface a direct structural influence of the hinge region on M4 binding appears less likely (21). As the increased binding was only observed for galactose-deficient polymeric IgA1 but not the galactose-deficient monomeric form of IgA1 it seems that the critical change within IgA1 is caused by polymerization and that this effect may be enhanced by galactose-deficient O-glycans of the IgA1 hinge region. An altered glycosylation in the hinge region could indirectly influence the tertiary structure of the IgA1 molecule and may thus affect polymerization and the conformation of the IgA molecule so as to facilitate binding of streptococcal IgA-binding M proteins.
IL-6 has been implicated in the pathogenesis of IgAN, as up-regulation of IL-6 mediates mesangial cell proliferation and matrix expansion and contributes to inflammatory cell infiltration in the kidney (23-25, 56). Immune complexes from patients with IgAN containing galactose-deficient polymeric IgA1 have been shown to stimulate mesangial IL-6 synthesis and secretion (26). In the current study we show that the streptococcal IgA-binding M4 protein binds to human mesangial cells, and enhances their IL-6 secretion. Induction of IL-6 secretion was also induced by the non-IgA binding mutant variant M4Δ451 suggesting that this effect was not dependent on IgA binding to M4. In addition, we found an enhanced effect on IL-6 secretion from mesangial cells, when these cells were co-stimulated with a combination of M4 and galactose-deficient polymeric IgA1. The effect on IL-6 secretion was even more pronounced using galactose-deficient polymeric IgA1 from patients with IgAN, which may have contained more IgA-IgA complexes than IgA purified from controls.
A similar effect to the effect of co-stimulation with M4 and galactose-deficient polymeric IgA1 was detected when cells were co-stimulated with the non-IgA binding mutant variant M4Δ451 and galactose-deficient polymeric IgA. These results suggest that the IgA-binding property of the streptococcal M4 protein, which may be a prerequisite for mesangial co-deposition with IgA, is not involved in exerting an inflammatory response. The results also indicate that the IgA-binding domain in M4 is not involved in cell binding and would thus, most probably, not be associated with cell signaling. We envisage that a fraction of IgA1 may circulate in vivo bound to M proteins, or their IgA-binding domain, and, upon reaching the mesangial cells, each agonist may exert a separate stimulatory effect, regardless if they are in complex or not. These findings suggest that streptococcal M proteins deposited in the mesangial region may contribute to the inflammatory response and could therefore be of importance in the pathogenesis of IgAN.
Mesangial deposits of C3 are commonly detected in kidney samples from patients with IgAN (1). C3 may originate from the circulation (hepatic and extra-hepatic synthesis) or mesangial cell secretion (32). Other complement components required for the formation and stabilization of the alternative pathway C3-convertase (factors B and D as well as properdin) are also locally synthesized (57, 58). The co-localization of C3 split products and C5b-9 in mesangial deposits together with in-situ detected C3 synthesis suggests that locally secreted C3 participates in the mesangial inflammation during IgAN (59). The finding herein that polymeric IgA1 induces C3 secretion by mesangial cells is novel. The prevalence of polymeric IgA1 in the mesangium during IgAN may thus explain the origin of local C3 deposits. C3 secretion occurs regardless of the type of O-glycosylation or source of the polymeric IgA1, but as galactose-deficient polymeric IgA1 is more prevalent in mesangial deposits in IgAN (55) the effect may be most relevant for this type of IgA. Previous publications have shown that immune complexes stimulate C3 secretion by mesangial cells (31). The IgA preparations used in our experiments did not contain detectable IgA-IgG complexes, but IgA-IgA complexes may possibly have induced a certain degree of mesangial C3 secretion. Mesangial stimulation with M4 did not induce C3 secretion. Co-stimulation with galactose-deficient polymeric IgA1, however, resulted in an enhanced C3 secretion compared to galactose-deficient polymeric IgA alone. The difference in response of mesangial cells to M proteins and galactose-deficient polymeric IgA1 with regard to IL-6 and C3 secretion suggest that these agonists utilize different signal pathways to exert their effects on mesangial cells.
Galactose-deficient IgA1 is present in the circulation in immune complexes and these complexes stimulate proliferation of mesangial cells in vitro whereas free IgA1, regardless of glycosylation and molecular form, does not induce cellular proliferation (reviewed in (3, 60)). However, free IgA1 binds to mesangial cells and induces cellular signaling (5, 26, 61). In our study, preparations of galactose-deficient polymeric IgA1 lacked IgG but may have contained IgA-IgA complexes. Patient IgA may contain more IgA-IgA complexes, thus explaining why this form of IgA1 induced mesangial cell proliferation (5).
The binding capacity of different forms of IgA to human mesangial cells has been previously described, showing that desialylated and degalactosylated IgA1 and polymeric IgA1 had higher binding affinity (62, 63). The manner by which IgA binds to mesangial cells is still a matter of debate. Two IgA-receptors have been described on human mesangial cells, the transferrin receptor CD71 and the Fcα/μ receptor (64, 65). In the current study, we found that the M4 protein bound to mesangial cells and enhanced secretion of IL-6. Binding to mesangial cells was mediated via the C-terminal conserved region of the M protein. As the structure of the conserved region is widely preserved between GAS strains (66) this finding implies that M proteins may be capable of binding to mesangial cells regardless of M-type. The putative receptor for M proteins on human mesangial cells remains to be elucidated and will be the subject of future studies.
We have previously shown that patients with IgAN were more likely than age-matched controls to have been infected with GAS serotypes expressing IgA-binding M proteins. We suggest, based on the results of our previous (16, 22) and current studies that, during GAS infection, M proteins, or fragments thereof, will bind to galactose-deficient polymeric IgA1 prevalent in the serum of patients with IgAN and thus circulate as an IgA1-M protein complex. IgA-binding M proteins in complex with IgA will deposit in the mesangial area (16). The IgA-binding domain of M proteins will enable the streptococcal protein, bound to galactose-deficient polymeric IgA1, to reach the mesangial region, where each of these proteins will bind to mesangial cells and exert a separate inflammatory response which is enhanced when both proteins act simultaneously. Streptococcal IgA-binding M proteins may thus partake in the initiation and propagation of an inflammatory response in the kidney during IgAN.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Margareta Stålhammar-Carlemalm, Division of Medical Microbiology, Department of Laboratory Medicine, Lund University, Lund, Sweden, for preparations of streptococcal proteins, and Dr. Ramesh Tati, Department of Pediatrics, Clinical Sciences Lund, Lund University, Lund, Sweden, for characterization of the mesangial cells, Stacy Hall, Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL, USA, for technical help with immunoblots, and Dr. Alice C. Smith, University of Leicester, UK, for the protocol of IgA1 purification. A preliminary version of the study appeared in the Ph.D. thesis of Dr. Roland Schmitt and was presented in poster form at the 45th annual meeting of ESPN in Kraków Poland September 6-8, 2012.
Financial support
This study was supported by grants from The Swedish Research Council (K2013-64X-14008 to DK and K2011-56X-09490-21-6 to GL), The Torsten Söderberg Foundation, Crown Princess Lovisa's Society for Child Care, Konung Gustaf V:s 80-årsfond (all to DK). The Foundations of Österlund and Olle Engkvist Byggmästare (both to GL). Skånes University Hospital (to AO) and funding from Region Skåne (to RS). JN was supported in part by grants from the National Institutes of Health DK078244, DK082753, DK083663, and GM098539.
Footnotes
Disclosure
The authors have no disclosures and no competing financial interests.
References
- 1.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–748. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 2.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 3.Novak J, Julian BA, Mestecky J, Renfrow MB. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol. 2012;34:365–382. doi: 10.1007/s00281-012-0306-z. [DOI] [PubMed] [Google Scholar]
- 4.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 5.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, Novak L, Matousovic K, Novak J. IgA nephropathy: molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–240. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 6.Tam KY, Leung JC, Chan LY, Lam MF, Tang SC, Lai KN. Macromolecular IgA1 taken from patients with familial IgA nephropathy or their asymptomatic relatives have higher reactivity to mesangial cells in vitro. Kidney Int. 2009;75:1330–1339. doi: 10.1038/ki.2009.71. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro RC, Moura IC, Launay P, Tsuge T, Haddad E, Benhamou M, Cooper MD, Arcos-Fajardo M. Pathogenic significance of IgA receptor interactions in IgA nephropathy. Trends Mol Med. 2002;8:464–468. doi: 10.1016/s1471-4914(02)02405-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen A, Chen WP, Sheu LF, Lin CY. Pathogenesis of IgA nephropathy: in vitro activation of human mesangial cells by IgA immune complex leads to cytokine secretion. J Pathol. 1994;173:119–126. doi: 10.1002/path.1711730208. [DOI] [PubMed] [Google Scholar]
- 9.Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schonlein purpura nephritis. Kidney Int. 2011;80:79–87. doi: 10.1038/ki.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feehally J. Immune mechanisms in glomerular IgA deposition. Nephrol Dial Transplant. 1988;3:361–378. doi: 10.1093/oxfordjournals.ndt.a091683. [DOI] [PubMed] [Google Scholar]
- 11.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldherr R, Rambausek M, Duncker WD, Ritz E. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant. 1989;4:943–946. doi: 10.1093/ndt/4.11.943. [DOI] [PubMed] [Google Scholar]
- 13.Rekola S, Bergstrand A, Bucht H, Lindberg A. Are beta-haemolytic streptococci involved in the pathogenesis of mesangial IgA-nephropathy? Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1985;21:698–702. [PubMed] [Google Scholar]
- 14.Kukuminato Y, Hamamoto M, Kataura A. Role of serum antibodies to streptococci in patients with IgA nephropathy. Acta Otolaryngol Suppl. 1993;508:6–10. doi: 10.3109/00016489309130259. [DOI] [PubMed] [Google Scholar]
- 15.Nakatsuka K. Serum anti-streptococcal IgA, IgG and IgM antibodies in IgA-associated diseases. Acta Paediatr Jpn. 1993;35:118–123. doi: 10.1111/j.1442-200x.1993.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt R, Carlsson F, Mörgelin M, Tati R, Lindahl G, Karpman D. Tissue deposits of IgA-binding streptococcal M proteins in IgA nephropathy and Henoch-Schonlein purpura. Am J Pathol. 2010;176:608–618. doi: 10.2353/ajpath.2010.090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki S, Nakatomi Y, Sato H, Tsukada H, Arakawa M. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet. 1994;343:12–16. doi: 10.1016/s0140-6736(94)90875-3. [DOI] [PubMed] [Google Scholar]
- 18.Koyama A, Sharmin S, Sakurai H, Shimizu Y, Hirayama K, Usui J, Nagata M, Yoh K, Yamagata K, Muro K, Kobayashi M, Ohtani K, Shimizu T. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int. 2004;66:121–132. doi: 10.1111/j.1523-1755.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Kawasaki Y, Yoshida K, Mochizuki K, Isome M, Honzumi K, Nozawa R, Suzuki S, Hosoya M, Suzuki J, Suzuki H. Detection of enteroviruses in renal biopsies from patients with immunoglobulin A nephropathy. Pediatr Nephrol. 2005;20:1578–1582. doi: 10.1007/s00467-005-2019-1. [DOI] [PubMed] [Google Scholar]
- 20.Frithz E, Hedén LO, Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol Microbiol. 1989;3:1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 21.Pleass RJ, Areschoug T, Lindahl G, Woof JM. Streptococcal IgA-binding proteins bind in the Calpha 2-Calpha 3 interdomain region and inhibit binding of IgA to human CD89. J Biol Chem. 2001;276:8197–8204. doi: 10.1074/jbc.M009396200. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt R, Lindahl G, Karpman D. Antibody response to IgA-binding streptococcal M proteins in children with IgA nephropathy. Nephrol Dial Transplant. 2010;25:3434–3436. doi: 10.1093/ndt/gfq346. [DOI] [PubMed] [Google Scholar]
- 23.Lim CS, Zheng S, Kim YS, Ahn C, Han JS, Kim S, Lee JS, Chae DW, Koo JR, Chun RW, Noh JW. Th1/Th2 predominance and proinflammatory cytokines determine the clinicopathological severity of IgA nephropathy. Nephrol Dial Transplant. 2001;16:269–275. doi: 10.1093/ndt/16.2.269. [DOI] [PubMed] [Google Scholar]
- 24.Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Dohi K, Ishikawa H, Ohmoto Y, et al. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949–3955. [PubMed] [Google Scholar]
- 25.Horii Y, Iwano M, Hirata E, Shiiki M, Fujii Y, Dohi K, Ishikawa H. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int Suppl. 1993;39:S71–75. [PubMed] [Google Scholar]
- 26.Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z, Novak L, Novak Z, Mayne R, Julian BA, Mestecky J, Wyatt RJ. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant. 2011;26:3451–3457. doi: 10.1093/ndt/gfr448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med. 2013;64:339–356. doi: 10.1146/annurev-med-041811-142014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt RJ, Julian BA. Activation of complement in IgA nephropathy. Am J Kidney Dis. 1988;12:437–442. doi: 10.1016/s0272-6386(88)80042-8. [DOI] [PubMed] [Google Scholar]
- 29.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 30.van den Dobbelsteen ME, Verhasselt V, Kaashoek JG, Timmerman JJ, Schroeijers WE, Verweij CL, van der Woude FJ, van Es LA, Daha MR. Regulation of C3 and factor H synthesis of human glomerular mesangial cells by IL-1 and interferon-gamma. Clin Exp Immunol. 1994;95:173–180. doi: 10.1111/j.1365-2249.1994.tb06033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmerman JJ, Van Gijlswijk-Janssen DJ, Van Der Kooij SW, Van Es LA, Daha MR. Antigen-antibody complexes enhance the production of complement component C3 by human mesangial cells. J Am Soc Nephrol. 1997;8:1257–1265. doi: 10.1681/ASN.V881257. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, Abe K, Koji T, Furusu A, Ozono Y, Harada T, Nakane PK, Yagame M, Endoh M, Nomoto Y, Sakai H. Intraglomerular C3 synthesis in human kidney detected by in situ hybridization. J Am Soc Nephrol. 1996;7:2428–2433. doi: 10.1681/ASN.V7112428. [DOI] [PubMed] [Google Scholar]
- 33.Johnsson E, Andersson G, Lindahl G, Hedén LO. Identification of the IgA-binding region in streptococcal protein Arp. J Immunol. 1994;153:3557–3564. [PubMed] [Google Scholar]
- 34.Morfeldt E, Berggård K, Persson J, Drakenberg T, Johnsson E, Lindahl E, Linse S, Lindahl G. Isolated hypervariable regions derived from streptococcal M proteins specifically bind human C4b-binding protein: implications for antigenic variation. J Immunol. 2001;167:3870–3877. doi: 10.4049/jimmunol.167.7.3870. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson F, Berggård K, Stålhammar-Carlemalm M, Lindahl G. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J Exp Med. 2003;198:1057–1068. doi: 10.1084/jem.20030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stålhammar-Carlemalm M, Waldemarsson J, Johnsson E, Areschoug T, Lindahl G. Nonimmunodominant regions are effective as building blocks in a streptococcal fusion protein vaccine. Cell Host Microbe. 2007;2:427–434. doi: 10.1016/j.chom.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Wall SB, Suzuki H, Smith A. D. t., Hall S, Poulsen K, Kilian M, Mobley JA, Julian BA, Mestecky J, Novak J, Renfrow MB. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franc V, Rehulka P, Raus M, Stulik J, Novak J, Renfrow MB, Sebela M. Elucidating heterogeneity of IgA1 hinge-region O-glycosylation by use of MALDI-TOF/TOF mass spectrometry: role of cysteine alkylation during sample processing. J Proteomics. 92:299–312. doi: 10.1016/j.jprot.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, Julian BA, Novak J. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcon GS, Kimberly RP, Tomino Y, Mestecky J, Novak J. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordström T, Movert E, Olin AI, Ali SR, Nizet V, Varki A, Areschoug T. Human Siglec-5 inhibitory receptor and immunoglobulin A (IgA) have separate binding sites in streptococcal beta protein. J Biol Chem. 2011;286:33981–33991. doi: 10.1074/jbc.M111.251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manea M, Kristoffersson A, Schneppenheim R, Saleem MA, Mathieson PW, Mörgelin M, Bjork P, Holmberg L, Karpman D. Podocytes express ADAMTS13 in normal renal cortex and in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2007;138:651–662. doi: 10.1111/j.1365-2141.2007.06694.x. [DOI] [PubMed] [Google Scholar]
- 46.Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ståhl AL, Vaziri-Sani F, Heinen S, Kristoffersson AC, Gydell KH, Raafat R, Gutierrez A, Beringer O, Zipfel PF, Karpman D. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111:5307–5315. doi: 10.1182/blood-2007-08-106153. [DOI] [PubMed] [Google Scholar]
- 48.Zoja C, Wang JM, Bettoni S, Sironi M, Renzi D, Chiaffarino F, Abboud HE, Van Damme J, Mantovani A, Remuzzi G, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am J Pathol. 1991;138:991–1003. [PMC free article] [PubMed] [Google Scholar]
- 49.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 50.Colman G, Tanna A, Efstratiou A, Gaworzewska ET. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J Med Microbiol. 1993;39:165–178. doi: 10.1099/00222615-39-3-165. [DOI] [PubMed] [Google Scholar]
- 51.Berge A, Björck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. doi: 10.1074/jbc.270.17.9862. [DOI] [PubMed] [Google Scholar]
- 52.Herwald H, Cramer H, Morgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Björck L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004;116:367–379. doi: 10.1016/s0092-8674(04)00057-1. [DOI] [PubMed] [Google Scholar]
- 53.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 54.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen AC, Bailey EM, Barratt J, Buck KS, Feehally J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J Am Soc Nephrol. 1999;10:1763–1771. doi: 10.1681/ASN.V1081763. [DOI] [PubMed] [Google Scholar]
- 56.Coletta I, Soldo L, Polentarutti N, Mancini F, Guglielmotti A, Pinza M, Mantovani A, Milanese C. Selective induction of MCP-1 in human mesangial cells by the IL-6/sIL-6R complex. Exp Nephrol. 2000;8:37–43. doi: 10.1159/000059327. [DOI] [PubMed] [Google Scholar]
- 57.Timmerman JJ, Verweij CL, van Gijlswijk-Janssen DJ, van der Woude FJ, van Es LA, Daha MR. Cytokine-regulated production of the major histocompatibility complex class-III-encoded complement proteins factor B and C4 by human glomerular mesangial cells. Hum Immunol. 1995;43:19–28. doi: 10.1016/0198-8859(94)00122-7. [DOI] [PubMed] [Google Scholar]
- 58.Song D, Zhou W, Sheerin SH, Sacks SH. Compartmental localization of complement component transcripts in the normal human kidney. Nephron. 1998;78:15–22. doi: 10.1159/000044876. [DOI] [PubMed] [Google Scholar]
- 59.Abe K, Miyazaki M, Koji T, Furusu A, Shioshita K, Tsukasaki S, Ozono Y, Harada T, Sakai H, Kohno S. Intraglomerular synthesis of complement C3 and its activation products in IgA nephropathy. Nephron. 2001;87:231–239. doi: 10.1159/000045920. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamouza H, Chemouny JM, Raskova Kafkova L, Berthelot L, Flamant M, Demion M, Mesnard L, Paubelle E, Walker F, Julian BA, Tissandie E, Tiwari MK, Camara NO, Vrtovsnik F, Benhamou M, Novak J, Monteiro RC, Moura IC. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 2012;82:1284–1296. doi: 10.1038/ki.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao YH, Xu LX, Zhang JJ, Zhang Y, Zhao MH, Wang HY. Differential binding characteristics of native monomeric and polymeric immunoglobulin A1 (IgA1) on human mesangial cells and the influence of in vitro deglycosylation of IgA1 molecules. Clin Exp Immunol. 2007;148:507–514. doi: 10.1111/j.1365-2249.2007.03374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai KN, To WY, Li PK, Leung JC. Increased binding of polymeric lambda-IgA to cultured human mesangial cells in IgA nephropathy. Kidney Int. 1996;49:839–845. doi: 10.1038/ki.1996.116. [DOI] [PubMed] [Google Scholar]
- 64.McDonald KJ, Cameron AJ, Allen JM, Jardine AG. Expression of Fc alpha/mu receptor by human mesangial cells: a candidate receptor for immune complex deposition in IgA nephropathy. Biochem Biophys Res Commun. 2002;290:438–442. doi: 10.1006/bbrc.2001.6218. [DOI] [PubMed] [Google Scholar]
- 65.Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol. 2004;15:622–634. doi: 10.1097/01.asn.0000115401.07980.0c. [DOI] [PubMed] [Google Scholar]
- 66.Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 67.Areschoug T, Stålhammar-Carlemalm M, Karlsson I, Lindahl G. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J Biol Chem. 2002;277:12642–12648. doi: 10.1074/jbc.M112072200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.