Abstract
The adaptor proteins Crk, including CrkI, CrkII, and CrkL, are important signal molecules that regulate a variety of cellular processes. Considerable progress has been made in understanding the roles of the Crk family proteins in signal transduction, with a focus on cellular transformation and differentiation. However, since Crk was identified in 1988, very few studies have addressed how Crk regulates the immune response. Recent work demonstrates that Crk proteins function as critical signal molecules in regulating immune cell functions. Emerging data on the roles of Crk in activation and inhibitory immunoreceptors signaling suggest that Crk proteins are potential immunotherapeutic targets in cancer and infectious diseases. The aim of this review is to summarize recent key findings regarding the role of Crk in immune responses mediated by T, B, and natural killer (NK) cells. In particular, the roles of Crk in NK cells function are discussed.
Keywords: Immune response, Adaptor protein, Natural killer cell, CT10 regulator of kinase, Inhibitory receptor, Cytotoxicity
INTRODUCTION
The v-crk oncogene was isolated by two separate groups: Mayer et al.1 from chicken tumor virus no.10 (CT10) retrovirus, and Tsuchie et al.2 from avian sarcoma virus (ASV1). Based on its ability to increase cellular tyrosine phosphorylation and transform primary chicken embryo fibroblasts, Mayer et al.1 named this new oncogene crk, for CT10 regulator of kinase, and the oncogene product p47gag-crk.3 Three years later, Reichman et al.4 cloned and sequenced a complementary DNA encoding the cellular homologue of the transforming oncogene v-crk of avian sarcoma virus CT10. Following the sequencing of the cellular Crk (c-Crk) in chickens,4 two isoforms of Crk (CrkI and CrkII, hereinafter referred to as ‘Crk’) and Crk-like (CrkL), which are encoded by separate genes, were identified in humans by two different groups.5, 6 Since then, extensive studies have been focused on the roles played by Crk in cell growth, motility, apoptosis, transcription, and transformation in the field of cell biology.7–9 Finding the proteins that interact with Crk in normal or transformed fibroblasts has represented a main effort during the past three decades. Several reviews have discussed the physiological and pathological function of Crk,8, 10, 11 signaling complex formation,7, 12 roles of Crk in human cancer,13 and roles of Crk in cytoskeletal actin dynamics.14 Few studies on Crk have discussed the function of Crk in lymphocytes.15, 16 The field of immunology now needs to direct attention to the role of Crk in the regulation of lymphocytes.
The interactions between Crk/CrkL and other signal proteins have been discussed already in other reviews.7, 8, 16 This review focuses on the roles of Crk and CrkL in immune responses mediated by T, B, and NK cells, with a focus on human NK cells. This review divides into seven main parts: (1) the structure of the Crk family proteins, (2) the regulation of Crk activities, (3) the role of Crk in regulating T cell functions, (4) the role of Crk in regulating B cell functions, (5) the role of Crk in NK cell functions, (6) the role of Crk in other immune cell functions, and (7) perspectives. The main points that this review addresses are that:
Crk not only plays numerous roles in cell biology but also Crk has several specific roles in regulating lymphocyte functions.
Our understanding of Crk has been advanced by the recent published studies on the roles of Crk in human primary NK cells, of which Crk may function as a 2-way molecular switch to control NK cell-mediated cytotoxicity.
THE STRUCTURE OF THE CRK FAMILY PROTEINS
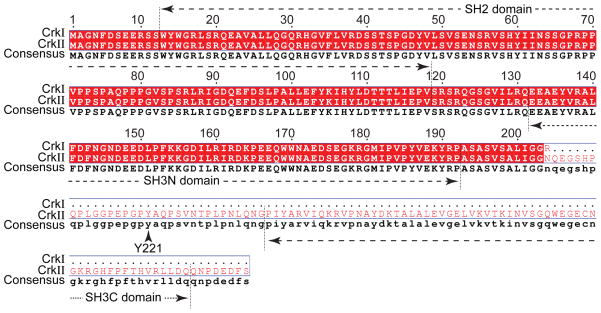
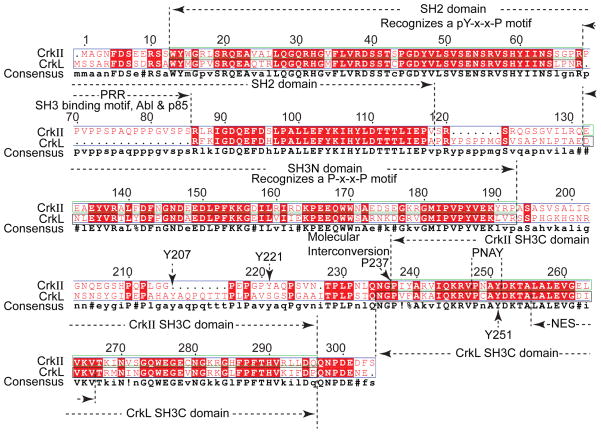
In 1992, Matsuda et al.5 isolated two species of human Crk cDNAs, named CrkI and CrkII, from human embryonic lung cells. CrkII is located on chromosome 17p13.3 in humans.4, 5 In 1993, ten Hoeve et al.6 identified the Crk-like gene, CrkL. CrkL is located on chromosome 22q11.21 in humans.6 The CrkL gene product is predicted to have a molecular mass of 36 kDa.6 CrkI (28 kDa), an alternately spliced form of CrkII (40 kDa), contains only one Src homology 2 (SH2) and one Src homology 3 (SH3) domain illustrated by multiple sequence alignment17, as shown in Figure 1. CrkI lacks the regulatory phosphorylation site and the c-terminal SH3 domain. CrkII and CrkL proteins contain one SH2 domain and two SH3 domains, named SH3N (N-terminal SH3 domain) and SH3C (C-terminal SH3 domain), respectively, as shown in Figure 2. A comparison of the structures of CrkII and CrkL reveals that the SH2 domain of CrkII has an extra stretch of 17 amino acids (N′-PPVPPSPAQPPPGVSPS-C′) that is rich in proline residues (proline-rich region, PRR), as shown in Figure 3. According to the nuclear magnetic resonance (NMR) structural studies by Kobashigawa et al.18, the PRR is located on the CrkII’s outer surface. The PRR is a specific SH3 domain-binding motif, such as Abelson murine leukemia kinase (Abl) and p85.19 However, this finding cannot explain the observation that CrkL (which possesses an SH2 domain, but without PRR in SH2), but not CrkII, is a preferred substrate of the breakpoint cluster region (Bcr)-Abl protein tyrosine kinases (PTKs) in Bcr-Abl-transformed cells.6, 20, 21 Recent studies by Jankowski et al.22 reveal that CrkL forms a constitutive complex with Abl because of different domain organizations between CrkL and CrkII, which may explain the strong preference of Bcr-Abl for CrkL. Table 1 summarizes the comparison of CrkII and CrkL in different aspects. Based on comparisons of the structures of CrkII and CrkL and their regulatory mechanisms, the consensus is that CrkII and CrkL each plays its own essential role in the regulation of cell function. CrkII and CrkL may not have redundant functions and cannot be compensated by each other. Recently, Prosser et al.,23 using the cloning of receptor targets (CORT) technique, identified a novel member of the Crk family proteins, CrkIII that contains a truncated SH3C. In summary, Crk family proteins are relatively small proteins that contain both SH2 and SH3 domains; however, the functions of the different domains are controversial and complex, as detailed below.
Figure 1. Comparison of CrkI and CrkII in sequence.
CrkI and CrkII sequences are aligned by Multalin version 5.4.1 software (http://multalin.toulouse.inra.fr/multalin/). The consensus sequences are marked in Red.
Figure 2. Domains of Crk and its binding partners.
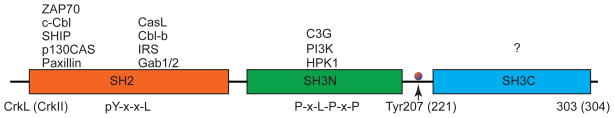
Schematic diagram of the domains of CrkL (303 aa) and CrkII (304 aa). The binding partners of SH2 and SH3 domains are listed on the top.
Figure 3. Domain Comparison of CrkL and CrkII.
Sequence alignment of CrkL and CrkII by Multalin version 5.4.1 (http://multalin.toulouse.inra.fr/multalin/). The consensus symbols are as follows: ! is anyone of IV. % is anyone of FY. # is anyone of NDQEBZ.
NES = nuclear export signal. PRR = proline-rich region.
Table 1.
Comparison of human CrkII and CrkL
| CrkII | CrkL | |
|---|---|---|
| Gene | CRK | CRKL |
| Gene location | Chromosome 17p13.3 | Chromosome 22q11.21 |
| Structure of un-phosphorylated Crk | Binding to Bcr-Abl in a repressed structure Full accessible SH2 binding site Masked SH3N binding site SH3C interacts extensively with other SH2 |
Constitutively associates with Bcr-Abl Partially masked SH2 binding site Fully accessible SH3N binding site Independent SH3C domain |
| Bcr-Abl binding to phosphorylated Crk | Abrogated | Not abrogated |
| SH3N accessibility in phosphorylated Crk | Masked | Fully accessible |
| Function in transforming fibroblasts | does not transform fibroblasts. | Transforms fibroblasts. |
| Number of amine acid (aa) | 304 | 303 |
Similarities:
| ||
THE REGULATION OF CRK ACTIVITIES
Generally, the intracellular signaling by Crk proteins is mediated by the interaction of the SH2 domain with phosphotyrosine residues, as well as the interaction of the SH3 domain with PRRs. Because Crk proteins lack the intrinsic catalytic activity that kinases possess, Crk proteins usually are classified as the adaptor protein family. Functionally, CrkI exhibits more transforming activity than does CrkII, as evidenced by the important observation that the cell lines expressing the CrkI protein grow as massive tumors in nude mice, whereas CrkII-expressing cells do not grow in nude mice.5 Several studies show that the SH3C domain and the linker region in CrkII are responsible for this difference, in which the SH3C domain can downregulate CrkII activity.18, 24–26 As for the regulation of CrkII and CrkL, the control of CrkII and CrkL activity is multilayered, as summarized as follows:
The first layer of regulation is mediated by phosphorylation at Tyrosine 207 (Tyr207) in CrkL and Tyrosine 211 (Tyr211) in CrkII.7 In 1994, Feller et al.27 proposed that the phosphorylated Tyr221 of CrkII could bind to its own SH2 domain, which prevents the SH3N of CrkII from binding other proteins or signal molecules. Tyr207 in CrkL is phosphorylated by Abl kinase.28, 29 Upon phosphorylation of CrkL at Tyr207, the SH2 domain interacts with the pTyr207-X-X-P region in the same molecule,22 similar to CrkII.18, 30 Consistent with this concept, using a fluorescence resonance energy transfer-based probe by sandwiching a truncated form of CrkL with a variant of yellow fluorescent protein (YFP), also called Venus, and an enhanced cyan fluorescent protein (ECFP), Mizutani et al.31 demonstrated that intramolecular binding of the SH2 domain to phosphorylated Tyr 207 in CrkL increases FRET efficiency. A similar result is observed in CrkII by fusing YFP and CFP with the N- and C-termini of a truncated Crkll molecule.32 Hofmann et al.33 and Cotton et al.,34 using the site-specific chemical modification of protein to label the N- and C-termini of a truncated CrkII molecule (lacking the regulatory C-terminal SH3 domain), observed a decrease in the fluorescence resonance energy transfer efficiency (indicating an increase in distance between SH2 and SH3N domains) upon phosphorylation of the truncated CrkII by Abl. Binding pTyr207 to its own SH2 domain results in preventing the SH2 domain of CrkL from interacting with other phosphorylated signaling molecules, such as paxillin,22 which indicates that phosphorylated CrkL may function as a negative regulator of signaling pathway, This concept is in line with studies from human NK cells, in which phosphorylated Crk dissociates from signal molecules a 125–135 kDa scaffold protein (p130) Crk-associated substrate (p130Cas) and Casitas B-lineage lymphoma (Cbl) protein during NK cells inhibition induced by engagement of two structurally distinct inhibitory receptors, CD94-NKG2A and killer-cell immunoglobulin-like receptor (KIR).35 Therefore, pTyr207/221 and the SH2 domain collaboratively function as the first layer of regulation. However, when and where phosphorylated Crk dissociates with signal molecules at the immunological synapses during inhibition in immune cells remains unclear.
The second layer of regulation is mediated by the regulatory SH3C domain. In general, SH3C functions as a negative regulatory unit because the SH3C contains elements that constrain binding to the SH2 and SH3N domains.18, 36 Indeed, CrkI, which lacks the SH3C domain, shows more transforming activity than does CrkII, which may involve phosphorylation of Y221 in CrkII and the SH3C domain.8, 25 However, a recent study demonstrated that the SH3C domain has positive effects via phosphorylation of Tyr251 residue in the RT-loop structure (a loop in SH3 structure named after arginine, R, and threonine, T residues), as shown in Figure 3. Phosphorylation of Tyr251 in a conserved PNAY sequence by Abl in vitro enhances activation of Abl by providing binding sites for the SH2/PTB (phosphotyrosine-binding domain) containing proteins.37, 38 In contrast to this observation, Jankowski et al.22 used molecular mass determination to demonstrate that CrkL exists as a monomer in solution and that only one Tyrosine site (Tyr207) is phosphorylated by Abl kinase in vitro. The more detailed discussion of the regulatory role of SH3C has been described.8 In summary, the regulatory SH3C domain provides the second layer of regulation, and the exact role of SH3C in regulating CrkII and CrkL proteins remains controversial.
The third layer of regulation is mediated by prolyl cis-trans isomerization.39 A recent finding by Sarkar et al.36, 39 demonstrates that isomerization of chicken CrkII requires the SH3N-SH3C linker region, which CrkI lacks. The authors found that a proline (Pro238 in chicken, similar to conserved residue Pro237 in humans) on the linker tethering the two SH3 domains of the Crk adaptor protein interconverts between the cis and the trans conformations (Figure 3). In the cis conformation, the two SH3 domains interact intramolecularly, forming an auto-inhibitory conformation. In this auto-inhibition, SH2 may interact with SH3 domains, and other signal molecules cannot bind to Crk. In the trans conformation, Crk exists in an extended conformation (also referred to as uninhibited conformation) that constitutes approximately 10% of total Crk molecules in the absence of any intermolecular binding partners, but it serves to activate the protein upon ligand binding. Interconversion between the cis (auto-inhibited) and trans (activated) conformations is accelerated by the action of peptidyl-prolyl isomerase A, also known as cyclophilin A (CypA).36 Functionally, phosphorylated Crk is similar to the cis form in vitro, in which Crk cannot bind to other signal molecules. Whether Crk molecules in the cis form have the same physiological outcomes as that of increased phosphorylation in the cell system or in vivo is not clear. The concept needs to be verified to determine if human CrkL or CrkII has similar proline isomerization in live cells and what receptor controls this process. Given the conserved Pro238 feature among all Crk proteins, the possibility exists that proline isomerization is present in other Crk proteins, such as CrkL or CrkII in humans. It will be of interest to examine the correlation of cis and trans conformations with the phosphorylation and un-phosphorylation of Crk in vivo and to determine how a cytosolic protein, CypA, affects the biological outcome in immune cells.
In summary, the activities of CrkII and CrkL are regulated not only by the tyrosine within the CrkII/CrkL and SH3C, but also by the key proline mediated isomerization (Pro238 in chicken), which further complicates the regulation of the Crk family proteins.
THE ROLE OF CRK IN REGULATING T-CELL FUNCTIONS
The function of Crk in T lymphocytes has been reported to form multi-protein complexes through T cell receptor (TCR) stimulation with a specific focus on the CD4+ T cell line - the Jurkat cell line. For example, Cbl becomes tyrosine-phosphorylated and associated with Crk upon TCR stimulation.40–43 The formation of complex is summarized in Table 2. The detailed interaction of Crk family proteins in lymphocytes has been reviewed by Isakov15 and Gelkop et al.16 In the following sections, several critical signal molecules involved with Crk family proteins will be classified on the basis of TCR signaling, costimulatory receptor signaling, cytokine signaling, and T cell development.
Table 2.
Proteins interact with Crk in T, B, and NK cells.
| Protein | Molecular Weight (kDa) | Stimulation | Cell Type | Biological function |
|---|---|---|---|---|
| p130cas | 130 | Stimulated with 721.221 | NK Cell | NK cell activation |
| BCR cross-linking | Ramos B Cell | B cell activation | ||
| p120-C-Cbl | 120 | NIH-3T3 fibroblasts transfected with HLA-DR7 | Human T cell clones | T cell anergy |
| SDF-1 (Stromal cell-derived factor SDF-1) | Jurkat Cell | Chemokine receptor signaling | ||
| Cbl-b | 120 | Anti-CD3 | Jurkat Cell | Negative regulator of TCR |
| Gab3 | 97 | Interleukin-2 | T, NK cell | IL-2 signaling |
| ZAP70 | 70 | Anti-CD3 | Jurkat Cell | T lymphocytes activation |
| HPK1 | 97 | Anti-CD3 | Jurkat Cell | T lymphocytes activation |
| PI3K | 85 | Anti-CD3 | Jurkat Cell | T lymphocytes activation |
| C3G | 120 | Stimulated with 721.221 | NK Cell | NK cell activation |
| BCR and integrin ligation | B Cell | B cell activation |
Crk in TCR signaling
Cbl, an E3-ligase that catalyzes protein ubiquitination,44, 45 is the first protein found to regulate TCR signaling via Crk interaction upon anti-CD3 signaling.41–43, 46 Following T cell activation, Crk binds phosphorylated Cbl and p85 (a regulatory subunit of PI3-K) with the Crk-SH2 domain and SH3N domain, respectively.47 In NIH-3T3 fibroblasts, endogenous Crk migrates similarly to a monomeric, non-complexed protein and is tyrosine-phosphorylated.27 It will be of interest to understand the state of endogenous Crk in resting primary lymphocytes. CrkL, but not Crk, is constitutively associated with C3G (Crk SH3-binding guanine nucleotide-releasing protein) in thymocytes. The association of C3G with CrkL is unaffected by CD3 stimulation of thymocytes and is not induced to associate with Crk.48 Interestingly, other studies have shown that binding of Cbl-b with CrkL interferes with its efficacy to interact with the other binding partner-C3G. C3G is also a guanine exchange factor of Rap-1 (Ras-related GTP-binding protein 1).49 Therefore, Cbl-b functions as a negative regulator of CrkL-C3G-mediated Rap1 (a member of the Ras family of small GTPases) activation in TCR-stimulated T cells.50 In both Jurkat and normal human peripheral blood-derived T cells, TCR stimulation induces the association of Cbl with all three forms of the human Crk protein, most prominently with CrkL.43 In anti-CD3-stimulated Jurkat T cells, TCR stimulation does not induce the phosphorylation of Crk or CrkL.43 In summary, Crk family proteins involve TCR signaling by forming multi-complex with other signal molecules such as C3G. However, how phosphorylated Crk regulates TCR signaling and whether TCR stimulation modulates the ratio of Crk and phosphorylated Crk remain unclear. Additional in vivo studies in primary T lymphocytes will be required to confirm the physiological function of Crk in TCR signaling and associations between Crk and other signal molecules.
Crk in T cell co-stimulatory receptor signaling
In Jurkat cells, co-receptor CD2 engagement, not by CD3 engagement, induces the association of tyrosine-phosphorylated p62, dok1 (downstream of Tyrosine Kinase a member of the Dok family of adaptor molecules) to CrkL.51 CrkL also plays an important role in lymphocyte function-associated antigen 1 (LFA-1) integrin (αLβ2) adhesion in T lymphocytes.52 In T cells, stimulation of TCR and beta1 integrin induces the association of p105CasL, a p130Cas homologous protein, with the SH2 domain of Crk or CrkL.53–55 In primary T lymphocytes, LFA-1 does not bind to its ligand ICAM-1 without TCR engagement that delivers ‘inside-out’ signals to LFA-1. The CrkL-C3G complex is required for TCR-stimulated activation of Rap1, leading to integrin adhesion.52 Similar to the roles of Crk in TCR signaling, the majority of work on the roles of Crk in T cells co-stimulatory receptors has relied on classical biochemical approaches to identify the binding partners such as p130Cas. However, the signaling pathways initiated by TCR that result in the role of Crk in integrin adhesion or other co-stimulatory receptors remain relatively obscure.
Crk in T cell cytokine signaling
Crk not only modulates the TCR and co-receptors signaling, but also plays a critical role in the cytokine secretion. Crk proteins interact with the hematopoietic progenitor kinase 1(HPK1), a serine-threonine kinase, which plays a critical role in IL-2 secretion.56 Interestingly, stimulation by IL-2 alone induces the specific phosphorylation of CrkL, but not of Crk, in human IL-2-dependent Kit 225 T lymphocytes.57 How IL-2 alone induces the specific phosphorylation of CrkL remains unclear. It will be of interest to investigate the roles of Crk family proteins in cytokine signaling.
Crk in T cell development
In 1999, Imaizumi et al.58 used the gene trap insertional mutagenesis approach to generate a mutant mouse line that is considered a Crk-II-specific disrupted mouse line as these mice express the truncated Crk proteins (similar to CrkI). Mice with CrkII-specific disruption (still expressing CrkL and CrkI) do not exhibit any obvious abnormalities, suggesting either that Crk-II is not essential for embryonic development or that CrkL may play a redundant and complementary role in embryonic development.58 Park et al.59 generated a complete null allele of Crk mice (still expressing CrkL) by using the Cre-loxP recombination approach. Crk-deficient mice die perinatally.59 No Crk-null mice can survive to adulthood.59 The Crk−/− embryonic data show that Crk is involved in cardiac and craniofacial development and plays an essential role in embryonic development,59 which is reminiscent of the phenotype of CrkL−/− mice.60 In 2001, Guris et al.60 generated CrkL−/− mice. However, the majority of homozygous CrkL−/− mice die at late stages of embryonic development.60 Only a small number of CrkL−/− mice (< 2%) survive, which suggests that CrkL is also an essential gene for embryonic development.60 The phenotypes of Crk- and CrkL-deficient mice are summarized in Table 3. No data report T cell development in Crk−/− mice due to the lack of Crk−/− adult mice.
Table 3.
Phenotypes of Crk and CrkL-deficient mice
| CrkI −/− | CrkII mutant mice lacking CrkII by the Gene Trap strategy | Crk−/− | CrkL−/− | |
|---|---|---|---|---|
| General phenotypes | ND* | No obvious abnormalities |
|
|
| Viability | ND | Normal | 95% of mice die. The reminder (5%) die shortly after birth. | 98% of mice die. |
| B cell development | ND | ND | ND | ND |
| T cell development | ND | ND | ND | Normal |
| T cell function | ND | ND | ND |
|
| NK cell development | ND | ND | ND | ND |
no data
In 2003, Peterson et al.61 investigated the role of CrkL in T-cell development and function. In CrkL−/− mice, the peripheral blood counts and functions are normal, whereas immunization induces comparable levels of IgG2a and IgG1 antibodies.61 Transferring CrkL−/− fetal liver cells into irradiated Rag2−/− recipients resulted in the functions of T cells from these chimeric mice being normal, revealing that CrkL is not essential for T cell development or function.61 Interestingly, the steady-state level of the Crk protein is not altered in the CrkL−/− mice, which indicates that Crk does not compensate for CrkL function in vivo. Taken together, both Crk and CrkL genes are required for embryonic development in mice.
Intriguingly, CrkL−/− mice also show a phenotype characteristic of human DiGeorge/velocardiofacial syndrome (DGS/VCFS).60, 62, 63 Three genes (Tbx1, CrkL, and Erk2) in chromosome 22q11.2 are responsible for the phenotypic features of DGS/VCFS.60, 63–66 Tbx1 and CrkL are two main genes for DGS/VCFS.64 Heterozygous deletions within human chromosome 22q11 are the genetic basis of DGS/VCFS.67 More than 80% of cases of DGS/VCFS are associated with a deletion within chromosome 22q11.64 Not only multiple candidate genes (30–50 genes) located within the region,68 but also dose-sensitive interaction of these genes, such as Tbx1 and CrkL in mice, play roles in DGS/VCFS,63 which adds an additional layer of complexity to the role of CrkL in DGS/VCFS. In general, decreased numbers of T cells with variable functional deficits (such as reduced proliferation capability to phytohemagglutinin) and normal production of immunoglobulin are observed in DGS/VCFS.69, 70 Therefore, DGS/VCFS may provide a tool to study the role of Crk in human immunobiology and immunodeficiency disease.
THE ROLE OF CRK IN REGULATING B CELL FUNCTIONS
Crk in B-cell antigen receptor (BCR) signaling
In the DT40 B cell line, B cell antigen receptor (BCR) ligation induces the formation of the Cbl-CrkII complex.71 The association of Cbl and CrkII is transient, depends on ligand-induced signaling, and does not require tyrosine phosphorylation of CrkII but does require the tyrosine phosphorylation of Cbl. The dissociation of Cbl-CrkII over time is due to the dephosphorylation of Cbl.71 CrkII also plays a role in BCR endocytosis.72 BCR ligation reduces the phosphorylation of CrkII in the DT40 B cell line. At least, the phosphorylation of CrkII did not increase after BCR ligation, which may be related to the constitutive phosphorylation of CrkII in resting the DT40 B cell line. Similarly, CrkL is not phosphorylated upon engagement of the BCR in the human Burkitt lymphoma lines Ramos and Daudi.73 Generally, stimulation of activation receptors, such as BCR and TCR, does not induce the phosphorylation of Crk. In the Ramos B cell line, BCR ligation induces the SH2 domains of CrkL and CrkII to bind to Cas and Cbl. In addition to the formation of Crk-Cas-Cbl, Crk also interacts with C3G in Ramos cells via its SH3 domains, which can translocate C3G from the cytosol to the plasma membrane, where Rap is located upon BCR ligation.74 In human tonsillar B cells and Nalm-6/ARH-77 B cell lines, ligation of either beta1 integrin or BCR induces tyrosine phosphorylation of human enhancer of filamentation-1 (HEF1), which forms a complex with CrkL.75 BCR stimulation also induces the interaction of Src homology 2 domain containing (Shc)/growth factor receptor-bound protein 2 (Grb2) complex and Crk.76 In human primary tonsillar B cells, BCR stimulation induces the association of Vav with Crk.73 BCR stimulation cannot induce the tyrosine phosphorylation of either Crk or CrkL.73 Overall, one of the main efforts directed toward Crk in BCR signaling is finding the binding partners of Crk in B cell lines. In summary, the association of Vav and Crk in human primary tonsillar B cells upon BCR stimulation is very intriguing, given the multiple roles of Vav in immune responses. Similar to the signal pathways of Crk in TCR signaling, Cbl and C3G are the major players in BCR signaling among Crk binding partners.
Crk in B cells co-stimulatory receptors signaling
Stimulation of BCR and LFA-1 induces the tyrosine phosphorylation and association of p130Cas with the Crk/CrkL SH2 domain.26 In CD21-expressing Raji cells, a Burkitt B lymphoma cell line, activation of CD21 induces interaction of Cbl with the SH2 domain of CrkL.77 Whether Crk also plays a role in the development of B cells remains unclear. In the 22q11.2 deletion syndrome (DiGeorge syndrome), in which the total B cell numbers are unaffected, some patients shows a significantly decreased proportion of memory B cells,78 which indicates a possible role of CrkL in B cell development and memory. In summary, Crk adaptor proteins play a role in BCR signaling, BCR endocytosis, and co-stimulatory receptor signaling by interacting with other signal molecules such as Cbl. The spatial-temporal details of Crk signal and physiological signaling events in primary B cells need to be elucidated.
THE ROLE OF CRK IN NK CELL FUNCTIONS
Crk in NK cells inhibitory receptors signaling
NK cell inhibitory receptors block activating signaling by recruiting phosphatases that dephosphorylate critical molecules such as Vav1 involved in activation.79 The detail of inhibitory receptor signaling has been described previously.79, 80 In 2008, Peterson and Long35 demonstrated that engagement of inhibitory receptors, including CD94-NKG2A and KIR, induces phosphorylation of Crk in both NK cell lines and primary NK cells. Crk phosphorylation leads to disassociation of the c-Cbl-Crk-p130CAS-C3G complex in NK cells in conjugates with their target cells expressing human leukocyte antigen class I. This c-Cbl-Crk-p130CAS-C3G complex occurs during NK cells activation induced by NK cells susceptible target cells such as 721.221 cells (an EBV transfected B cell line), which is similar to the complex formation between Crk and Cbl upon TCR and BCR ligation in T and B cells.15 Interestingly, during inhibition of NK cells by engagement of inhibitory receptors killer-cell immunoglobulin-like receptor (e.g. KIR2DL1) and CD94-NKG2A, phosphorylated Crk dissociates from other signaling molecules such as C3G and p130cas, which is reminiscent of the status of auto-inhibition of chicken CrkII.39 However, the status of Crk and ratio of Crk/pCrk in resting NK cells and the similarity in structure and function between cis conformation of Crk and phosphorylated Crk remain unclear.
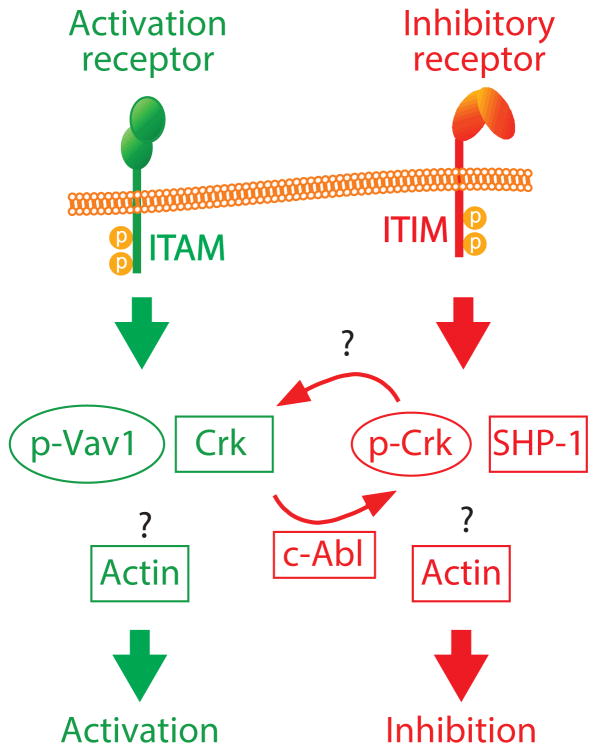
In 2012, Liu et al. demonstrated that inhibitory receptor CD94-NKG2A alone induces phosphorylation of Crk.81 In this study, the authors used a reductionist model of lipid bilayers containing ligand for activating receptor CD16 (Fc) and ligand for inhibitory receptor CD94-NKG2A (HLA-E) to selectively induce activation and inhibitory signaling, respectively.81 Combining live cell imaging with lipid bilayers, the authors demonstrated the distinct roles of un-phosphorylation and phosphorylation of Crk in the control of actin polymerization in the inhibitory synapse induced by HLA-E and Fc containing lipid bilayer. The discovery of the ability of NK inhibitory receptor alone to induce phosphorylation of the adaptor proteins-Crk/CrkL marks a true paradigm shift in which inhibitory receptor alone is sufficient to induce tyrosine phosphorylation, an event usually indicating cellular activation.81 Therefore, a new model for inhibitory receptor function is proposed (Figure 4): Crk phosphorylation prevents Crk-dependent activation signals from their intended remodeling of the filamentous-actin (F-actin) network.81 However, whether Crk can interact directly with the cytoplasmic domain of the inhibitory receptor, CD94-NKG2A/KIR, and how un-phosphorylated Crk and phosphorylated Crk affect actin dynamics at immunological synapses remain unclear. Overall, studies on the role of Crk in NK cell function have just begun. The role of the Crk family proteins in inhibitory receptors signaling and their roles in the cell biology of NK cells such as education/memory remain unclear.
Figure 4. Signaling mediated by Crk in human NK cell.
Phosphorylation of Crk induced by inhibitory receptors blocks NK cell activation. At activating synapses, Crk is required for the movement of activation receptor microclusters and their ability to trigger activation signals (not shown). During inhibition triggered by inhibitory receptor, Crk is phosphorylated and associates with the tyrosine kinase c-Abl. Interconversion between un-phosphorylated Crk (Green) and phosphorylated Crk (Red) functions as a molecular switch in the control of NK cell activation via interaction between Crk and pVav-1 or interaction between pCrk and SHP-1. Green indicates receptors and signal molecules that contribute to activation. Red indicates receptors and signal molecules that contribute to inhibition.
Crk in NK cell activating receptor signaling
NK cell activation is controlled by integration of signals from activation and inhibition.82 Crk not only plays a role in NK cells inhibitory receptor signaling as described above, but also modulates the signaling of activating receptors. During NK cell activation induced by susceptible target cells, Crk forms a complex with C3G (presumably via the SH3N domain) and p130cas (presumably via the SH2 domain). During activation, the majority of Crk or CrkL is in the un-phosphorylated state. The un-phosphorylated CrkL may constitutively associate with endogenous Abl via the fully accessible SH3N domain, which indicates that the SH3N domain in un-phosphorylated CrkL may not be available during NK cell activation.35 The un-phosphorylated CrkII forms a very different structure, compared to CrkL.22 It is not clear which isoform of Crk is dominant in activated NK cells. In 2009, Segovis et al.83 found that CrkL is important in NK cell-target cell conjugate formation, microtubule organization center (MTOC) polarization, NKG2D (activating receptor) -dependent cellular cytotoxicity, and antibody-stimulated granule release. In the conjugation between human NK clones with target cells expressing MICA (a ligand for NKG2D), CrkL was recruited to cytotoxic immunological synapses in an NKG2D-dependent manner. However, how CrkL is engaged with activating receptors signaling is not clear, and whether CrkL serves a functionally redundant role with CrkII in NK cellular cytotoxicity remains to be determined. In the recent study by Liu et al.81, Crk also is involved in CD16 (activating receptor)-induced degranulation. Silencing of Crk family of proteins expression with siRNAs targeted to both Crk and CrkL in human primary NK cells has profound effects on NK cells activation by CD16 signaling. Imaging of NK cells on lipid bilayers carrying Fc alone reveals that movement of Fc microclusters, phosphorylation of Vav1, and accumulation of F-actin are greatly reduced.81 In a human NK cell line, NK3.3, stimulation of NK3.3 with IL-2 or crosslinking of CD2 or CD16 induces the interactions between Crk and Cbl upon silencing of Crk family of proteins.84 The combination of IL-2 with CD2 crosslinking further increases tyrosine phosphorylation and association of Cbl with Crk.84, 85 Taken together, Crk family proteins not only contribute to NKG2D mediated natural cytotoxicity signaling for adhesion, granulation polarization, and degranulation, but also play a critical role in the CD16 mediated cytotoxicity, also known as antibody-dependent cellular cytotoxicity.
THE ROLE OF CRK IN OTHER IMMUNE CELL FUNCTIONS
CrkL is a major specific substrate for Abl in neutrophils. CrkL is heavily tyrosine phosphorylated in neutrophils of patients with Philadelphia chromosome positive chronic myelogenous leukemia (CML).21 This finding is similar to the observation from v-Crk-transformed 3Y1 cells.27 In Philadelphia chromosome negative peripheral blood cells, CrkL is present only in the non-phosphorylated form.86 In contrast, all Bcr/Abl+ CML and acute lymphoblastic leukemia patient samples examined show clear tyrosine-phosphorylation of CrkL.20, 86 What role Crk plays in CML remains unclear. In summary, the role of Crk in other immune cellssuch as dentritic cells and macrophages remains to be determined.
PERSPECTIVES
In this review, the roles of the Crk family of proteins in T, B, and NK cells receptor function have been briefly summarized. Since the identification of Crk in 1980s, great progress has been made in addressing the physiological and pathological roles of Crk in normal and transformed fibroblasts. Most research on Crk has focused on the cell apoptosis, proliferation, transformation processes, and identification of Crk binding partners.7, 8 Although some of the signaling properties of Crk have been identified in the Jurkat cell line or other transformed lymphocyte cell lines by traditional biochemical and cell biological approaches, the role of Crk in immune response, including immune cytokine secretion, cytotoxicity, immunological synapse formation, and inhibitory receptor signaling on primary immune cells, as well as in infectious and primary immunodeficiency diseases, is poorly understood. Four important and significant questions about Crk in immune cells are listed as follows:
Do different isoforms of Crk family of proteins play different roles in immune cells?
Given the different isoforms of Crk that exist in immune cells, an obvious question that remains to be answered is which isoform in particular types of immune cells, such as T, B, and NK cells, plays a role in different immune responses. Whether these isoforms play a complementary or redundant role in immunoreceptor signaling remains unclear. Addressing these questions will be helpful to design a specific target to harness the immune cells during infection and to induce the effective immune responses against cancer.
What is the role of phosphorylated Crk in NK cell licensing?
In human primary NK cells, the phosphorylation of Crk induced by inhibitory receptor CD94-NKG2A alone is unexpected, considering that phosphorylation events usually indicate cell activation.81 Given that the MHC class I-specific inhibitory receptors not only control NK cell cytotoxicity but also have a role in tuning intrinsic responsiveness of NK cells to subsequent activation signaling (a process referred to as ‘licensing’, ‘arming’, or ‘education’ 87–90), this finding indicates that the phosphorylated Crk may play a role in NK cell licensing. However, how phosphorylated Crk family proteins function in NK cell education or NK licensing remains unclear, as does the status/amount of Crk (phosphorylated Crk, un-phosphorylated Crk, or de-phosphorylated Crk) in resting immune cells, IL-2 activated NK cells, and activating or inhibitory receptors-stimulated NK cells.
What is the role of Crk in NK memory?
NK memory is an emerging topic in the field of immunology.91, 92 One of the immunological features of the 22q11.2 deletion syndrome (DiGeorge syndrome) is the significantly reduced number of CD27+ IgM+ IgD+ memory B-lymphocytes, which suggests that Crk family of proteins may play a role in B cell memory.67, 93, 94 Therefore, it will be of interest to determine if Crk also plays a role in NK cell memory.
How do Crk family proteins impact cytoskeletal organization?
Cytoskeletal remodeling plays a critical role in controlling cytotoxicity of cytotoxic T lymphocytes and NK cells.95, 96 During activation of human primary NK cells induced by activating receptor CD16, Crk silencing has profound effects on actin organization, of which F-actin appears as disorganized patches at cytotoxic immunological synapses.81 During inhibition of NK cells induced by inhibitory receptor CD94/NKG2A, F-actin is disrupted through Crk phosphorylation.81 The molecular mechanism of cytoskeletal actin remodeling by Crk or phosphorylated Crk is not clear. Crk may regulate the F-actin dynamics via two distinct pathways. One is that Crk interacts with several downstream signal molecules such as Paxillin, C3G/Rap-1, and p130Cas/DOCK180/Rac-1 to regulate F-actin.14 The second pathway could be the interaction between Crk and pVav-1, as shown in Figure 4. Vav-1 is a well-known molecule that can regulate actin cytoskeletal reorganization in both T and B cells.97–99 Therefore, it will be of interest to investigate the possible mechanisms of Crk regulating actin dynamics in human NK cells, which will greatly enhance our understanding of how Crk functions as a master regulatory switch operating upstream of Vav-1 which in turn directly influences actin dynamics.
In summary, finding and identifying the proteins associated with Crk by classical biochemical approaches has been a major effort in the field during the past several decades. The time has come for immunologists to implement new cutting-edge technologies to explore the roles of Crk in immune response. The emerging super resolution imaging of live cells such as stimulated emission depletion microscopy and photoactivated localization microscopy approaches should elucidate the spatiotemporal details of Crk in the immune responses.
Acknowledgments
I thank Dr. Jordan Orange for comments and critical reading of the manuscript and Dr. Lee Ligon for editorial assistance. Work in the author’s laboratory was supported in part by the Baylor-UTHouston Center for AIDS Research Core Support Grant number AI36211 from the National Institute of Allergy and Infectious Diseases and the Caroline Wiess Law Fund for Research in Molecular Medicine.
Abbreviations
- Abl
Abelson murine leukemia kinase
- Bcr
Breakpoint cluster region
- BCR
B-cell antigen receptor
- Cas
Crk-associated substrate
- Cbl
Casitas B-lineage lymphoma
- Crk
CT10 (chicken. tumor virus no. 10) regulator of kinase
- CrkL
CT10 (chicken. tumor virus no. 10) regulator of kinase like
- C3G
Crk SH3-binding GNRP (guanine nucleotide-releasing protein)
- CypA
cyclophilin A
- DOCK180
180-kDa protein downstream of Crk
- Dok1
downstream of tyrosine kinases protein 1
- Erk
Extracellular signal-regulated kinase
- F-actin
filamentous –actin
- Gab1
growth factor receptor-bound protein 2 (GRB2)-associated binding protein 1
- HEF1
human enhancer of filamentation 1
- HPK1
hematopoietic progenitor kinase 1
- KIR
killer-cell immunoglobulin-like receptor
- PTB
phosphotyrosine-binding domain
- PTKs
protein tyrosine kinases
- PI3-K
phosphoinositide 3-kinase
- Rac-1
Ras-related C3 botulinum toxin substrate 1
- Rap-1
Ras-related GTP-binding protein 1
- RT-loop
loop in SH3 structure named after arginine, R, and threonine, T residues commonly found in it
- SH2 and SH3
Src homology 2 and 3
- SHP-1
SH2 domain-containing phosphatase 1
- TCR
T cell receptor
References
- 1.Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988;332(6161):272–5. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchie H, Chang CH, Yoshida M, Vogt PK. A newly isolated avian sarcoma virus, ASV-1, carries the crk oncogene. Oncogene. 1989;4(11):1281–4. [PubMed] [Google Scholar]
- 3.Mayer BJ, Hamaguchi M, Hanafusa H. Characterization of p47gag-crk, a novel oncogene product with sequence similarity to a putative modulatory domain of protein-tyrosine kinases and phospholipase C. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):907–14. doi: 10.1101/sqb.1988.053.01.104. [DOI] [PubMed] [Google Scholar]
- 4.Reichman CT, Mayer BJ, Keshav S, Hanafusa H. The product of the cellular crk gene consists primarily of SH2 and SH3 regions. Cell Growth Differ. 1992;3(7):451–60. [PubMed] [Google Scholar]
- 5.Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T, Shibuya M. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol Cell Biol. 1992;12(8):3482–9. doi: 10.1128/mcb.12.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ten Hoeve J, Morris C, Heisterkamp N, Groffen J. Isolation and chromosomal localization of CRKL, a human crk-like gene. Oncogene. 1993;8(9):2469–74. [PubMed] [Google Scholar]
- 7.Feller SM. Crk family adaptors-signalling complex formation and biological roles. Oncogene. 2001;20(44):6348–71. doi: 10.1038/sj.onc.1204779. [DOI] [PubMed] [Google Scholar]
- 8.Birge RB, Kalodimos C, Inagaki F, Tanaka S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun Signal. 2009;7:13. doi: 10.1186/1478-811X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austgen K, Johnson ET, Park TJ, Curran T, Oakes SA. The adaptor protein CRK is a pro-apoptotic transducer of endoplasmic reticulum stress. Nature cell biology. 2012;14(1):87–92. doi: 10.1038/ncb2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oehrl W, Kardinal C, Ruf S, Adermann K, Groffen J, Feng GS, et al. The germinal center kinase (GCK)-related protein kinases HPK1 and KHS are candidates for highly selective signal transducers of Crk family adapter proteins. Oncogene. 1998;17(15):1893–901. doi: 10.1038/sj.onc.1202108. [DOI] [PubMed] [Google Scholar]
- 11.Sattler M, Salgia R. Role of the adapter protein CRKL in signal transduction of normal hematopoietic and BCR/ABL-transformed cells. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 1998;12(5):637–44. doi: 10.1038/sj.leu.2401010. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda M, Kurata T. Emerging components of the Crk oncogene product: the first identified adaptor protein. Cellular signalling. 1996;8(5):335–40. doi: 10.1016/0898-6568(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.Sriram G, Birge RB. Emerging roles for crk in human cancer. Genes & cancer. 2010;1(11):1132–9. doi: 10.1177/1947601910397188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, Maruoka M, Takeya T. Functional mechanisms and roles of adaptor proteins in abl-regulated cytoskeletal actin dynamics. Journal of signal transduction. 2012;2012:414913. doi: 10.1155/2012/414913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakov N. A new twist to adaptor proteins contributes to regulation of lymphocyte cell signaling. Trends in immunology. 2008;29(8):388–96. doi: 10.1016/j.it.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Gelkop S, Babichev Y, Kalifa R, Tamir A, Isakov N. Involvement of crk adapter proteins in regulation of lymphoid cell functions. Immunologic research. 2003;28 (2):79–91. doi: 10.1385/IR:28:2:79. [DOI] [PubMed] [Google Scholar]
- 17.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic acids research. 1988;16(22):10881–90. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobashigawa Y, Sakai M, Naito M, Yokochi M, Kumeta H, Makino Y, et al. Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nat Struct Mol Biol. 2007;14(6):503–10. doi: 10.1038/nsmb1241. [DOI] [PubMed] [Google Scholar]
- 19.Anafi M, Rosen MK, Gish GD, Kay LE, Pawson T. A potential SH3 domain-binding site in the Crk SH2 domain. J Biol Chem. 1996;271(35):21365–74. doi: 10.1074/jbc.271.35.21365. [DOI] [PubMed] [Google Scholar]
- 20.Nichols GL, Raines MA, Vera JC, Lacomis L, Tempst P, Golde DW. Identification of CRKL as the constitutively phosphorylated 39-kD tyrosine phosphoprotein in chronic myelogenous leukemia cells. Blood. 1994;84(9):2912–8. [PubMed] [Google Scholar]
- 21.Oda T, Heaney C, Hagopian JR, Okuda K, Griffin JD, Druker BJ. Crkl is the major tyrosine-phosphorylated protein in neutrophils from patients with chronic myelogenous leukemia. J Biol Chem. 1994;269(37):22925–8. [PubMed] [Google Scholar]
- 22.Jankowski W, Saleh T, Pai MT, Sriram G, Birge RB, Kalodimos CG. Domain organization differences explain Bcr-Abl’s preference for CrkL over CrkII. Nature chemical biology. 2012;8(6):590–6. doi: 10.1038/nchembio.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prosser S, Sorokina E, Pratt P, Sorokin A. CrkIII: a novel and biologically distinct member of the Crk family of adaptor proteins. Oncogene. 2003;22 (31):4799–806. doi: 10.1038/sj.onc.1206714. [DOI] [PubMed] [Google Scholar]
- 24.Muralidharan V, Dutta K, Cho J, Vila-Perello M, Raleigh DP, Cowburn D, et al. Solution structure and folding characteristics of the C-terminal SH3 domain of c-Crk-II. Biochemistry. 2006;45(29):8874–84. doi: 10.1021/bi060590z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa S, Toyoshima H, Kozutsumi H, Hagiwara K, Sakai R, Tanaka T, et al. The C-terminal SH3 domain of the mouse c-Crk protein negatively regulates tyrosine-phosphorylation of Crk associated p130 in rat 3Y1 cells. Oncogene. 1994;9(6):1669–78. [PubMed] [Google Scholar]
- 26.Harkiolaki M, Gilbert RJ, Jones EY, Feller SM. The C-terminal SH3 domain of CRKL as a dynamic dimerization module transiently exposing a nuclear export signal. Structure. 2006;14(12):1741–53. doi: 10.1016/j.str.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Feller SM, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. Embo J. 1994;13(10):2341–51. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong R, ten Hoeve J, Heisterkamp N, Groffen J. Tyrosine 207 in CRKL is the BCR/ABL phosphorylation site. Oncogene. 1997;14(5):507–13. doi: 10.1038/sj.onc.1200885. [DOI] [PubMed] [Google Scholar]
- 29.Senechal K, Heaney C, Druker B, Sawyers CL. Structural requirements for function of the Crkl adapter protein in fibroblasts and hematopoietic cells. Mol Cell Biol. 1998;18(9):5082–90. doi: 10.1128/mcb.18.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen MK, Yamazaki T, Gish GD, Kay CM, Pawson T, Kay LE. Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature. 1995;374(6521):477–9. doi: 10.1038/374477a0. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani T, Kondo T, Darmanin S, Tsuda M, Tanaka S, Tobiume M, et al. A novel FRET-based biosensor for the measurement of BCR-ABL activity and its response to drugs in living cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(15):3964–75. doi: 10.1158/1078-0432.CCR-10-0548. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa K, Mochizuki N, Ohba Y, Mizuno H, Miyawaki A, Matsuda M. A pair of fluorescent resonance energy transfer-based probes for tyrosine phosphorylation of the CrkII adaptor protein in vivo. J Biol Chem. 2001;276(33):31305–10. doi: 10.1074/jbc.M104341200. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann RM, Cotton GJ, Chang EJ, Vidal E, Veach D, Bornmann W, et al. Fluorescent monitoring of kinase activity in real time: development of a robust fluorescence-based assay for Abl tyrosine kinase activity. Bioorganic & medicinal chemistry letters. 2001;11(24):3091–4. doi: 10.1016/s0960-894x(01)00650-3. [DOI] [PubMed] [Google Scholar]
- 34.Cotton GJ, Muir TW. Generation of a dual-labeled fluorescence biosensor for Crk-II phosphorylation using solid-phase expressed protein ligation. Chemistry & biology. 2000;7(4):253–61. doi: 10.1016/s1074-5521(00)00100-9. [DOI] [PubMed] [Google Scholar]
- 35.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29(4):578–88. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar P, Reichman C, Saleh T, Birge RB, Kalodimos CG. Proline cis-trans isomerization controls autoinhibition of a signaling protein. Mol Cell. 2007;25 (3):413–26. doi: 10.1016/j.molcel.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriram G, Reichman C, Tunceroglu A, Kaushal N, Saleh T, Machida K, et al. Phosphorylation of Crk on tyrosine 251 in the RT loop of the SH3C domain promotes Abl kinase transactivation. Oncogene. 2011;30(46):4645–55. doi: 10.1038/onc.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sriram G, Birge RB. Commentary: The carboxyl-terminal Crk SH3 domain: Regulatory strategies and new perspectives. FEBS letters. 2012 doi: 10.1016/j.febslet.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar P, Saleh T, Tzeng SR, Birge RB, Kalodimos CG. Structural basis for regulation of the Crk signaling protein by a proline switch. Nat Chem Biol. 2011;7(1):51–7. doi: 10.1038/nchembio.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisner H, Czech MP. Coupling of the proto-oncogene product c-Cbl to the epidermal growth factor receptor. J Biol Chem. 1995;270(43):25332–5. doi: 10.1074/jbc.270.43.25332. [DOI] [PubMed] [Google Scholar]
- 41.Sawasdikosol S, Ravichandran KS, Lee KK, Chang JH, Burakoff SJ. Crk interacts with tyrosine-phosphorylated p116 upon T cell activation. J Biol Chem. 1995;270(7):2893–6. doi: 10.1074/jbc.270.7.2893. [DOI] [PubMed] [Google Scholar]
- 42.Buday L, Khwaja A, Sipeki S, Farago A, Downward J. Interactions of Cbl with two adapter proteins, Grb2 and Crk, upon T cell activation. J Biol Chem. 1996;271(11):6159–63. doi: 10.1074/jbc.271.11.6159. [DOI] [PubMed] [Google Scholar]
- 43.Reedquist KA, Fukazawa T, Panchamoorthy G, Langdon WY, Shoelson SE, Druker BJ, et al. Stimulation through the T cell receptor induces Cbl association with Crk proteins and the guanine nucleotide exchange protein C3G. J Biol Chem. 1996;271(14):8435–42. doi: 10.1074/jbc.271.14.8435. [DOI] [PubMed] [Google Scholar]
- 44.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209(1):21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 45.Ryan PE, Davies GC, Nau MM, Lipkowitz S. Regulating the regulator: negative regulation of Cbl ubiquitin ligases. Trends in biochemical sciences. 2006;31 (2):79–88. doi: 10.1016/j.tibs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Sawasdikosol S, Chang JH, Pratt JC, Wolf G, Shoelson SE, Burakoff SJ. Tyrosine-phosphorylated Cbl binds to Crk after T cell activation. J Immunol. 1996;157(1):110–6. [PubMed] [Google Scholar]
- 47.Gelkop S, Babichev Y, Isakov N. T cell activation induces direct binding of the Crk adapter protein to the regulatory subunit of phosphatidylinositol 3-kinase (p85) via a complex mechanism involving the Cbl protein. J Biol Chem. 2001;276(39):36174–82. doi: 10.1074/jbc.M100731200. [DOI] [PubMed] [Google Scholar]
- 48.Amsen D, Kruisbeek A, Bos JL, Reedquist K. Activation of the Ras-related GTPase Rap1 by thymocyte TCR engagement and during selection. European journal of immunology. 2000;30(10):2832–41. doi: 10.1002/1521-4141(200010)30:10<2832::AID-IMMU2832>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 49.Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, et al. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem. 1999;274(20):14376–81. doi: 10.1074/jbc.274.20.14376. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Shao Y, Fang D, Huang J, Jeon MS, Liu YC. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. J Biol Chem. 2003;278(26):23978–83. doi: 10.1074/jbc.M212671200. [DOI] [PubMed] [Google Scholar]
- 51.Martelli MP, Boomer J, Bu M, Bierer BE. T cell regulation of p62(dok) (Dok1) association with Crk-L. J Biol Chem. 2001;276(49):45654–61. doi: 10.1074/jbc.M105777200. [DOI] [PubMed] [Google Scholar]
- 52.Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, et al. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. The Journal of cell biology. 2008;182(6):1231–44. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanda H, Mimura T, Morino N, Hamasaki K, Nakamoto T, Hirai H, et al. Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk. European journal of immunology. 1997;27(8):2113–7. doi: 10.1002/eji.1830270840. [DOI] [PubMed] [Google Scholar]
- 54.Ohashi Y, Iwata S, Kamiguchi K, Morimoto C. Tyrosine phosphorylation of Crk-associated substrate lymphocyte-type is a critical element in TCR- and beta 1 integrin-induced T lymphocyte migration. J Immunol. 1999;163(7):3727–34. [PubMed] [Google Scholar]
- 55.Ohashi Y, Tachibana K, Kamiguchi K, Fujita H, Morimoto C. T cell receptor-mediated tyrosine phosphorylation of Cas-L, a 105-kDa Crk-associated substrate-related protein, and its association of Crk and C3G. The Journal of biological chemistry. 1998;273(11):6446–51. doi: 10.1074/jbc.273.11.6446. [DOI] [PubMed] [Google Scholar]
- 56.Ling P, Yao Z, Meyer CF, Wang XS, Oehrl W, Feller SM, et al. Interaction of hematopoietic progenitor kinase 1 with adapter proteins Crk and CrkL leads to synergistic activation of c-Jun N-terminal kinase. Mol Cell Biol. 1999;19(2):1359–68. doi: 10.1128/mcb.19.2.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gesbert F, Garbay C, Bertoglio J. Interleukin-2 stimulation induces tyrosine phosphorylation of p120-Cbl and CrkL and formation of multimolecular signaling complexes in T lymphocytes and natural killer cells. J Biol Chem. 1998;273(7):3986–93. doi: 10.1074/jbc.273.7.3986. [DOI] [PubMed] [Google Scholar]
- 58.Imaizumi T, Araki K, Miura K, Araki M, Suzuki M, Terasaki H, et al. Mutant mice lacking Crk-II caused by the gene trap insertional mutagenesis: Crk-II is not essential for embryonic development. Biochemical and biophysical research communications. 1999;266(2):569–74. doi: 10.1006/bbrc.1999.1869. [DOI] [PubMed] [Google Scholar]
- 59.Park TJ, Boyd K, Curran T. Cardiovascular and craniofacial defects in Crk-null mice. Mol Cell Biol. 2006;26(16):6272–82. doi: 10.1128/MCB.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guris DL, Fantes J, Tara D, Druker BJ, Imamoto A. Mice lacking the homologue of the human 22q11. 2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nature genetics. 2001;27(3):293–8. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- 61.Peterson AC, Marks RE, Fields PE, Imamoto A, Gajewski TF. T cell development and function in CrkL-deficient mice. European journal of immunology. 2003;33(10):2687–95. doi: 10.1002/eji.200324294. [DOI] [PubMed] [Google Scholar]
- 62.Moon AM, Guris DL, Seo JH, Li L, Hammond J, Talbot A, et al. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Developmental cell. 2006;10(1):71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guris DL, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Developmental cell. 2006;10(1):81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Momma K. Cardiovascular anomalies associated with chromosome 22q11. 2 deletion syndrome. The American journal of cardiology. 2010;105(11):1617–24. doi: 10.1016/j.amjcard.2010.01.333. [DOI] [PubMed] [Google Scholar]
- 65.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nature genetics. 2001;27(3):286–91. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 66.Newbern J, Zhong J, Wickramasinghe RS, Li X, Wu Y, Samuels I, et al. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(44):17115–20. doi: 10.1073/pnas.0805239105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gennery AR. Immunological aspects of 22q11. 2 deletion syndrome. Cellular and molecular life sciences: CMLS. 2012;69(1):17–27. doi: 10.1007/s00018-011-0842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meechan DW, Maynard TM, Gopalakrishna D, Wu Y, LaMantia AS. When half is not enough: gene expression and dosage in the 22q11 deletion syndrome. Gene expression. 2007;13(6):299–310. doi: 10.3727/000000006781510697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jawad AF, McDonald-Mcginn DM, Zackai E, Sullivan KE. Immunologic features of chromosome 22q11. 2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) The Journal of pediatrics. 2001;139(5):715–23. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- 70.Junker AK, Driscoll DA. Humoral immunity in DiGeorge syndrome. The Journal of pediatrics. 1995;127(2):231–7. doi: 10.1016/s0022-3476(95)70300-4. [DOI] [PubMed] [Google Scholar]
- 71.Jacob M, Todd L, Sampson MF, Pure E. Dual role of Cbl links critical events in BCR endocytosis. International immunology. 2008;20(4):485–97. doi: 10.1093/intimm/dxn010. [DOI] [PubMed] [Google Scholar]
- 72.Jacob M, Todd LA, Majumdar RS, Li Y, Yamamoto K, Pure E. Endogenous cAbl regulates receptor endocytosis. Cellular signalling. 2009;21(8):1308–16. doi: 10.1016/j.cellsig.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Smit L, van der Horst G, Borst J. Sos, Vav, and C3G participate in B cell receptor-induced signaling pathways and differentially associate with Shc-Grb2, Crk, and Crk-L adaptors. J Biol Chem. 1996;271(15):8564–9. doi: 10.1074/jbc.271.15.8564. [DOI] [PubMed] [Google Scholar]
- 74.Ingham RJ, Krebs DL, Barbazuk SM, Turck CW, Hirai H, Matsuda M, et al. B cell antigen receptor signaling induces the formation of complexes containing the Crk adapter proteins. J Biol Chem. 1996;271(50):32306–14. doi: 10.1074/jbc.271.50.32306. [DOI] [PubMed] [Google Scholar]
- 75.Manie SN, Beck AR, Astier A, Law SF, Canty T, Hirai H, et al. Involvement of p130(Cas) and p105(HEF1), a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells. J Biol Chem. 1997;272(7):4230–6. doi: 10.1074/jbc.272.7.4230. [DOI] [PubMed] [Google Scholar]
- 76.Smit L, van der Horst G, Borst J. Formation of Shc/Grb2- and Crk adaptor complexes containing tyrosine phosphorylated Cbl upon stimulation of the B-cell antigen receptor. Oncogene. 1996;13(2):381–9. [PubMed] [Google Scholar]
- 77.Lottin-Divoux S, Jean D, Le Romancer M, Frade R. Activation of Epstein-Barr virus/C3d receptor (gp140, CR2, CD21) on human B lymphoma cell surface triggers Cbl tyrosine phosphorylation, its association with p85 subunit, Crk-L and Syk and its dissociation with Vav. Cellular signalling. 2006;18(8):1219–25. doi: 10.1016/j.cellsig.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 78.McLean-Tooke A, Spickett GP, Gennery AR. Immunodeficiency and autoimmunity in 22q11. 2 deletion syndrome. Scandinavian journal of immunology. 2007;66(1):1–7. doi: 10.1111/j.1365-3083.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 79.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunological reviews. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Long EO. Regulation of immune responses through inhibitory receptors. Annual review of immunology. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 81.Liu D, Peterson ME, Long EO. The adaptor protein crk controls activation and inhibition of natural killer cells. Immunity. 2012;36(4):600–11. doi: 10.1016/j.immuni.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual review of immunology. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Segovis CM, Schoon RA, Dick CJ, Nacusi LP, Leibson PJ, Billadeau DD. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J Immunol. 2009;182(11):6933–42. doi: 10.4049/jimmunol.0803840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang JY, Umehara H, Inoue H, Tabassam FH, Okazaki T, Kono T, et al. Differential interaction of Cbl with Grb2 and CrkL in CD2-mediated NK cell activation. Molecular immunology. 2000;37(17):1057–65. doi: 10.1016/s0161-5890(01)00020-7. [DOI] [PubMed] [Google Scholar]
- 85.Umehara H, Inoue H, Huang J, Kono T, Minami Y, Tanaka Y, et al. Role for adapter proteins in costimulatory signals of CD2 and IL-2 on NK cell activation. Molecular immunology. 2002;38(8):587–96. doi: 10.1016/s0161-5890(01)00099-2. [DOI] [PubMed] [Google Scholar]
- 86.ten Hoeve J, Arlinghaus RB, Guo JQ, Heisterkamp N, Groffen J. Tyrosine phosphorylation of CRKL in Philadelphia+ leukemia. Blood. 1994;84(6):1731–6. [PubMed] [Google Scholar]
- 87.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nature reviews Immunology. 2010;10(10):724–34. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 89.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunological reviews. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunological reviews. 2006;214:143–54. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 91.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186(4):1891–7. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paust S, von Andrian UH. Natural killer cell memory. Nature immunology. 2011;12(6):500–8. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 93.McLean-Tooke A, Barge D, Spickett GP, Gennery AR. Immunologic defects in 22q11. 2 deletion syndrome. The Journal of allergy and clinical immunology. 2008;122(2):362–7. 367e1–4. doi: 10.1016/j.jaci.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 94.Finocchi A, Di Cesare S, Romiti ML, Capponi C, Rossi P, Carsetti R, et al. Humoral immune responses and CD27+ B cells in children with DiGeorge syndrome (22q11. 2 deletion syndrome) Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2006;17(5):382–8. doi: 10.1111/j.1399-3038.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 95.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nature reviews Immunology. 2008;8(9):713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annual review of cell and developmental biology. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 97.Tybulewicz VL. Vav-family proteins in T-cell signalling. Current opinion in immunology. 2005;17(3):267–74. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Oberley MJ, Wang DS, Yang DT. Vav1 in hematologic neoplasms, a mini review. American journal of blood research. 2012;2(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 99.Harwood NE, Batista FD. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harbor perspectives in biology. 2011;3(2) doi: 10.1101/cshperspect.a002360. [DOI] [PMC free article] [PubMed] [Google Scholar]