Abstract
While miRNAs have been shown to participate in innate immune responses, it is not completely understood how miRNAs regulate negative immuno-modulatory events. IL-10 is an important anti-inflammatory mediator that prevents excessive inflammation and associated immunological pathologies. Although the regulation of IL-10 expression has been well studied at both the transcriptional and translational levels, it is less clear how miRNAs control IL-10 expression during inflammation. In this study, we found that miR-27a is downregulated in macrophages following stimulation through TLR2 and TLR4, but not TLR3. Upregulation of miR-27a enhanced the expression of pro-inflammatory cytokines in TLR2/4 activated macrophages. Conversely, knockdown of miR-27a diminished cytokine expression. Mechanistically, we found that miR-27a negatively regulates IL-10 expression in that upregulation of miR-27a decreases, whereas downregulation of miR-27a increases IL-10 expression in activated macrophages. Likely due to the decreased expression of IL-10, upregulation of miR-27a diminished IL-10-dependent STAT3 phosphorylation in TLR4 activated macrophages. Consistent with IL-10 being a potential mediator for the role of miR-27a in immune response, blocking IL-10 abolished the enhancing effect of miR-27a on TLR4 activated inflammation. In conclusion, our study identified miR-27a downregulation as a negative regulatory mechanism that prevents overly exuberant TLR2 and TLR4 driven inflammatory responses.
Keywords: miR-27a, IL-10, innate immunity, TLR4, TLR2
Introduction
Innate immunity constitutes a first line of immune defense against host invasion by microbial pathogens. Immune cells, such as macrophages and neutrophils, respond to micro-organisms through a series of unique receptors that detect pathogen associated molecular patterns (PAMPs) (1-4). Among these, Toll-like receptor 4 (TLR4) and TLR2 recognize lipopolysaccharide (LPS) and lipoproteins that are important bacterial cell-wall components (1-4). Engagement of TLRs with PAMPs activates immune cells to produce pro-inflammatory mediators, such as cytokines and reactive oxygen species, that are essential to the eradication of microbial infection (1-5). However, overly exuberant inflammation can cause collateral tissue damage that leads to organ injury, such as acute lung injury. Multiple negative regulatory mechanisms that are employed by the immune system, such as induced expression of IL-10 and IκB-α, have been shown to prevent excessive inflammatory response (6-9).
IL-10 is a potent anti-inflammatory cytokine that plays important role in preventing inflammatory and autoimmune pathologies (7, 8, 10). This role of IL-10 is evidenced by spontaneously developing chronic enterocolitis in IL-10 knockout mice (11). IL-10 is expressed by various inflammatory cell types, including macrophages, neutrophils, dendritic cells, mast cells, natural killer (NK) cells and T cell subsets (7, 8, 10). Diminished expression of IL-10 has been shown to be associated with a variety of immunological disorders, such as cancer, rheumatoid arthritis, asthma, and inflammatory bowel disease (7, 8, 10).
microRNAs (miRNAs) are a class of small non-coding RNAs that target the 3′ UTR of mRNAs and thereby inhibiting their expression (12-16). A number of miRNAs have been found to participate in innate immune responses (17-20). However, how miRNAs target negative regulatory mechanisms in innate immunity is not completely understood (21). Given that IL-10 has been found to be underexpressed in numerous inflammatory pathologies, we set to identify regulatory mechanisms of IL-10 expression by miRNAs. We were particularly interested in miRNAs whose expression is decreased in inflammatorily activated macrophages and that potentially target IL-10. Inferably, aberrant expression of those miRNAs could lead to diminished IL-10 and thereby contributing to the development of inflammatory diseases. In the process of characterizing miRNAs that had altered expression in LPS treated macrophages, we found that miR-27a demonstrates such a role in TLR2/4 induced innate immune response. Our data suggest that miR-27a downregulation serves as an important negative feedback mechanism by which macrophages restrain excessive inflammatory response.
Materials and methods
Reagents
LPS from Escherichia coli 0111:B4 was from Sigma-Aldrich. Ultra-pure LPS from Salmonella minnesota R595, PAM3CSK4 and poly I:C were from Invivogene. Isotype rat IgG and rat anti-IL-10 blocking antibody were from eBioscience. RAW 264.7 cells were from American Type Culture Collection (ATCC).
Generation of mouse bone marrow derived macrophages (BMDMs), mouse peritoneal macrophages, and human peripheral blood mononuclear cell (PBMC) derived macrophages
Mouse BMDMs were derived from bone marrow cells of C57BL/6 mice (NCR-Fredrick). Briefly, after lysis of red blood cells, bone marrow cells were cultured in DMEM media containing 10% FBS and 50 ng/ml murine M-CSF (R&D Systems) for 5 days. The cells were then trypsinized and plated for treatment or transfection. Peritoneal macrophages were elicited from C57BL/6 mice by i.p. injection of 1 ml sterile 4% Brewer thioglycollate. Cells were harvested 4 days later by peritoneal lavage and plated on plates. After 1 hour at 37°C, non-adherent cells were removed by washing and adherent macrophages were used for treatment or transfection. Human peripheral blood mononuclear cells (PBMCs) were purchased from ZenBio Inc. PBMCs were cultured in DMEM media containing 10% FBS and 50 ng/ml human M-CSF (R&D Systems) for 5 days. The cells were then trypsinized and plated for treatment or transfection. The animal protocol was approved by the UAB Institutional Animal Care and Use Committee (IACUC).
miRNA array
Total RNAs were purified from macrophages with miRNeasy Mini Kit (Qiagen). The miRNA array was performed by Exiqon using miRCURY LNA™ microRNA Array (Exiqon). The data were deposited at Gene Expression Omnibus (GEO) with an accession number GSE55414 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE55414).
Quantitative real-time PCR
Probe Master Mix kit (Roche) was used for amplification of miRNAs. Taqman probes for miR-27a and internal references, small nucleolar RNA 135 (sno135) (mouse) and small nucleolar RNA U47 (human), were purchased from Life Technologies. SYBR Green Master Mix kit (Roche) was used to amplify the following genes. Primer sequences were: mouse GAPDH: sense, 5′ CGACTTCAACAGCAACTCCCACTCTTCC 3′; antisense, 5′ TGGGTGGTCCAGGGTTTCTTACTCCTT 3′; mouse Tubulin: sense, 5′ GGATGCTGCCAATAACTATGCTCGT 3′; antisense, 5′ GCCAAAGCTGTGGAAAACCAAGAAG 3′; mouse TNF-α: sense, 5′ AGAGCTACAAGAGGATCACCAGCAG 3′; antisense, 5′ TCAGATTTACGGGTCAACTTCACAT 3′; mouse IL-1β: sense, 5′ AAGGAGAACCAAGCAACGACAAAATA 3′; antisense, 5′ TTTCCATCTTCTTCTTTGGGTATTGC; mouse IL-6: sense, 5′ CCCAATTTCCAATGCTCTCCTA 3′; antisense, 5′ AGGAATGTCCACAAACTGATATGCT; mouse IL-10: sense, 5′ AGCATTTGAATTCCCTGGGTGA 3′; antisense, 5′ CCTGCTCCACTGCCTTGCTCTT 3′; mouse IL-12 p40: sense, 5′ CCAAATTACTCCGGACGGTTCAC 3′; antisense, 5′ CAGACAGAGACGCCATTCCACAT 3′. To normalize the expression of miRNAs or cytokines and calculate fold change, ΔCt values were first obtained as follows: ΔCt = Ct of GAPDH, Tubulin, sno135, or U47 - Ct of miRNAs or cytokines. ΔΔCt values were then obtained as follows: ΔΔCt = ΔCt of treated groups - ΔCt of untreated control groups. Fold change was calculated as 2ΔΔCt, with control groups regarded as 1 fold.
Enzyme-linked immunosorbent assay (ELISA) for cytokines
Levels of TNF-α, IL-6 and IL-10 in supernatants were quantified using DuoSet ELISA Development kits (R&D Systems) according to the manufacturer’s instructions.
Western blotting
Western blotting was performed as previously described (22). Anti-p-STAT3 and anti-STAT3 antibodies were from Cell Signaling.
Luciferase assay
Mouse and human IL-10 3′ UTR sequences that contain the site potentially bound by miR-27a were obtained by PCR amplification using mouse genomic DNA as template and cloned into pMir-Report Luciferase vector (Life Technologies). Mouse IL-10 3′ UTR that had mutations at the miR-27a binding site was created by site mutagenesis. HEK-293T or RAW 264.7 cells were transfected with 20 nM control mimics or 20 nM miR-27a mimics. 6 hours after transfection, cells were transfected again with 5 or 100 ng pMir-Report constructs. After 48 hours of transfection, luciferase activity in the cells was determined using a Luciferase Assay System (Promega).
Transfection of miRNAs
Macrophages were transfected with 20 nM miRNA mimics or 20 nM miRNA inhibitors using HiperFect transfection reagent (Qiagen) according to the manufacturer’s instructions. Control mimics, miR-27a mimics, control inhibitors, anti-miR-27a inhibitors were from Life Technologies.
Statistical analysis
One-way ANOVA followed by the Bonferroni test was performed for multiple group comparisons. The Student t test was used for comparison between two groups. p < 0.05 was considered statistically significant.
Results
miR-27a is downregulated in macrophages after TLR4 and TLR2 stimulation
To study the role of miRNAs in innate immune response, we performed a miRNA array assay on RNAs of untreated and LPS treated macrophages (GSE55414). We found that a number of miRNAs had altered expression in LPS treated cells. Of those upregulated miRNAs, miR-147 and miR-125a-5p were found to inhibit inflammatory response through a negative feed-back mechanism (23, 24). Among those downregulated miRNAs, miR-27a was particularly interesting because it was predicted to potentially target IL-10.
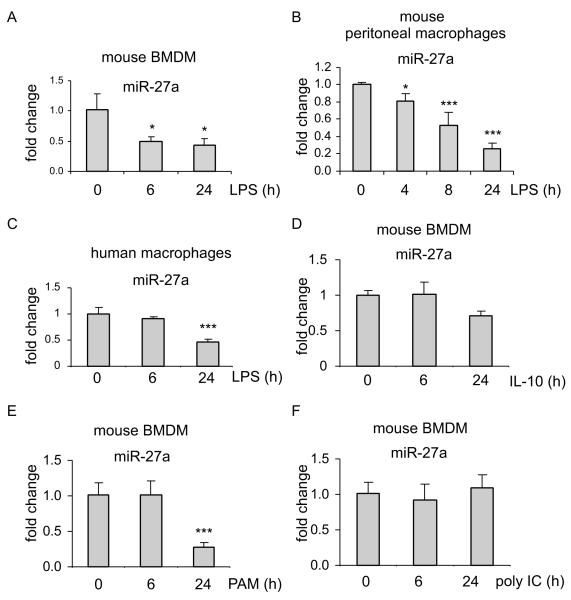
We first validated the expression of miR-27a by real-time PCR and found that miR-27a expression is indeed downregulated in LPS treated mouse BMDMs (Figure 1A). To determine whether miR-27a downregulation is a response that only occurs in BMDMs or is a more general phenomenon, we treated mouse peritoneal macrophages with LPS and found that miR-27a is also downregulated after LPS exposure (Figure 1B). Furthermore, miR-27a expression was decreased in LPS treated human macrophages (Figure 1C). These data suggest that miR-27a downregulation is a response to LPS stimulation shared by all types of macrophages.
Figure 1. miR-27a is downregulated in macrophages after TLR4 and TLR2 stimulation.
(A-C) mouse BMDMs (A), mouse peritoneal macrophages (B), or human PBMC derived macrophages (C) were treated with 100 ng/ml LPS for the indicated length of time. Total RNA in the cells were isolated and levels of miR-27a were determined by real-time PCR assay. *p<0.05, **p<0.01, ***p<0.001 compared to time “0”. (D) BMDMs were treated with 2 ng/ml IL-10 for the indicated length of time. Levels of miR-27a were determined by real-time PCR assay. (E-F) BMDMs were treated with 1 μg/ml TLR2 ligand, PAM, or 1 μg/ml TLR3 ligand, poly I:C (F) for the indicated length of time. Levels of miR-27a were determined by real-time PCR assay. Representative of two experiments are shown.
Because IL-10 is induced by LPS stimulation, and has been shown to regulate the expression of several miRNAs (25-30), we asked if miR-27a is subject to similar regulation by IL-10. We found no change in miR-27a expression in macrophages treated with IL-10 for 6h (Figure 1D). These data suggest that miR-27a downregulation at early time points after LPS treatment is unlikely to be an autocrine effect of IL-10. However, IL-10 treatment for 24h decreased miR-27a level in macrophages (Figure 1D), suggesting that IL-10 may still contribute to miR-27a downregulation at later time points after LPS treatment.
To determine if activation of other TLRs also downregulates miR-27a, we treated macrophages with the synthetic TLR2 ligand, Pam(3)CSK(4) (PAM), and the TLR3 ligand, poly I:C, and found that treatment with PAM, but not poly:IC, decreased the expression of miR-27a in macrophages (Figure 1E and 1F).
Upregulation of miR-27a enhances inflammatory response of macrophages after TLR4 stimulation
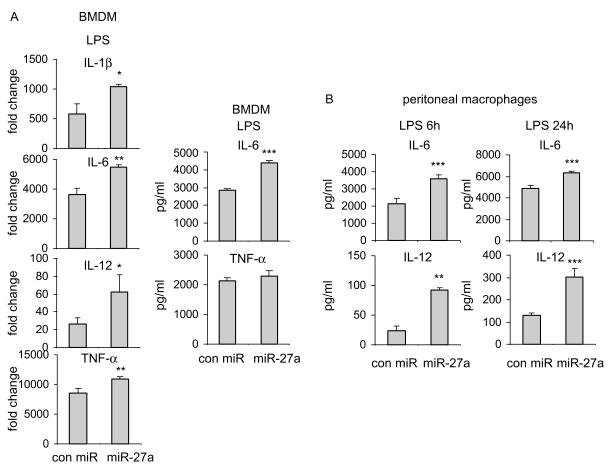
In our initial experiments, we found that miR-27a is downregulated in LPS treated macrophages. These data suggest that miR-27a may participate in inflammatory responses associated with TLR4 activation. To test this hypothesis, we increased the level of miR-27a in BMDMs by transfecting the cells with miR-27a mimics. We found that upregulation of miR-27a increases LPS induced expression of pro-inflammatory cytokines, including IL-1β, IL-6, IL-12, and TNF-α, compared to that found in BMDMs transfected with control mimics (Figure 2A). Consistent with the findings in BMDMs, upregulation of miR-27a also enhanced LPS induced expression of IL-6 and IL-12 in peritoneal macrophages (Figure 2B). To rule out the possibility of non-specific effects caused by impurity of LPS from Escherichia coli 0111:B4, we performed the same experiments using ultra-pure LPS from Salmonella minnesota R595. As shown in Supplementary Figures 1A and 1B and consistent with those found with LPS from Escherichia coli 0111:B4, upregulation of miR-27a increased ultra-pure LPS induced expression of pro-inflammatory cytokines, including IL-6, IL-12, and TNF-α, compared to that found in BMDMs transfected with control mimics. These data suggest that miR-27a is a positive regulator of TLR4 induced inflammatory response.
Figure 2. Upregulation of miR-27a enhances inflammatory response of macrophages after TLR4 stimulation.
(A) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS for 6h. The mRNA and protein levels of pro-inflammatory cytokines, IL-1β, IL-6, IL-12 and TNF-α, were determined by real-time PCR and ELISA assays. (B) Mouse peritoneal macrophages were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS for 6h or 24h. The protein levels of IL-6 and IL-12 were determined by ELISA assays. *p<0.05, **p<0.01, ***p<0.001 compared to the “con miR” group. Experiments were done 2-3 times with similar results obtained.
Upregulation of miR-27a enhances inflammatory response to TLR2 stimulation
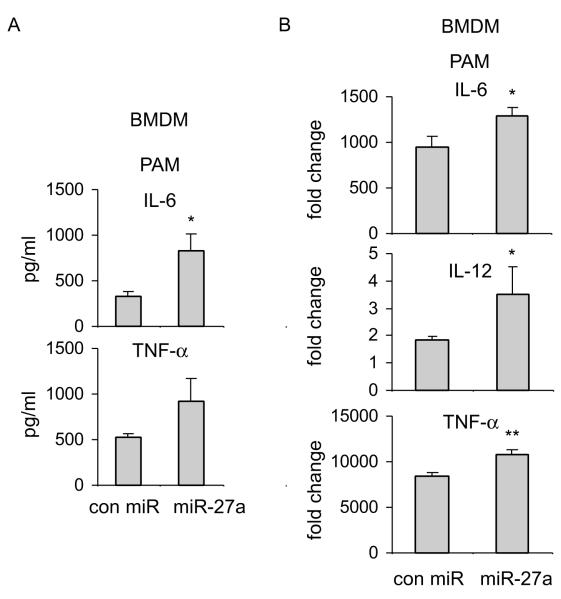
Because miR-27a is also downregulated in PAM treated macrophages, we next investigated if miR-27a participates in TLR2 induced inflammatory response. As shown in Figures 3A and 3B, upregulation of miR-27a enhanced the expression of pro-inflammatory cytokines, including IL-6, IL-12 and TNF-α, compared to that found in PAM treated macrophages infected with control mimics. Taken together, these data suggest that miR-27a regulates the immune response after both TLR4 and TLR2 engagement.
Figure 3. Upregulation of miR-27a enhances inflammatory response to TLR2 stimulation.
BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 1 μg/ml PAM for 6h. The mRNA and protein levels of IL-6, IL-12 and TNF-α, were determined by ELISA assays (A) and real-time PCR (B). *p<0.05 compared to the “con miR” group. Experiments were done 2-3 times with similar results obtained.
Downregulation of miR-27a attenuates inflammatory response to TLR4 stimulation
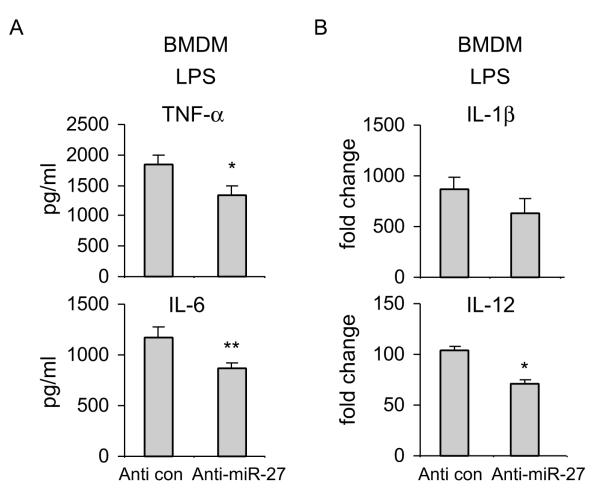
Since we have found that upregulation of miR-27a increases the inflammatory response to TLR4 activation, we asked if downregulation of miR-27a would act in an opposite manner. As shown in Figure 4A and 4B, downregulation of miR-27a by anti-miR-27a inhibitors diminished LPS induced expression of TNF-α and IL-6 in macrophages, as compared to that found in cells transfected with control inhibitors. These data provide further evidence that miR-27a is a positive regulator of inflammatory response in macrophages.
Figure 4. Downregulation of miR-27a attenuates inflammatory response to TLR4 stimulation.
BMDMs were transfected with 20 nM control inhibitors or specific anti-miR-27a inhibitors. 48h after transfection, cells were treated with 100 ng/ml LPS for 6h. The mRNA and protein levels of IL-1β, IL-6, IL-12 and TNF-α, were determined by real-time PCR and ELISA assays. *p<0.05, **p<0.01 compared to the “Anti con” group. Experiments were done twice with similar results obtained.
miR-27a negatively regulates the expression of IL-10
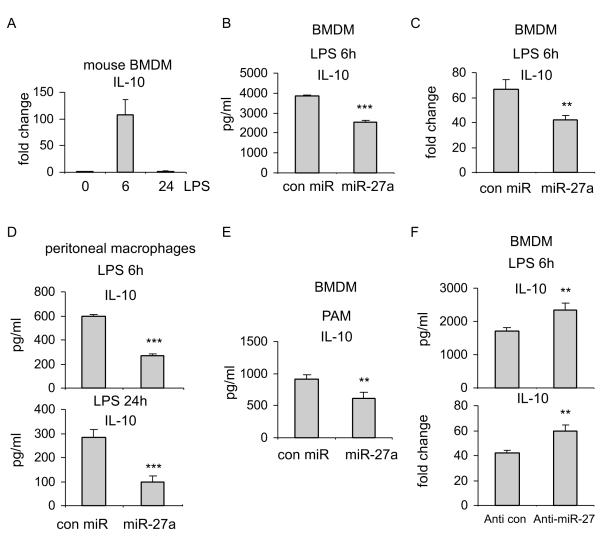
We have found that miR-27a is downregulated in TLR4 and TLR2 activated macrophages. Furthermore, miR-27a downregulation diminished the inflammatory response to TLR4 activation. These data suggest that miR-27a downregulation may be a negative feedback mechanism in macrophages that prevents overly activated inflammatory responses. Because IL-10 is one of the most important anti-inflammatory mediators (7, 8), we asked if miR-27a exerts its effect on immune response through regulating IL-10. We showed that IL-10 expression is upregulated early in LPS treated macrophages (Figure 5A), in line with the decreased expression of miR-27a at the same time point (Figures 1A and 1B). Of more importance, upregulation of IL-10 in LPS treated BMDMs was diminished by miR-27a, at both the protein and the mRNA levels (Figures 5B and 5C and Supplementary Figure 1C). Additionally, miR-27a also downregulated IL-10 expression in LPS treated peritoneal macrophages (Figure 5D). Similar to its effect in LPS stimulated cells, miR-27a decreased IL-10 expression in PAM treated macrophages (Figure 5E). These data suggest that miR-27a is a negative regulator of IL-10. Consistent with the findings with miR-27a upregulation, downregulation of miR-27a enhanced the expression of IL-10 in LPS treated macrophages (Figure 5F).
Figure 5. miR-27a negatively regulates the expression of IL-10.
(A) BMDMs were treated with 100 ng/ml LPS for the indicated length of time. Total RNA in the cells were isolated and levels of IL-10 were determined by real-time PCR assay. (B-C) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS for 6h. The protein (B) and the mRNA (C) levels of IL-10 were determined by real-time PCR and ELISA assays. **p<0.01 compared to the “con miR” group. (D) Peritoneal macrophages were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS for 6h or 24h. The protein levels of IL-10 were determined by ELISA assays. ***p<0.001 compared to the “con miR” group. (E) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 1 μg/ml PAM for 6h. The protein levels of IL-10 were determined by ELISA assays. **p<0.01 compared to the “con miR” group. (F) BMDMs were transfected with 20 nM control inhibitors or specific anti-miR-27a inhibitors. 48h after transfection, the cells were treated with 100 ng/ml LPS for 6h. The protein and the mRNA levels of IL-10 were determined by real-time PCR and ELISA assays, respectively. **p<0.01 compared to the “Anti con” group. Experiments were done twice with similar results obtained.
miR-27a targets IL-10
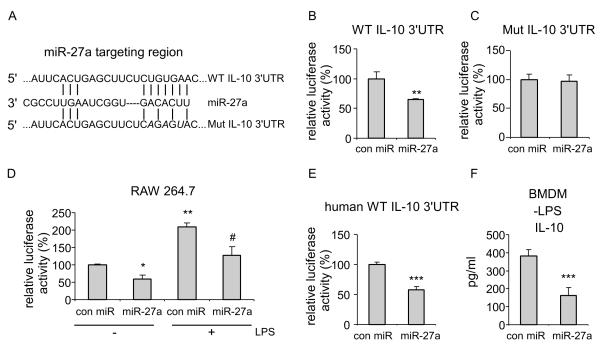
As we have shown that miR-27a decreases IL-10 expression, we asked if miR-27a does so by targeting IL-10 3′ UTR, a general mechanism by which miRNAs regulate target expression (12-16). Using TargetScan and miRWalk (31), computational programs predicting miRNA targets, we identified a site at the 3′ UTR of the IL-10 transcript that is predicted to be targeted by miR-27a (Figure 6A), with a p value of the miRNA/target association less than 0.05. We cloned the 3′ UTR of the IL-10 mRNA into a luciferase reporter vector downstream of the luciferase gene. We also created mutations in the 3′ UTR of the IL-10 mRNA at the site targeted by miR-27a (Figure 6A) and cloned the mutant 3′ UTR into the same luciferase reporter. We found that miR-27a decreases the activity of the luciferase reporter that contains wild-type 3′ UTR of IL-10 mRNA (Figure 6B). However, miR-27a had no effect on the activity of a luciferase reporter that contains the mutant 3′ UTR of IL-10 mRNA (Figure 6C). To determine if LPS enhancing IL-10 expression is mediated, at least in part, by regulating the 3′ UTR of IL-10, we performed the luciferase reporter assay in macrophage cell line, RAW 264.7. As shown in Figure 6D, miR-27a decreased the activity of the luciferase reporter that contains wild-type 3′ UTR of IL-10 mRNA in untreated RAW cells, consistent with those found in HEK-293 cells. More importantly, we found that LPS treatment enhances the activity of the luciferase reporter (Figure 6D), consistent with decreased expression of miR-27a in LPS treated macrophages. Such enhanced activity of the luciferase reporter in LPS treated RAW cells was found again to be diminished by miR-27a mimics (Figure 6D). miR-27a also decreased the activity of the luciferase reporter that contains human wild-type 3′ UTR of IL-10 mRNA (Figure 6E), suggesting the conservation of IL-10 targeting by miR-27a across species. Of note, miR-27a also downregulated basal expression of IL-10 in BMDMs (Figure 6F). These data suggest that IL-10 is a direct target of miR-27a.
Figure 6. miR-27a targets IL-10.
(A) Schematic illustration of the miR-27a targeting site at the 3′ UTR of the mouse IL-10 gene. The created point mutations were depicted in italic letters. (B) HEK-293 cells were transfected with 20 nM control mimics or specific miR-27a mimics. 6h after transfection, cells were transfected again with 5 ng pMir-Report luciferase constructs that contain the wild-type IL-10 3′UTR. Two days after transfection, the cells were lysed and luciferase activity measured. The absolute luciferase activity in the “con miR” group was regarded as “100%”. **p<0.01 compared to the “con miR” group. (C) HEK-293 cells were transfected with 20 nM control mimics or specific miR-27a mimics. 6h after transfection, cells were transfected again with 5 ng pMir-Report luciferase constructs that contain the mutant IL-10 3′UTR. Two days after transfection, Luciferase activity in the cells was measured. Experiments were done twice with similar results obtained. (D) RAW 264.7 cells were transfected with 20 nM control mimics or specific miR-27a mimics. 6h after transfection, cells were transfected again with 100 ng pMir-Report luciferase constructs that contain the wild-type IL-10 3′UTR. Two days after transfection, the cells were treated without or with ultrapure LPS for 6h. Cells were then lysed and luciferase activity measured. The absolute luciferase activity in the untreated “con miR” group was regarded as “100%”. *p<0.05, **p<0.01 compared to the untreated “con miR” group. # p<0.05 compared to the LPS treated “con miR” group. (E) Experiments were performed as in “A” except for the pMir-Report luciferase construct that contained the human wild-type IL-10 3′UTR. ***p<0.001 compared to the “con miR” group. (F) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, protein levels of IL-10 in the supernatants were determined by ELISA assay. ***p<0.001 compared to the “con miR” group.
miR-27a regulates IL-10 dependent signaling event in LPS treated macrophages
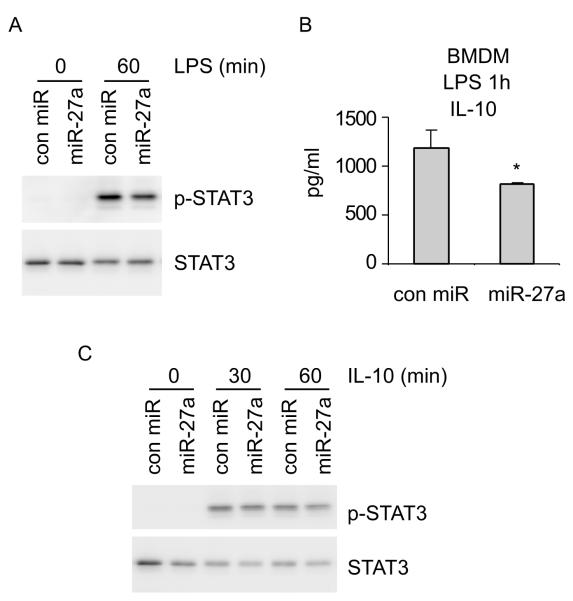
It has been previously shown that LPS induced phosphorylation of STAT3 is dependent on IL-10 (32). Because we found that miR-27a diminishes LPS induced IL-10 expression, we asked if miR-27a regulates STAT3 phosphorylation in LPS treated macrophages. As shown in Figure 7A, upregulation of miR-27a diminished LPS induced STAT3 phosphorylation. We reasoned that downregulation of STAT3 phosphorylation by miR-27a in LPS treated macrophages is likely due to decreased IL-10 expression in the same cells. We first confirmed that miR-27a does indeed decrease IL-10 expression at one hour after LPS treatment (Figure 7B). However, there remains the possibility that the diminished STAT3 phosphorylation in LPS treated macrophages may be caused by miR-27a targeting IL-10 signaling events. To test this hypothesis, we examined if miR-27a affects IL-10 induced downstream events. As shown in Figure 7C, miR-27a had no effect on IL-10 induced STAT3 phosphorylation. Taken together, these data suggest that miR-27a selectively targets IL-10 expression, but not IL-10 downstream signaling events, to limit the anti-inflammatory capacity of macrophages.
Figure 7. miR-27a regulates IL-10 dependent signaling event in LPS treated macrophages.
(A) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS for 0 and 1h. Cells were then harvested and levels of p-STAT3 and total STAT3 were determined by Western blotting. (B) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS for 1h. Levels of IL-10 were determined by ELISA. *p<0.05 compared to the “con miR” group. (C) BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 2 ng/ml IL-10 for 0, 1, and 2h. Levels of p-STAT3 and total STAT3 were determined by Western blotting. Experiments were done twice with similar results obtained.
Blocking IL-10 abolishes the regulation of inflammatory response by miR-27a in macrophages
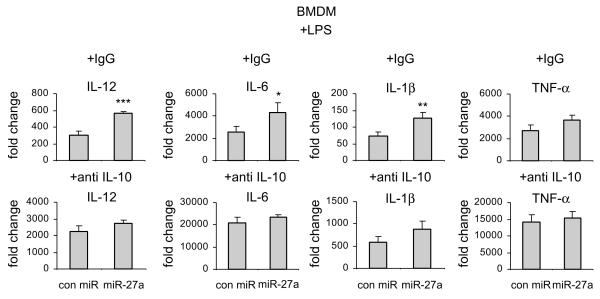
The findings above suggest that the mechanism by which miR-27a regulates immune responses in macrophages is through targeting IL-10. If this is true, blocking IL-10 should abolish the effects of miR-27a. To test this hypothesis, we treated miR-27a mimics transfected macrophages with anti-IL-10 blocking antibody or control isotype IgG. As shown in Figure 8, upregulation of miR-27a enhanced LPS induced expression of IL-12, IL-6, IL-1β, and TNF-α in control isotype IgG treated macrophages. Anti-IL-10 antibody enhanced LPS induced expression of these pro-inflammatory cytokines, consistent with IL-10 acting as an anti-inflammatory molecule (Figure 8). More importantly, the enhancement of LPS induced expression of IL-12, IL-6, IL-1β, and TNF-α in macrophages with miR-27 overexpression was attenuated by anti-IL-10 antibodies (Figure 8). Taken together, these data suggest that regulation of inflammatory response by miR-27a is mediated, at least partially, by IL-10.
Figure 8. Blocking IL-10 abolishes the regulation of inflammatory response by miR-27a in macrophages.
BMDMs were transfected with 20 nM control mimics or miR-27a mimics. 48h after transfection, cells were treated with 100 ng/ml LPS, plus 2.5 μg/ml control isotype IgG or anti-IL-10 antibody for 6h. The levels of IL-12, IL-6, IL-1β and TNF-α were determined by real-time PCR. *p<0.05, **p<0.01, ***p<0.001 compared to the “con miR” group. Experiments were done twice with similar results obtained.
Discussion
Inflammatory responses mediated by TLR2 and TLR4 play a crucial role in combating bacterial infection (1-4). However, excessive inflammation is a major causal factor that leads to clinically important morbidities in infected patients (1-4). The immune system is equipped with a wide range of negative regulatory mechanisms that dampen inappropriately excessive inflammatory responses at multiple levels in the activation process (1-4). These include transcriptional factors and co-repressors, protein modification enzymes and RNA binding proteins.
miRNAs have been shown to be major regulators in innate immune response (17-20). However, only a limited number of miRNAs have been identified to function in negative feedback loops that ameliorate inflammation. In this study, we found that miR-27a is downregulated in macrophages activated through TLR2 or TLR4. Downregulation of miR-27a diminishes activation of macrophages by TLR2/4 stimulation and coincides with increased IL-10 expression. Therefore, miR-27a downregulation appears to be a prerequisite to keep inflammatory response under control during macrophage activation.
IL-10 is one of the most important anti-inflammatory mediators (7, 8). Dysregulation of IL-10 has been shown to participate in inflammatory pathologies (7, 8). Therefore, appropriate regulation of IL-10 expression appears required in order to maintain immunological homeostasis (7, 8, 10). IL-10 expression is subject to both transcriptional and translational regulation (7, 8). For example, Sp1 and Sp3 play an indispensable role in controlling IL-10 expression at the transcriptional level. In addition, IL-10 expression is subject to translational regulation through adenylate-uridylate-rich (AU-rich) elements in the 3′ UTR of the IL-10 mRNA (7, 8). However, additional mechanisms that participate in the post-transcriptional control of IL-10 expression are still waiting to be elucidated. In this study, we identified miR-27a as a negative regulator of IL-10 expression, and highlight the complexity of the immunoregulatory network involved in modulation of IL-10 expression. It needs to be noted that IL-10 was returned to the basal level at the late time point of LPS treatment when miR-27a expression was still suppressed. These data suggest that miR-27a is not a predominant regulator of IL-10 expression at the late stage of inflammatory response and downregulation of miR-27a is insufficient for sustainably high IL-10 expression.
Binding of IL-10 to its receptor results in activation of the JAK1-STAT3 pathway (33). The transcriptional factor, STAT3, then enters nucleus to diminish inflammatory response through incompletely characterized mechanisms (7, 8). We found that miR-27a upregulation decreases STAT3 phosphorylation in LPS treated macrophages, and concluded that such decrease in STAT3 phosphorylation is due to reduced IL-10 expression caused by miR-27a upregulation. This conclusion is supported by the finding that miR-27a upregulation does not affect STAT3 phosphorylation in macrophages treated with exogenous IL-10. These data also suggest that inhibition of IL-10 expression is directly responsible for the ability of miR-27a to enhance inflammatory responses.
Although miR-27a decreases IL-10 expression in LPS treated macrophages, the increase in the expression of pro-inflammatory cytokines by upregulation of miR-27a is smaller than that induced by IL-10 blocking antibody. This can be explained by the finding that miR-27a only partially decreases, but does not completely block the expression of IL-10 in LPS treated macrophages. However, anti-IL-10 antibodies at the concentration used in these studies are able to completely block secreted IL-10 in LPS treated macrophages (data not shown).
Upregulation of miR-27a has been found in various types of cancers and is often associated with adverse outcome from malignancy (34-37). A chronic inflammatory micro-environment is a risk factor that contributes to cell transformation, proliferation and resistance to programmed cell death (38, 39). Given that miR-27a is a positive regulator of inflammation, it is possible that miR-27a functions as an oncomir by promoting a deleteriously proinflammatory environment that drives pathological progression of cancer. Importantly, our study lends further evidence to the correlation between inflammatory diseases and cancer (38, 39).
In summary, our study identified miR-27a as a potential therapeutic target for treatment of inflammatory diseases and cancer. Our data also provide solid rationale for future studies that investigate correlations between the expression of miR-27a and/or single-nucleotide polymorphisms of the miR-27a gene and clinicopathological features of inflammatory diseases.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL114470 (to V. J. T.), HL105473 and HL076206 (to G. L.).
Grant support: NIH grants HL114470, HL105473 and HL076206.
Footnotes
Author contributions: GL designed the study; NX, HC, SB, ZT performed the experiments; MF, RS, EA, VJT and GL wrote the manuscript.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 4.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008;20:530–537. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Salomao R, Brunialti MK, Rapozo MM, Baggio-Zappia GL, Galanos C, Freudenberg M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock. 2012;38:227–242. doi: 10.1097/SHK.0b013e318262c4b0. [DOI] [PubMed] [Google Scholar]
- 6.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 7.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, Libby P, Hansson GK, Shortman K, Dong C, Gabrilovich D, Gabrysova L, Howes A, O’Garra A. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol. 2011;11:693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ, Smale ST. Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nat Immunol. 2012;13:916–924. doi: 10.1038/ni.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Zeng Y. Principles of micro-RNA production and maturation. Oncogene. 2006;25:6156–6162. doi: 10.1038/sj.onc.1209908. [DOI] [PubMed] [Google Scholar]
- 14.Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25:6163–6169. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- 15.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 16.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:170–177. doi: 10.1161/ATVBAHA.112.300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 21.Alam MM, O’Neill LA. MicroRNAs and the resolution phase of inflammation in macrophages. Eur J Immunol. 2011;41:2482–2485. doi: 10.1002/eji.201141740. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542–6549. doi: 10.4049/jimmunol.1202496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, Thannickal VJ, Abraham E, Liu G. miR-125a-5p Regulates Differential Activation of Macrophages and Inflammation. Journal of Biological Chemistry. 2013;288:35428–35436. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossato M, Curtale G, Tamassia N, Castellucci M, Mori L, Gasperini S, Mariotti B, De Luca M, Mirolo M, Cassatella MA, Locati M, Bazzoni F. IL-10-induced microRNA-187 negatively regulates TNF-alpha, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proc Natl Acad Sci U S A. 2012;109:E3101–3110. doi: 10.1073/pnas.1209100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung ST, So EY, Chang D, Ming-Lum A, Mui AL. Interleukin-10 inhibits lipopolysaccharide induced miR-155 precursor stability and maturation. PLoS One. 2013;8:e71336. doi: 10.1371/journal.pone.0071336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Hou J, Ma F, Wang P, Liu X, Li N, Wang J, Wang Q, Cao X. Type I IFN inhibits innate IL-10 production in macrophages through histone deacetylase 11 by downregulating microRNA-145. J Immunol. 2013;191:3896–3904. doi: 10.4049/jimmunol.1203450. [DOI] [PubMed] [Google Scholar]
- 28.Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci U S A. 2013;110:11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer JS, Montufar-Solis D, Vigneswaran N, Klein JR. Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10−/− mice precedes expression in the colon. J Immunol. 2011;187:5834–5841. doi: 10.4049/jimmunol.1100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCoy CE, Sheedy FJ, Qualls JE, Doyle SL, Quinn SR, Murray PJ, O’Neill LA. IL-10 inhibits miR-155 induction by toll-like receptors. J Biol Chem. 2010;285:20492–20498. doi: 10.1074/jbc.M110.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Pattison MJ, Mackenzie KF, Arthur JS. Inhibition of JAKs in macrophages increases lipopolysaccharide-induced cytokine production by blocking IL-10-mediated feedback. J Immunol. 2012;189:2784–2792. doi: 10.4049/jimmunol.1200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Jie Z, Ye S, Li Z, Han Z, Wu J, Yang C, Jiang Y. Genetic variations in miR-27a gene decrease mature miR-27a level and reduce gastric cancer susceptibility. Oncogene. 2012 doi: 10.1038/onc.2012.569. [DOI] [PubMed] [Google Scholar]
- 35.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Li DC, Li ZF, Liu CX, Xiao YM, Zhang B, Li XD, Zhao J, Chen LP, Xing XM, Tang SF, Lin YC, Lai YD, Yang P, Zeng JL, Xiao Q, Zeng XW, Lin ZN, Zhuang ZX, Zhuang SM, Chen W. Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen. Oncogene. 2011;30:3875–3886. doi: 10.1038/onc.2011.103. [DOI] [PubMed] [Google Scholar]
- 37.Jones KB, Salah Z, Del Mare S, Galasso M, Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, Volinia S, Stein GS, Croce CM, Lian JB, Aqeilan RI. miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012;72:1865–1877. doi: 10.1158/0008-5472.CAN-11-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.