Abstract
During dentate gyrus development the early embryonic radial glial scaffold is replaced by a secondary glial scaffold around birth. In contrast to neocortical and early dentate gyrus radial glial cells these postnatal glial cells are severely altered with regard to position and morphology in reeler mice lacking the secreted protein Reelin. In this study we focus on the functional impact of these defects. Most radial glial cells throughout the nervous system serve as scaffolds for migrating neurons and precursor cells for both neurogenesis and gliogenesis. Precursor cell function has been demonstrated for secondary radial glial cells but the exact function of these late glial cells in granule cell migration and positioning is not clear. No data exist concerning the interplay between granule neurons and late radial glial cells during dentate gyrus development. Here we show that despite the severe morphological defects in the reeler dentate gyrus the precursor function of secondary radial glial cells is not impaired during development in reeler mice. In addition, selective ablation of Disabled-1, an intracellular adaptor protein essential for Reelin signaling, in neurons but not in glial cells allowed us to distinguish effects of Reelin signaling on radial glial cells from possible secondary effects based on defective granule cells positioning.
Keywords: gliogenesis, hippocampus, reeler, development, differentiation
Introduction
Radial glial cells in different brain areas usually serve two different functions – they are multipotent precursor cells giving rise to neurons and glial cells and they facilitate glial guided migration (Campbell and Gotz 2002). These radial glial cells express proteins of the Reelin signaling cascade (Förster et al. 2002; Hartfuss et al. 2003; Luque et al. 2003), which is important for neuronal migration. Reeler mice lacking Reelin show severe defects in neuronal positioning throughout the brain (D’Arcangelo et al. 1997; Falconer 1951; Rice and Curran 2001). The molecular basis of the Reelin signaling cascade was first discovered in neurons: Reelin, a large secreted glycoprotein, binds to the lipoprotein receptors ApoE receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR) (Trommsdorff et al. 1999), which induces phosphorylation of the adaptor protein Disabled-1 (Dab1) by Src family kinases (Arnaud et al. 2003; Bock and Herz 2003; Howell et al. 1999; Kuo et al. 2005). This, in turn, activates a multitude of signaling cascades that modulate cytoskeletal dynamics (Beffert et al. 2002; Chai et al. 2009; Leemhuis and Bock 2011). Mice lacking both Reelin receptors ApoER2 and VLDLR, as well as single knockout mice deficient for the intracellular adaptor protein Dab1, phenocopy the reeler mutant.
Radial glial cells express these proteins of the Reelin signaling cascade. In addition, Reelin has a direct effect on glial cells as shown by stripe choice assays (Förster et al. 2002) and Reelin stimulation of isolated radial glial cells (Hartfuss et al. 2003). Although radial glial cells are Reelin responsive, those in the developing neocortex of reeler mice are only mildly affected, being normally positioned with less straight and slightly shortened processes (Hack et al. 2007; Hartfuss et al. 2003). In addition, mice with a neuron-specific knockout of Dab1 (Nex-Cre positive Dab1fl/fl) show a neocortical morphology that is virtually indistinguishable from completely Dab1-deficient mice (Franco et al. 2011), suggesting that Reelin signaling to radial glial cells alone is not sufficient to rescue neuronal migration defects in the neocortex. Furthermore, the glial guided migration of neurons proceeds normally in the absence of Reelin signaling, whereas only somal translocation is disturbed (Franco et al. 2011).
The development of the dentate gyrus differs from that of the neocortex and can be subdivided into two major phases. In the prenatal phase of dentate gyrus development proliferation takes place in the neuroepithelium near the fimbria. Early (primary) radial glial cells span the whole length from the fimbria to the pial surface of the dentate gyrus, and young neurons as well as precursor cells migrate along their fibers (Nakahira and Yuasa 2005) from the neuroepithelium into the dentate anlage (Altman and Bayer 1990a). In the second postnatal phase, the precursor cells build up a new proliferation zone within the dentate gyrus that becomes more and more restricted to the subgranular zone. Within this first postnatal week, a late secondary radial glial scaffold develops whose processes traverse the forming granule cell layer (Rickmann et al. 1987). This scaffold is fully developed around P10 to P14 (Brunne et al. 2010). Afterwards most of these cells start a final transformation and become astrocytes of the molecular layer. Only few of them remain into adulthood and constitute the stem cells for adult neurogenesis in the dentate gyrus (Christie and Cameron 2006). In reeler mice secondary radial glial cells in the dentate gyrus are severely altered with respect to their positioning and morphology. They are distributed throughout the dentate gyrus and fail to establish radial processes (Förster et al. 2002; Weiss et al. 2003) and have a more stellate, astrocyte-like morphology.
Using immunohistochemical markers for glial maturation in the dentate gyrus (Brunne et al. 2010) and BrdU labeling studies, we demonstrate here that in reeler mice, despite the severe morphological phenotype, the maturation and differentiation of radial glial cells are not affected during dentate gyrus development. This contrasts with the adult situation where reeler mice show an increase in astrogliogenesis at the expense of neurogenesis (Zhao et al. 2007). In addition, using conditional knockout mice with Dab1 deleted only in neurons (Franco et al. 2011), we were able to show that loss of Reelin signaling in granule cells was not sufficient to produce the full neuronal and glial phenotypes observed in the null mutants. This suggests that Reelin also has direct effects on the secondary glia.
Methods
Animals
For timed matings, the day of vaginal plug detection was considered as embryonic day 0.5 (E0.5), and the day of birth as postnatal day 0 (P0). The animals were either on a C57BL/6JxSv129Ev mixed background (reeler, Dab1-KO, Dab1-flox) or only C57BL/6J (Nestin-Cre, Nex-Cre, tdTomato-Reporter Ai9, hGFAP-Cre, Dab1-5Fki). All animals were maintained in accordance with the institutional and national guidelines for animal care. Genotypes were confirmed by PCR analysis as described (Franco et al. 2011; Leemhuis et al. 2010; Pramatarova et al. 2008).
Tissue preparation for immunohistochemistry
Brains for immunohistochemistry were obtained from 14 young reeler (Falconer 1951) and 14 age-matched wildtype mice from the same litters (E16.5, P0, P3, P6, P10, P14, P21; two animals/age). In addition, brains from three P10 and three P15 mice of each of the following genotypes were used: conditional Dab1 mice (Dab1fl/fl), Nex-Cre positive Dab1fl/fl mice and Nestin-Cre positive Dab1fl/fl mice from the lab of Ulrich Müller (Franco et al. 2011). Nestin-Cre mice and Nex-Cre mice have been described elsewhere (Belvindrah et al. 2007; Goebbels et al. 2006; Graus-Porta et al. 2001). Furthermore brains from two P10 and two P15 Nex-Cre positive tdTomato reporter mice (Madisen et al. 2010) and brains from two P15 mice of each of the following genotypes, were used: Dab15F/5F, hGFAP-Cre positive Dab1fl/fl, hGFAP-Cre negative Dab1fl/fl and hGFAP-Cre positive Dab1fl/wt. The hGFAP-Cre (Zhuo et al. 2001) Dab1fl/fl and Dab15F/5F mice were from the lab of Brian Howell (Howell et al. 2000; Pramatarova et al. 2008). Animals older than P6 were killed with CO2 and perfused intracardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Brains were postfixed in 4% PFA at 4°C overnight. The brains of younger animals were dissected after decapitation under hypothermic anaesthesia and immersion-fixed in 4% PFA in PBS at 4°C overnight.
Immunohistochemistry and microscopy
Immunohistochemistry and microscopy were carried out as described earlier (Brunne et al. 2010). In brief, brains were embedded in 5% agar and cut into 50 μm coronal sections using a Leica-VT1000S vibratome (Leica, Nussloch, Germany). Sections were blocked with a mixture of 10% horse serum (HS) and 0.5% Triton X-100 in PBS for 1h at room temperature (RT) and incubated in a mixture of primary antibodies diluted in PBS +10% HS, followed by secondary fluorochrome-conjugated antibodies (Alexa-Fluor series, Invitrogen, Karlsruhe, Germany) in PBS for 2–3 h. Triton-X-100 in the blocking step was omitted in the GLT1 staining. The primary antibodies used in this study are listed in Table 1.
Table 1.
List of primary antibodies.
| Antigen | Dilution | Company | Order Number | Staining pattern in the dentate gyrus (Brunne et al. 2010) |
|---|---|---|---|---|
| BLBP | 1:500 | Millipore | AB9558 | glial cells (radial glia and immature astrocytes) |
| BrdU | 1:1000 | Abcam | 6326-250 | BrdU labeled cells |
| Doublecortin | 1:1000 | Santa Cruz | sc-8066 | immature neurons |
| GFAP1 | 1:500 | Dako Cytomation | Z0334 | radial glia and astrocytes throughout development |
| GFAP2 | 1:500 | Sigma-Aldrich | G3893 | GFAP specific, secondary radial glia beginning from P10 on and astrocytes |
| GLT1 | 1:4000 | Millipore | AB1783 | radial glial cells and astrocytes |
| GS | 1:500 | Millipore | MAB302 | mature astrocytes |
| Nestin (Rat401) | 1:30 | DSHB | Rat401 | precursor cells including radial glial cells at early developmental stages |
| NeuN | 1:1000 | Millipore | MAB377 | postmitotic neurons |
| Prox1 | 1:500 | Millipore | AB5475 | young and mature granule cells |
| Reelin | 1:500 | Chemicon | MAB5364 | Reelin expression |
| S100β | 1:500 | Sigma-Aldrich | S2532 | mature astrocytes |
| Vimentin (40EC) | 1:20 | DSHB | 40EC | glial cells, especially between P3 and P10 |
referred to as GFAP_rp (rabbit polyclonal)
referred to as GFAP_mm (mouse monoclonal)
Abbreviations: DSHB, Developmental Studies Hybridoma Bank.
BrdU injection, immunohistochemical detection and quantification of marker co-expression
12 reeler mice and 12 age-matched wildtype mice received a single intraperitoneal (i.p.) injection of 50 Rg/g body weight of 5-bromo-2-deoxyuridine (BrdU; Sigma, Munich, Germany) in 0.9% NaCl (P0, P3 and P10; four animals/age). The tissue was collected 21days post-injection (p.i.). 50 μm vibratome sections were treated with 2M HCl at 37°C for 30 min and washed in 0.1M phosphate buffer (PB, pH 7.4) at RT. The sections were blocked in 0.1M PB containing 10% normal donkey serum, 1% Triton X-100 and 10% avidin (Vector Laboratories, Burlingame, CA/USA) for 1h, followed by incubation in a mixture of primary antibodies including rat anti-BrdU diluted in PB containing 10% biotin (Vector Laboratories) at 4°C. The sections were incubated with a rat IgG-specific biotin-conjugated antibody (Vector Laboratories), followed by incubation for 2h with a mixture of fluorochrome-labeled avidin (AMCA-Avidin 1:200, biotin-avidin reagents from Vector Laboratories) and fluorochrome-conjugated secondary antibodies (Alexa-Fluor series, Invitrogen) in PB. To evaluate the percentage of BrdU-labeled cells expressing neuronal (NeuN) or glial (GFAP) markers, 50 BrdU-positive cells per animal from 3–4 different animals were analyzed. Results are presented as mean ±SEM.
Production of recombinant Reelin
Preparation of Reelin-containing supernatants and control supernatants was carried out as described previously (Leemhuis et al. 2010). In brief conditioned medium from HEK-293 cells stably transfected with full-length Reelin cDNA and control cells transfected with a control vector was collected and concentrated 10-fold using 100 kDa cutoff centrifugal filters (Millipore/Merck, Darmstadt, Germany). Reelin- and control-conditioned supernatants were tested for their ability to stimulate tyrosine phosphorylation of Dab1 as described (Bock and Herz 2003).
Primary hippocampal cell culture and immunocytochemistry
Hippocampi from four reeler mice, four Dab1 knockout mice (Howell et al. 1997) and four corresponding wildtype mice were dissected and trypsinized in 450 μl Hanks’ Balanced Salt Solution (HBSS) (Lonza, Basel, Switzerland) supplemented with trypsin-EDTA (Sigma) at 37°C for 10 min. The digestion was stopped by two short washes with 1 ml Dulbecco’s Modified Eagle Medium (DMEM, 4.5 g/L glucose, Lonza). Thereafter, the tissue was dissociated in trituration medium as described (Bock and Herz 2003). 150 μl of this cell suspension were diluted in DMEM +4.5 g/L glucose, 2 mM Glutamax, and 10% fetal calf serum. The cells were plated at a density of 12000 cells/cm2 on poly-D-lysine-coated coverslips and maintained in a 5% CO2 atmosphere at 37°C. After 4 h the cells were fixed with 4% PFA for 20 min and analyzed by immunocytochemistry. For Reelin stimulation experiments, the culture medium was supplemented with Reelin or control concentrates. The medium was exchanged after four hours against fresh Reelin-containing or control medium. These cells were fixed after 24 h. The cells were permeabilized, blocked with PBS + 10 % HS + 0.1 % Triton-X-100 for 30 min, and incubated with primary antibodies (Table 1) diluted in PBS + 10 % HS at 4°C overnight, followed by incubation with appropriate fluorochrome-conjugated secondary antibodies (Alexa-Fluor series, Invitrogen, Karlsruhe, Germany). After mounting with Mowiol, the cells were imaged as described above. Single- and double-labeled cells were counted using Adobe Photoshop and ImageJ (http://rsbweb.nih.gov/ij) processing software. Using the DAPI stain as a mask assured that no cells were counted twice.
Quantification of process orientation and morphology during astrocytic transformation
Brains were obtained from 3 reeler and 3 age-matched wildtype mice at P21. A combination of brain lipid binding protein (BLBP)– and glial fibrillary acidic protein (GFAP)-specific antibodies was used to stain cell bodies (BLBP) and cell processes (GFAP) of transforming glial cells. At least twenty double-labeled cells from each animal were reconstructed using Adobe Photoshop software and the number of processes per cell orientated either towards the molecular layer or the hilus was determined. In addition morphological aspects (area, perimeter, Ferets diameter and aspect ratio) of transforming radial glial cells were analysed using ImageJ.
Results
Dentate gyrus development in reeler mice
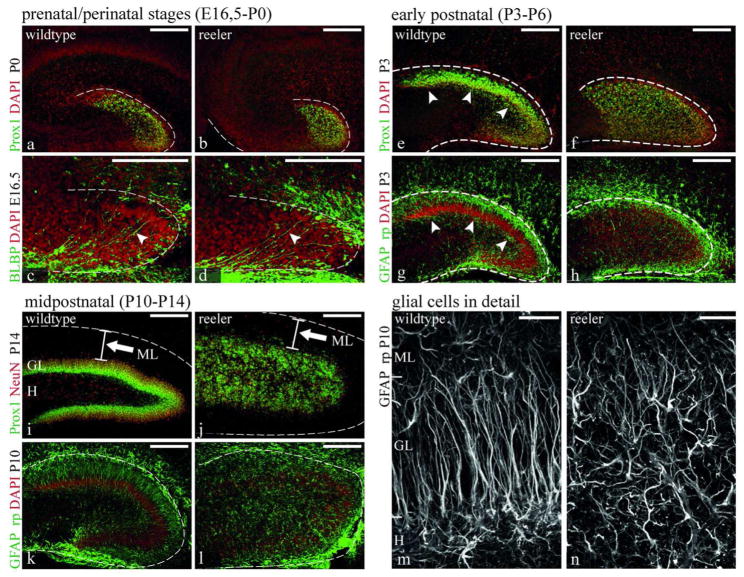
In the present study of Reelin effects on secondary radial glial cells and precursor differentiation, we first confirmed and extended previous studies concerning Reelin expression in wildtype mice and morphological defects in reeler mice during dentate gyrus development. For this purpose immunohistochemical labeling of glial cells (GFAP, BLBP), granule neurons (Prox1) and Reelin expressing cells was performed on brain sections from reeler and wildtype mice at different developmental stages, ranging from E16.5 to P21 (E16.5, P0, P3, P6, P10, P14, P21), thus covering the peri- and postnatal phases of dentate gyrus development. At birth Reelin expression was very strong but restricted to the suprapyramidal region of the dentate gyrus (Fig. 1b). Although Reelin expression was high at birth, no obvious defects concerning radial glial morphology (Fig. 2c,d) and neuronal positioning (Fig. 2a,b) were detectable in the reeler dentate gyrus. At P3 Reelin expression was much weaker but Reelin positive Cajal-Retzius (CR) cells covered the pial surface all around the dentate gyrus (Fig. 1c). At the same time, compaction of the granule layer became obvious in wildtype but not in reeler mice (Fig. 2e,f), accompanied by the formation of radial glial cell processes spanning the incipient granule layer (Fig. 2g). In reeler mice GFAP-positive cells were scattered throughout the dentate gyrus without any obvious orientation and showed short, branched processes (Fig. 2h). Reelin expression at the pial surface rapidly decreased during further development, while Reelin expressing interneurons in the hilar region appeared around P10 (arrows Fig. 1e,f). In wildtype mice, the granule cell layer became more and more compacted resulting in the typical lamination of the dentate gyrus consisting of the hilus, granule layer, and molecular layer (Fig 2i). In reeler mice, granule cells remained scattered in the dentate gyrus but, as in wildtype mice, a cell-sparse molecular layer forms (Fig. 2j). Glial cells remained scattered throughout the dentate gyrus showing several short, irregular processes (Fig. 2l,n). In summary, highest Reelin expression around birth preceded apparent developmental defects in the reeler dentate gyrus. The reeler defect in the dentate gyrus became obvious when the secondary radial glial scaffold assembled in wildtype mice around P3 and compaction of granule cells began. Although a small number of granule neurons were malpositioned in the molecular layer, this cell-sparse area is formed in reeler mice whereas the hilar region could not be clearly distinguished.
Fig. 1. Reelin expression in the developing dentate gyrus.
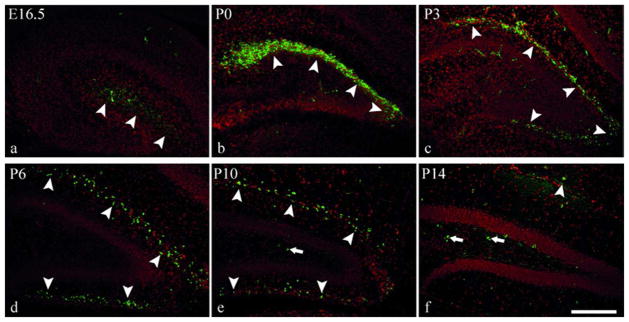
Immunohistochemical visualization of Reelin (green) in coronal hippocampal sections at different developmental stages in wildtype mice. All sections are counterstained with DAPI (red). At E16.5 (a) and P0 (b) Reelin expression is restricted to suprapyramidal regions of the pial surface (arrowheads in a,b), while Reelin expressing cells can be found at the pial surface around the whole dentate gyrus from P3 onwards (arrowheads in c–f). At P10, Reelin positive cells become apparent in the hilus (arrows in e). Reelin expression is strongest at P0 (b) and rapidly declines during further development. The staining pattern at P14 (f) resembles that seen in adult mice (not shown). Scale bar 200μm.
Fig. 2. Dentate gyrus development in reeler mice.
Staining of dentate gyrus granule cells (Prox1) and radial glial cells (BLBP or GFAP) in reeler and wildtype mice at different developmental stages. At early prenatal and perinatel stages no obvious defects are apparent in reeler mice neither with regard to neuronal positioning (a,b) nor radial glial cell morphology (c,d). At the onset of granule cell compaction at early postnatal stages in wildtype mice (e), the reeler phenotype develops with deficient granule cell lamination (f) and an irregular assembly of the secondary radial glial scaffold (g,h). At later postnatal stages, when the granule cell layer (GL) and the secondary radial glial scaffold are fully developed in wildtype mice (i,k,m) no similarly ordered granule cell lamination and radial glial scaffold are visible in reeler mice (j,l,n). The hilar region (H) is densely populated with granule cells in reeler mice (j) whereas the molecular layer (ML) is clearly delineated as cell sparse area at this later stage (arrows in i and j). Higher magnification of secondary radial glial cells in reeler (n) and wildtype (m) mice at P14 (extended focus z-stack 32 × 0,58 μm) reveals the different morphology of individual glial cells. (a–l) counterstained with DAPI or coimmunostained with NeuN (red) as indicated. Scale bars 200μm in a–l, 25μm in m–n.
Maturation of secondary radial glial cells in the reeler dentate gyrus
Brain sections from 14 reeler and corresponding wildtype animals at different developmental stages were immunolabeled with markers characterizing secondary radial glia maturation (Brunne et al. 2010) thus providing a developmental sequence of glial maturation that was independent of structural criteria. Although the morphology of secondary radial glial cells was severely altered in reeler mice, the expression profile of intermediate filaments revealed that the overall maturation of the glial population took place normally. In reeler mice, Nestin was first detected around P0 to P3 (Fig. 3a), while Vimentin expression was strongest between P3 and P14 (Fig. 3b and Suppl. Fig. 1). Strong GFAP expression was not observed before P10 (Fig. 3c). This was exactly the same time course as identified in wildtype animals (Fig. 3) (Brunne et al. 2010). Another important marker protein expressed in radial glial cells is BLBP. Staining for BLBP was of particular interest as it has been reported to be differently expressed in radial glial cells in reeler mice (Hartfuss et al. 2003; Sibbe et al. 2009). Decreased BLBP staining was not observed in the dentate gyrus, for neither primary (Fig. 2c,d) nor secondary radial glial cells (Fig. 3c).
Fig. 3. Maturation of secondary radial glial cells.
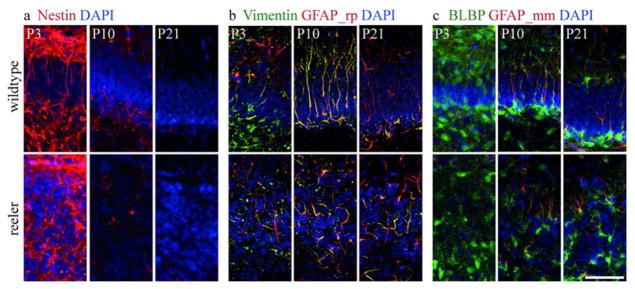
Immunohistochemical staining for Nestin (a), Vimentin (b), GFAP (c) and BLBP (c) reveals the same developmental expression profile in reeler and wildtype mice. Nestin is highly expressed at early stages (a), Vimentin expression is pronounced between P3 and P10 (b, see also Suppl. Fig. 1) and GFAP expression coming up late around P10 (c). Strong BLBP expression is observed throughout development in wildtype and reeler animals (c). Scale bar 50 μm.
Terminal astrocytic differentiation of radial glial cells in the reeler dentate gyrus
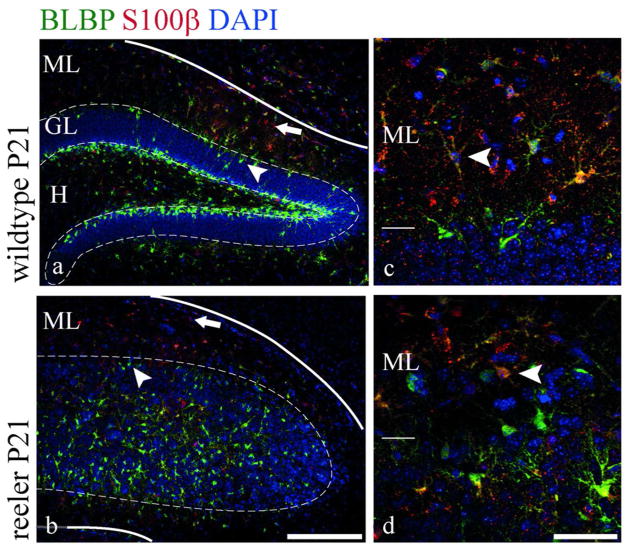
At late developmental stages many radial glial cells of the dentate gyrus undergo a final astroglial transformation and migration into the molecular layer (Brunne et al. 2010). Double labeling for BLBP and S100β at this late stage (P21) revealed a gradient of astrocyte maturation in the molecular layer of reeler animals similar to that seen in wildtype mice, with young BLBP-positive astrocytes next to the granule cell layer and S100β-positive mature astrocytes near the pial surface (Fig. 4a,b). Double labeled cells were found in between (Fig. 4c,d). This distribution of astrocytes of different maturational stages in the molecular layer of reeler mice pointed to a normal transformation of secondary radial glial cells populating the molecular layer. Transforming radial glial cells had been characterized as GFAP-BLBP coexpressing cells within the granule cell layer in wildtype mice (Brunne et al. 2010). Therefore, coimmunohistochemistry for these markers was used to study earlier stages of this astroglial transformation (Fig. 5a), and GFAP-BLBP double-labeled cells in close proximity to the molecular layer (less than 80μm) were reconstructed (Fig. 5b) and analyzed in relation to different morphological parameters. Overall 353 reconstructed wildtype cells and 297 reconstructed reeler cells were analyzed with ImageJ and the results depicted as box-and-whisker plots (Fig. 5c). In reeler as well as wildtype mice the analysis revealed a high degree of cell shape diversity (Fig. 5b,c), with a slightly greater diversity in wildtype mice. For example, the area occupied by the reconstructed cells varied between 24μm2 and 1350μm2 in wildtype mice and between 38μm2 and 776μm2 in reeler mice. Nevertheless, the interquartile range was very similar between wildtype (97–212μm2) and reeler mice (107–211μm2), showing that most of the cells have a comparable size. The same holds true for the perimeter, the Feret’s diameter and the aspect ratio, indicating that transforming radial glial cells are morphologically very similar in wildtype and reeler mice.
Fig. 4. Astrocyte arrangement in the molecular layer.
Immunohistochemical staining for BLBP and S100β at the end of dentate gyrus development (a–d). A gradient of astrocyte maturation within the molecular layer, which is obvious in wildtype mice at this stage (a, c), can also be identified in reeler mice (b, d). BLBP positive cells are found near the granule cell layer (arrowheads a,b) and S100β positive cells near the pial surface (arrows in a,b). Double-labeled cells are observed in the inner portions of the molecular layer (arrowheads at higher magnification in c, d). Scale bar 200μm in a,b; 50 μm in c,d.
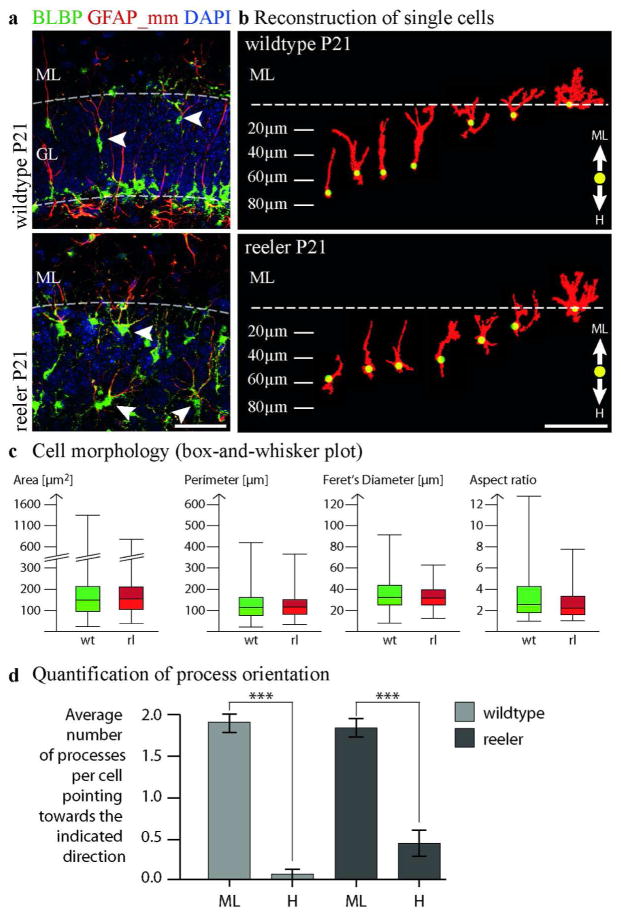
Fig. 5. Terminal astrocytic transformation of radial glial cells in reeler mice.
(a) Double-labeling for GFAP (glial processes) and BLBP (somata of glial cells) at the end of dentate gyrus development (P21) in wildtype and reeler mice showed radial glial cells during their final transformation into molecular layer astrocytes. Although cell processes are branched and less straight in reeler, most processes of the glial cells are orientated towards the molecular layer at this late stage of development. (b) GFAP-BLBP positive cells next to the molecular layer (less than 80μm distance) in wildtype and reeler dentate gyrus were reconstructed (red) and the position of the cell soma was identified by DAPI and BLBP staining (yellow). (c) Box-and-Whisker plots describing the morphology of the reconstructed cells with respect to area, perimeter, Ferets diameter and aspect ratio (n=353 wildtype cells and 297 reeler cells). (d) The number of processes directed towards the molecular layer versus those directed towards the hilus was quantified (n=3 animals and 20 reconstructed cells per animal). The quantification demonstrates orientation of BLBP-GFAP co-immunolabeled glial cells towards the molecular layer in reeler mice with significantly more processes pointing towards the molecular layer than towards the hilus (p<0,001). Scale bar 50μm in a,b.
As BLBP was mainly localized to glial cell bodies while GFAP staining was seen in glial processes, imaging both staining patterns allowed us to assess cell orientation in addition to cell morphology. Analysis of cell orientation (Fig. 5d) showed that transforming radial glial cells were in most cases correctly polarized in reeler mice, with their GFAP processes oriented towards the molecular layer (Fig. 5b,c). Together these findings suggest that terminal astrocytic transformation of secondary radial glial cells destined to the molecular layer took place normally in reeler mice.
Neurogenesis and astrogliogenesis in the reeler dentate gyrus
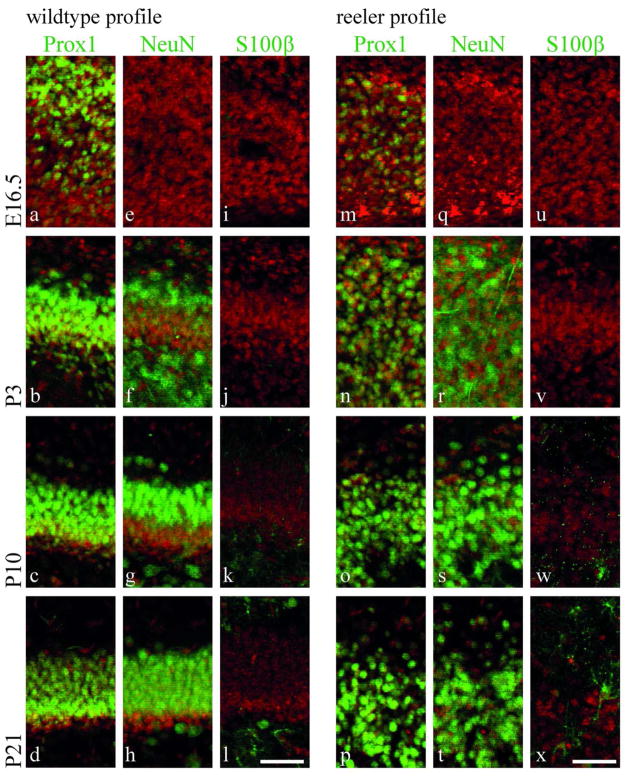
As secondary radial glial cells are part of the dentate gyrus precursor cell pool (Brunne et al. 2010), and since differentiation of precursor cells is altered in adult reeler mice (Zhao et al. 2007), the question arose to which extent precursor differentiation might be affected during dentate gyrus development. To analyze precursor cell differentiation during dentate gyrus development, brain sections from reeler and wildtype animals were immunostained for the granule cell marker Prox1, the pan-neuronal marker NeuN, and the marker of mature astrocytes S100β at different developmental stages (Fig. 6). The granule marker Prox1 was expressed from E16.5 on. The marker for mature neurons, NeuN, appeared at around P0–P3, whereas S100β, a marker for mature astrocytes, was not expressed before P10. No difference in the expression of these differentiation markers was observed between wildtype and reeler mice during dentate gyrus development. In addition, two proteins important for the role of astrocytes in glutamate homeostasis – the glial glutamate transporter GLT1 and glutamine synthetase - were normally expressed in the reeler dentate gyrus with one important exception. GLT1 levels are higher in glial cells of the subgranular zone when compared to hilar and molecular layer astrocytes in wildtype mice. In reeler mice, such cells with a higher GLT1 expression were only rarely seen, and they failed to build up a specialized layer (Suppl. Fig. 2).
Fig. 6. Cell differentiation in reeler mice.
Immunohistochemical labeling for Prox1, NeuN and S100β throughout dentate gyrus development in wildtype (a–l) and reeler (m–x) animals. The granule cell marker Prox1 is highly expressed from E16.5 on (a–d). NeuN expression is detected from P3 on (f–h). The marker for mature astrocytes, S100β, is expressed from P10 on (k–l). No differences can be observed between wildtype (a–l) and reeler (m–x) animals concerning the developmental expression profile of these differentiation markers. All sections counterstained with DAPI (red). Scale bar 50μm.
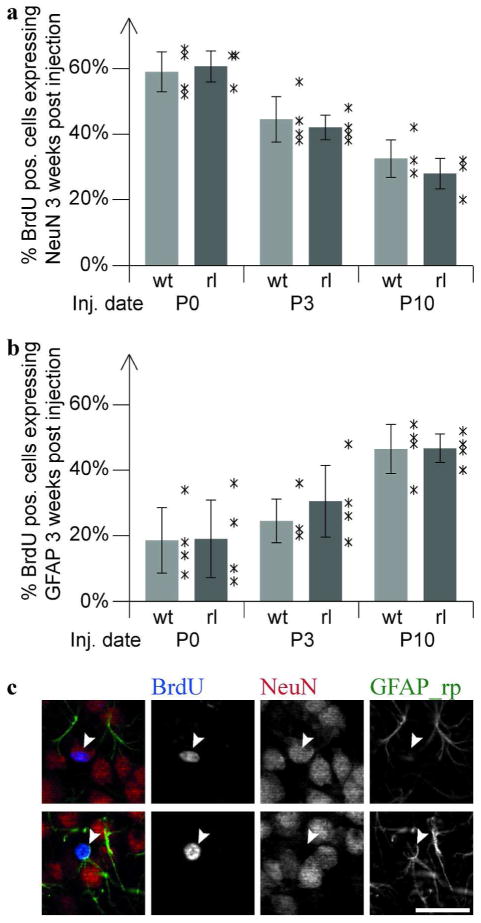
For a more comprehensive view on cell differentiation, proliferating cells were pulse-labeled in vivo with BrdU at different developmental stages (P0, P3, P10) in reeler and wildtype mice. The numbers of BrdU-positive neurons (expressing NeuN) and BrdU positive astrocytes (expressing GFAP) were quantified three weeks after injection, when most of the BrdU-labeled cells had completed differentiation. Only strongly BrdU-labeled cells were chosen for analysis to ensure that these cells had not undergone several additional cell divisions between injection and analysis. By P0 most of the BrdU-labeled cells had differentiated into neurons (Fig. 7a), while only few of them had differentiated into GFAP-positive astrocytes (Fig. 7b). Although Reelin expression was high at this stage there was no difference in cell differentiation between wildtype and reeler mice (Fig. 7a,b). At P3, when the compaction of granule cells and the arrangement of secondary radial glia became apparent in wildtype but not in reeler mice (Fig. 2e,f), astrogliogenesis slightly increased while neurogenesis declined. Again, no significant difference in neurogenesis or astrogliogenesis could be observed between reeler and wildtype animals. At P10 the granule cells built up an ordered layer, which was traversed by secondary radial glial cells (Fig. 2i,k) and proliferation was restricted to the subgranular and hilar regions in wildtype mice (Suppl. Fig. 3). Neurogenesis was low in reeler as well as in wildtype mice and most of the P10 BrdU-labeled cells differentiated to astrocytes. Even at this stage, neurogenesis and astrogliogenesis were indistinguishable between reeler and wildtype. In reeler as well as in wildtype animals we observed an increase in astrogliogenesis of about 180% (9,0±5,5% to 26,3±5,3%) and a decrease in neurogenesis of about 50% (29,9±2,8% to 15,1±2,8). Although Reelin expression was very high during these developmental stages, no differentiation defects in the reeler dentate gyrus were detectable, arguing against a direct role of Reelin in dentate gyrus precursor differentiation.
Fig. 7. Neurogenesis and astrogliogenesis during dentate gyrus development.
Quantification of BrdU-positive cells expressing NeuN (a) or GFAP (b) three weeks post injection (BrdU injection at P0, P3 and P10; n = 3–4 animals per condition and 50 BrdU-positive cells per animal; mean ±SEM; asterisks represent the individual values for each animal). No significant difference between wildtype and reeler mice is detectable during development neither for neurogenesis (a) nor astrogliogenesis (b). (c) Examples of NeuN and GFAP labeled, BrdU positive cells. Scale bar 25μm.
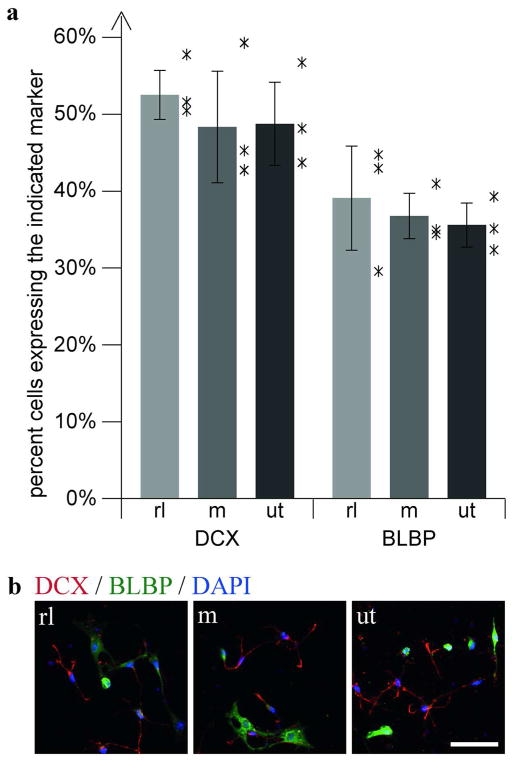
To substantiate this finding, dissociated hippocampal cell cultures from P2 reeler animals were stimulated with Reelin or a control medium directly after seeding. The expression of doublecortin, a marker for immature neurons, and BLBP, a marker for immature astrocytes as well as radial glial cells, was quantified 24 h after seeding. Incubation with Reelin-conditioned medium did not alter the amount of DCX- or BLBP-positive cells, indicating that Reelin signaling has no direct effect on precursor differentiation under these conditions (Fig 8).
Fig. 8. Stimulation of dissociated hippocampal cells with Reelin conditioned medium.
(a) Quantification of the differentiation of dissociated hippocampal cells from reeler animals (P2) into neurons (DCX, red) or glial cells (BLBP, green) following 24h of stimulation with Reelin (rl) or control medium (m) as well as in untreated (ut) cells (n = 3 animals; ~1000 cells per animal; mean ±SEM; asterisks represent the individual values for each animal). (b) Sample images from cultures used for quantification. Scale bar 50μm.
Morphology of secondary radial glial cells depends on direct Reelin signaling as well as neuronal positioning
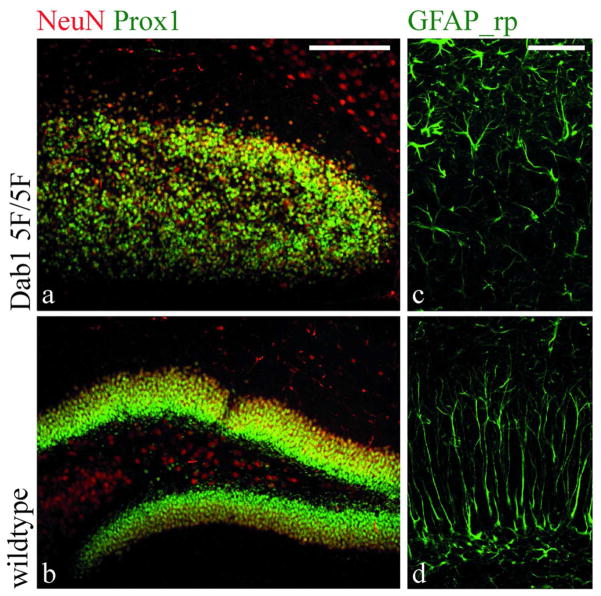
In the neocortex Reelin signaling primarily affects neurons. Hence, the question arose whether the severe morphological defects of secondary radial glial cells in the reeler dentate gyrus might be an indirect effect resulting from the malpositioning of granule neurons or whether it might result from a direct signaling effect of Reelin onto glial cells. As Reelin is a secreted extracellular signaling molecule, mice lacking the intracellular Reelin-transducing effector protein Dab1 were used instead of reeler mice to selectively abolish Reelin signaling in specific cell populations. Dab1 is phosporylated upon Reelin signaling leading to the activation of downstream signaling cascades. The role of Dab1 phosphorylation for dentate gyrus defects in Reelin deficient mice was confirmed using a Dab1-5F knockin mouse line, which express a non-phosphorylatable Dab1 protein that cannot transduce the Reelin signal (Howell et al. 2000). These mice showed the same dentate gyrus phenotype as reeler mice. Granule cells were scattered throughout the dentate gyrus and glial cells failed to assemble in a radial glial scaffold (Fig. 9). This confirmed the role of the canonical Reelin signaling cascade in dentate gyrus morphogenesis and allowed the switch to Dab1 mutants in the following experiments.
Fig. 9. Dentate gyrus morphology in Dab1-5F knockin mice.
Immunohistochemical staining with an antibody against the pan-neuronal marker NeuN or the more specific granule neuron marker Prox1 and the glial marker GFAP reveals a dentate gyrus phenotype in Dab15F/5F knockin mice indistinguishable from Dab1 knockout mice with granule cells beeing scattered throughout the dentate gyrus and the radial glia showing short, branched processes. Scalebar 100μm in a,b; 50μm in c,d.
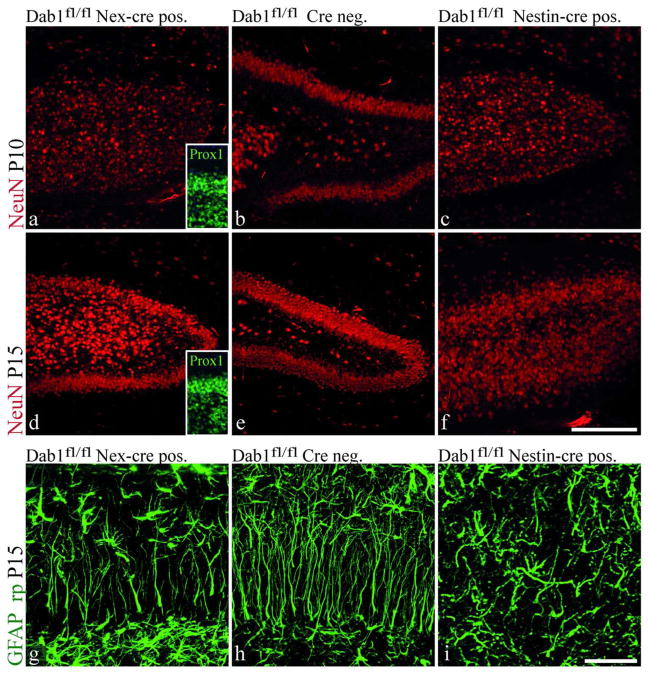
In Nex-Cre positive Dab1fl/fl mice Dab1 is specifically knocked out in immature neurons while it remains active in glial cells, including the secondary radial glial cells (Suppl. Fig. 4)(Franco et al. 2011; Goebbels et al. 2006). Cre-negative and Nestin-Cre positive Dab1fl/fl mice were used as controls. Cre-negative mice were indistinguishable from wildtype mice (Fig. 10b,e) while Nestin-Cre positive mice showed a Dab1 knockout phenotype (Fig. 10c,f) due to the expression of Cre in Nestin-positive precursor cells, leading to a knockout of Dab1 in neurons and glial cells. At P10 two of three Nex-Cre positive Dab1fl/fl mice displayed a phenotype indistinguishable from Nestin-Cre positive Dab1fl/fl mice (Fig. 10a) while the third one showed a weak rescue, with few granule cells compacted in a granule cell layer (insert in Fig. 10a). At P15, all Nex-Cre positive animals showed a dentate gyrus phenotype intermediate between wildtype and Dab1 knockout mice. Secondary radial glial cells had partially aligned radial processes directed towards the molecular layer (Fig. 10g). Granule cells formed a partially compacted layer (Fig. 10d and insert in Fig. 10d), which remained into adulthood (data not shown), although they did not express Dab1 and therefore could not translate the Reelin signal themselves. This suggests that secondary radial glial cells have a direct effect on the positioning of the granule cells, at least in part. On the other hand, although Reelin signaling to radial glial cells is not impaired in Nex cre Dab1fl/fl mice, these cells do not have a completely normal morphology. This suggests that the malpositioning of granule cells in these mice affects radial glial cells indirectly. Taken together, these results indicate a bidirectional interaction of neurons and glial cells in the postnatal dentate gyrus.
Fig. 10. Secondary radial glial cells and granule cell compaction in Nex Cre-Dab1fl/fl mice.
Staining for postmitotic neurons (NeuN) and secondary radial glial cells (GFAP_rp) in Dab1fl/fl Nex-cre positive mice lacking Dab1 in neurons but not glial or radial glial cells at P10 and P15. Nestin-cre positive Dab1fl/fl mice (c,f,i), with cre expression in neurons and glial cells, showing a Dab1 knockout phenotype and cre-negative Dab1fl/fl mice (b,e,h) showing a wildtype phenotype, are used as controls. At P10 Dab1fl/fl Nex-cre positive mice (a) show a dentate gyrus phenotype which resembles Dab1 knockout mice or Nestin-cre positive Dab1fl/fl mice (c). In contrast at P15 (d) an intermediate phenotype can be observed between mice that have Dab1 (e) and mice lacking Dab1 in neurons and glial cells (f). Staining for Prox1 identifies dislocated cells as granule cells (inserts in a and d). GFAP staining is depicted as high magnification extended focus images (Z-Stack 26×1μm) revealing the partially rescued secondary radial glial cell morphology in Nex Cre-Dab1fl/fl mice (g). For comparison Cre negative (h) and Nestin-cre positive Dab1fl/fl mice (i) are shown. Scale bar 200 μm in a; 50 μm in b)
The opposite approach, to disrupt the Reelin signaling cascade exclusively in radial glial cells but not in neurons, was precluded by the neural precursor properties of radial glial cells (Kriegstein and Alvarez-Buylla 2009; Malatesta and Gotz 2013). Accordingly, hGFAP-Cre positive Dab1fl/fl mice showed a phenotype indistinguishable from Dab1 knockout animals (Suppl. Fig. 5).
Discussion
The present study shows that the establishment of the secondary radial glial scaffold in the dentate gyrus depends on direct Reelin signaling to these glial cells as well as the correct layering of neurons, which is also Reelin dependent (Fig. 10). As both, Reelin signaling and granule cell positioning are altered in reeler mice, position and morphology of secondary radial glial cells are severely altered in this mutant (Fig. 2). However, despite these morphological alterations, the glial cell population shows a normal maturation (Fig. 3), a normal transformation process (Fig. 4+5), and normal differentiation (Fig. 6–8), indicating that an ordered cell arrangement is rather dispensable for early cell type-specific maturation. In contrast, in a previous study it was shown that cell differentiation and proliferation is severely altered in the dentate gyrus of adult reeler mice (Zhao et al. 2007). Together these findings lead to the hypothesis that the strictly ordered structure of the dentate gyrus, which is built up during the first three weeks of postnatal development, is a prerequisite for proper adult neurogenesis.
Primary and secondary radial glial cells in the reeler dentate gyrus
Dentate gyrus primary radial glial cells show many similarities to neocortical radial glial cells: They contact the ventricle and their long radial processes extend to the pial surface (Pinto and Gotz 2007). They act as precursor cells (Altman and Bayer 1990a; Altman and Bayer 1990b; Pleasure et al. 2000) and provide a scaffold for migrating neurons (Nakahira and Yuasa 2005). Like radial glial cells in the neocortex, primary radial glial cells of the dentate gyrus show no major defects in reeler mice. These cells extend long radial processes from the neuroepithelium to the pial surface as in wildtype mice (Fig. 2c,d; Fig. 11). Nevertheless we cannot rule out that there may be less obvious changes similar to those reported in the neocortex (Hartfuss et al. 2003). This is in agreement with earlier studies demonstrating that the positioning of neurons in the reeler dentate gyrus cannot be distinguished from wildtype until early postnatal stages (Stanfield and Cowan 1979). Presumably SDF1 rather than Reelin controls this early phase of development in the dentate gyrus (Bagri et al. 2002). Nevertheless, Reelin expression is high in the suprapyramidal region of the dentate gyrus pial surface, even at the earliest investigated embryonic stage (E16.5) and has its maximum around birth (Fig. 1). At these early stages Reelin expression is restricted to those portions of the dentate gyrus that are close to the CA1 region. As there are no significant defects in the dentate gyrus at this stage, early Reelin expression presumably plays a role for pyramidal cell migration in the CA1 region. This assumption is supported by the fact that pyramidal cells of CA1 migrate towards the Reelin-rich region of the dentate gyrus pial surface (Nakahira and Yuasa 2005) and show malpositioning as early as E15.5 in reeler mice (Stanfield and Cowan 1979). Secondary radial glial cells of the dentate gyrus do not have contact to the ventricle and exhibit comparatively short processes. Moreover, in contrast to neocortical radial glial cells and primary radial glia in the dentate gyrus, they are severely affected in reeler mice. Their cell bodies are scattered throughout the dentate gyrus and their processes are heavily branched and not aligned in an ordered scaffold as in wildtype animals (Fig. 2m,n; Fig. 11). However, despite their remarkable morphological defects, the maturation and final astroglial transformation of these cells proceeds surprisingly normal. The expression of intermediate filament proteins, which have been shown to characterize the maturation process of the dentate gyrus secondary radial glial population, is not altered in reeler mice (Fig. 3). Expression of BLBP, a glial protein that shows lower expression levels in reeler in neocortical radial glial cells but not in radial glial cells of the ganglionic eminence (Hartfuss et al. 2003), also appears unaltered in the reeler dentate gyrus (Fig. 3C).
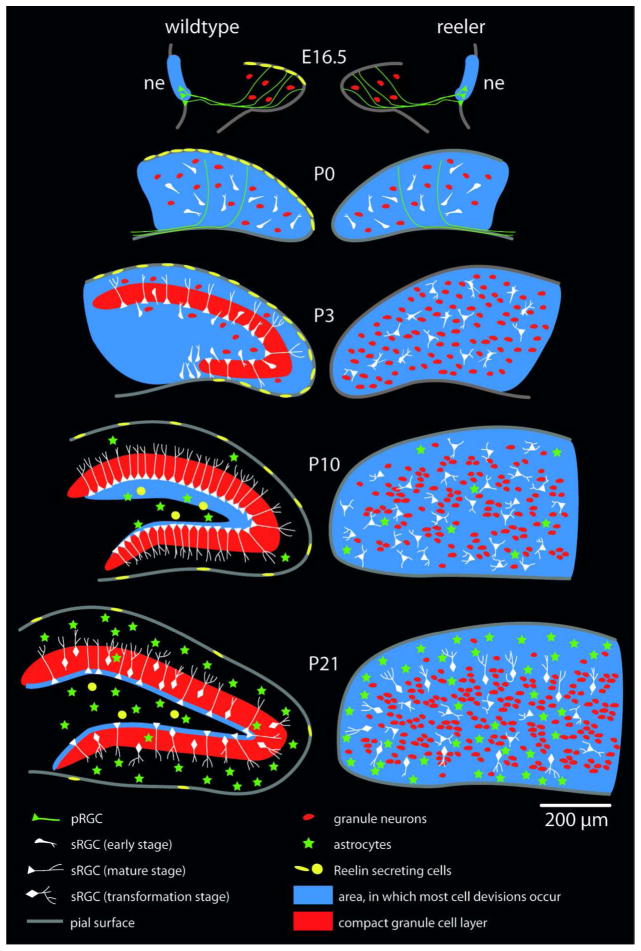
Fig. 11. Schematic diagram of dentate gyrus development in reeler and wildtype mice.
At E16.5 primary radial glial cells (pRGCs) span the whole length from the neuroepithelium (ne) to the pial surface. Cell proliferation mainly takes place in the neuroepithelium, and neurons and precursor cells migrate into the dentate anlage. P0 is a poorly organized intermediate stage, where pRGCs disappear while the secondary radial glial cells (sRGCs) do not yet form an ordered scaffold. Until P0, dentate gyrus development of reeler and wildtype mice is indistinguishable. Around P3 the secondary radial glial scaffold evolves and simultaneously granule neurons become organized in a compact cell layer. This step fails in reeler mice, where granule cells and glial cells remain scattered throughout the dentate anlage. Around P10 the sRGC scaffold is fully developed in wildtype mice and mature astrocytes become detectable in wildtype as well as reeler mice. From P14 to P21 sRGCs transform into molecular layer astrocytes which proceeds very similar in wildtype and reeler mice. Importantly, proliferating cells get confined to the subgranular zone in wildtype mice between P10 and P21, while they remain scattered throughout the dentate gyrus in reeler mice. For the sake of clarity, mossy cells, interneurons, oligodendrocytes and microglia have been omitted here.
Astrocytic transformation of secondary radial glial cells in the reeler dentate gyrus
During late stages of postnatal development (P14–P21) most secondary radial glial cells undergo a final astrocytic transformation (Brunne et al. 2010). Transforming cells migrate to the molecular layer, contributing to the high number of astrocytes in this region. Although there are a few more neurons populating the molecular layer in reeler animals, this layer clearly remains a region that is low in neurons and rich in astrocytes. Using double labeling for BLBP and GFAP to characterize the transformation process of secondary radial glial cells, we were able to show that transforming astroglial cells near the molecular layer exhibit correctly orientated processes towards this layer in reeler mice (Fig. 5). In addition astrocytes in the molecular layer express S100β, whereas glial cells inside the granule cell layer are immunoreactive for BLBP. In between there is a zone where double labeled cells are found, suggesting an inside-out gradient of molecular layer astrocytes that is similarly observed in wildtype animals (Fig. 4). In summary, during astrocytic transformation of secondary radial glial cells between P14–P21, positioning and morphology of the majority of these cells is corrected in the reeler dentate gyrus, leading to a virtually normal distribution of astrocytes in the molecular layer.
Reelin effects on dentate gyrus precursor differentiation
BrdU injections at different developmental stages and quantification of BrdU positive neurons and astrocytes three weeks post injection revealed a slight decrease in neurogenesis and an increase in astrogliogenesis from P0 to P10 (Fig. 7). These results are in agreement with previous studies concerning neurogenesis and astrogliogenesis in the mouse dentate gyrus (Cowan et al. 1980; Reznikov 1991). Oligodendrogliogenesis in the mouse dentate gyrus starts around postnatal day 9 (Reznikov 1991) and was therefore not addressed in this study, which deals mainly with earlier developmental stages. Nevertheless some of the unidentified (neither NeuN- nor GFAP-positive) BrdU-positive cells might become oligodendrocytes.
Of note, none of the investigated developmental stages showed a difference in cell differentiation between reeler and wildtype mice (Fig. 7). In addition we could show that Reelin treatment has no direct effect on dentate gyrus cell differentiation in vitro (Fig. 8). Taken together, these results do not support a strong direct effect of Reelin on cell differentiation in the developing dentate gyrus.
This contrasts to the adult reeler dentate gyrus where a significant decrease in neurogenesis and a slight increase in astrogliogenesis were found (Zhao et al. 2007). Of note, in this earlier study an outbred reeler line was used that showed an increased survival rate of reeler animals compared to the inbred line used in the present study.
The difference between adult and juvenile animals might be due to the different organization of proliferation zones. While progenitor cells are located in different positions during dentate gyrus development (Li et al. 2009), proliferation is mainly restricted to the subgranular zone in adult mice (Suppl. Fig. 3, Fig. 11). In adult neurogenesis, proliferation niches in the subgranular zone are believed to be important to protect precursor cells against an environment that overall is non-permissive to proliferation (Breunig et al. 2008; Fuentealba et al. 2012; Seri et al. 2004). These niches depend on cell arrangement and cell morphology which both are disturbed in the reeler dentate gyrus. There is no separation between the granule layer, the hilus and SGZ glial cells, which are loosely distributed in the reeler dentate gyrus at postnatal day 21 (Suppl. Fig. 2). Hence it is possible, that the disturbed morphology of the reeler dentate gyrus, in particular of the stem cell niche in the subgranular zone, contributes to the altered cell differentiation in the adult but not in the juvenile brain. A similar indirect effect of altered cell positioning is seen on the vasculature of reeler mice (Lindhorst et al. 2012). Furthermore, as neuronal progenitors in the adult dentate gyrus are lost throughout age due to the astrocytic differentiation of adult stem cells (Encinas et al. 2011), one may speculate that the reduced neurogenesis and increased astrogliogenesis in the adult reeler dentate gyrus may result from an intensified astrocytic turnover of stem cells. Slightly reduced levels of Foxg1 in the reeler dentate gyrus (Tian et al. 2012) may also contribute to the loss of progenitor cells during aging, as Foxg1 is a transcription factor necessary for the maintenance of the dentate gyrus progenitor pool. In addition, it cannot be ruled out that Reelin, via modulating interneuron circuitry, might influence adult stem cell proliferation (Song et al. 2012). As Reelin is mainly expressed in interneurons in adult animals but not during development, Reelin might regulate stem cell differentiation in adult but not juvenile animals.
Altogether, the presumable loss of stem cell niches might lead to a reduced sustainment of multipotent precursors and thus to a decrease in neurogenesis in adult reeler mice independent of direct Reelin signaling to precursor cells.
Morphological defects of secondary radial glial cells in reeler mice result from direct and indirect Reelin effects
In previous studies using slice cultures and stripe choice assays, it was shown that Reelin exerts a direct effect on dentate gyrus glial cells. These cells accumulate on Reelin containing stripes and develop longer processes after Reelin stimulation of hippocampal slice cultures from reeler animals (Förster et al. 2002; Frotscher et al. 2003; Zhao et al. 2004). Thus at least in vitro Reelin is able to influence glial cell morphology and positioning. On the other hand, radial glial cell morphology can also be influenced by the interaction with neurons (Gasser and Hatten 1990). Therefore, it is possible that the malpositioning of neurons in the reeler dentate gyrus affects radial glial morphology, too. Using Nex-Cre positive Dab1fl/fl mice missing the intracellular adaptor protein for Reelin signaling in neurons but not in glial cells, we could demonstrate that defects in secondary radial glial cells in the reeler dentate gyrus in vivo are indeed caused by a combination of direct Reelin signaling to these cells and by indirect effects, most likely resulting from the malpositioning of Dab1 negative neurons (Fig. 10). This is the first in vivo observation of a direct effect of neuronal positioning on radial glial morphology. In addition, this experiment also shows the importance of secondary radial glial cells for the correct positioning of granule neurons: The responsiveness of radial glial cells to Reelin led to a partial rescue of the positioning of neurons that are not Reelin-responsive in these mutants.
These findings also indicate that there may be major differences between the dentate gyrus and neocortex with respect to the interactions of radial glial cells and neurons. Nex-Cre positive Dab1fl/fl mice show a neocortical phenotype similar to Dab1 knockout mice (Franco et al. 2011). Thus, in contrast to the situation in the dentate gyrus, Reelin signaling to neocortical radial glial cells is not sufficient to rescue positioning of Reelin unresponsive neurons. In addition, in the neocortex the radially aligned glial scaffold is a prerequisite for proper radial migration of neurons, at least at later stages when neurons migrate via glial guided migration (Nadarajah et al. 2001). In contrast, in the dentate gyrus the granule cell layer and the secondary radial glial scaffold develop simultaneously. During all developmental stages, the radial processes of secondary radial glial cells span exactly the width of the granule cell layer (Fig. 2, Fig. 11). Together with the finding that secondary radial glial morphology depends on granule cell compaction, conversely that granule cell compaction depends on radial glial cell morphology (Fig. 10), our results indicate that the development of the dentate gyrus depends on a bidirectional interaction of radial glial cells and granule neurons.
Supplementary Material
Acknowledgments
We thank Jonathan Göldner (Center for Neuroscience, University of Freiburg, Germany) for excellent technical assistance and Manfred Olschewski (Institute of Medical Biometry and Statistics, University of Freiburg, Germany) for help with the statistical analysis. The monoclonal antibodies Rat-401 (developed by S. Hockfield) and 40E-C (developed by A. Alvarez-Buylla) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. This study was supported by the Deutsche Forschungsgemeinschaft (grants BO1806/2-1 to H.B., SFB780-B5 to J.H., M.F. and H.B., MA2410/1-2-4 to P.M., and FR 620/12-1 to M.F.). Support by the BMBF (e:bio ReelinSys, to H.B.) is acknowledged. J.H. is the recipient of a W. Paul award of the Humboldt Foundation. M.F. is Senior Research Professor of the Hertie Foundation. In addition this work was supported by the NIH (S.J.F., NS060355; U.M., NS046456 and MH078833), the Skaggs Institute for Chemical Biology (U.M.), and the Dorris Neuroscience Center (U.M.)
References
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990a;301:365–81. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990b;301:325–42. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–60. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–64. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–65. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–32. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunne B, Zhao S, Derouiche A, Herz J, May P, Frotscher M, Bock HH. Origin, maturation, and astroglial transformation of secondary radial glial cells in the developing dentate gyrus. Glia. 2010;14:14. doi: 10.1002/glia.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–8. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation of n-cofilin at the leading edge. Commun Integr Biol. 2009;2:375–7. doi: 10.4161/cib.2.4.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Stanfield BB, Kishi K. The development of the dentate gyrus. Curr Top Dev Biol. 1980;15(Pt 1):103–57. [PubMed] [Google Scholar]
- D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–79. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS. Two new mutants ‘Trembler’ and ‘Reeler’, with neurological actions in the house mouse. Journal of Genetics. 1951;50:192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- Förster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, Muller U, Frotscher M. Reelin, Disabled 1, and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:13178–83. doi: 10.1073/pnas.202035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–97. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex. 2003;13:634–40. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser UE, Hatten ME. Neuron-glia interactions of rat hippocampal cells in vitro: glial-guided neuronal migration and neuronal regulation of glial differentiation. J Neurosci. 1990;10:1276–85. doi: 10.1523/JNEUROSCI.10-04-01276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–21. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–79. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–91. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Forster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Gotz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–7. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–8. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–85. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–86. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemhuis J, Bock HH. Reelin modulates cytoskeletal organization by regulating Rho GTPases. Commun Integr Biol. 2011;4:254–7. doi: 10.4161/cib.4.3.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemhuis J, Bouche E, Frotscher M, Henle F, Hein L, Herz J, Meyer DK, Pichler M, Roth G, Schwan C, et al. Reelin signals through apolipoprotein E receptor 2 and Cdc42 to increase growth cone motility and filopodia formation. J Neurosci. 2010;30:14759–72. doi: 10.1523/JNEUROSCI.4036-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Kataoka H, Coughlin SR, Pleasure SJ. Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development. 2009;136:327–35. doi: 10.1242/dev.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhorst T, Kurz H, Sibbe M, Meseke M, Forster E. Congruence of vascular network remodeling and neuronal dispersion in the hippocampus of reelin-deficient mice. Histochem Cell Biol. 2012 doi: 10.1007/s00418-012-0912-9. [DOI] [PubMed] [Google Scholar]
- Luque JM, Morante-Oria J, Fairen A. Localization of ApoER2, VLDLR and Dab1 in radial glia: groundwork for a new model of reelin action during cortical development. Brain Res Dev Brain Res. 2003;140:195–203. doi: 10.1016/s0165-3806(02)00604-1. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Gotz M. Radial glia - from boring cables to stem cell stars. Development. 2013;140:483–6. doi: 10.1242/dev.085852. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nakahira E, Yuasa S. Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol. 2005;483:329–40. doi: 10.1002/cne.20441. [DOI] [PubMed] [Google Scholar]
- Pinto L, Gotz M. Radial glial cell heterogeneity--the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000;20:6095–105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramatarova A, Chen K, Howell BW. A genetic interaction between the APP and Dab1 genes influences brain development. Mol Cell Neurosci. 2008;37:178–86. doi: 10.1016/j.mcn.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov KY. Cell proliferation and cytogenesis in the mouse hippocampus. Adv Anat Embryol Cell Biol. 1991;122:1–74. doi: 10.1007/978-3-642-76447-9. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–39. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Amaral DG, Cowan WM. Organization of radial glial cells during the development of the rat dentate gyrus. J Comp Neurol. 1987;264:449–79. doi: 10.1002/cne.902640403. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Sibbe M, Forster E, Basak O, Taylor V, Frotscher M. Reelin and Notch1 cooperate in the development of the dentate gyrus. J Neurosci. 2009;29:8578–85. doi: 10.1523/JNEUROSCI.0958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489:150–4. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield BB, Cowan WM. The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:423–59. doi: 10.1002/cne.901850303. [DOI] [PubMed] [Google Scholar]
- Tian C, Gong Y, Yang Y, Shen W, Wang K, Liu J, Xu B, Zhao J, Zhao C. Foxg1 has an essential role in postnatal development of the dentate gyrus. J Neurosci. 2012;32:2931–49. doi: 10.1523/JNEUROSCI.5240-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Weiss KH, Johanssen C, Tielsch A, Herz J, Deller T, Frotscher M, Forster E. Malformation of the radial glial scaffold in the dentate gyrus of reeler mice, scrambler mice, and ApoER2/VLDLR-deficient mice. J Comp Neurol. 2003;460:56–65. doi: 10.1002/cne.10644. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chai X, Forster E, Frotscher M. Reelin is a positional signal for the lamination of dentate granule cells. Development. 2004;131:5117–25. doi: 10.1242/dev.01387. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chai X, Frotscher M. Balance between neurogenesis and gliogenesis in the adult hippocampus: role for reelin. Dev Neurosci. 2007;29:84–90. doi: 10.1159/000096213. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.