Abstract
AIM: To evaluate plasma levels of nitrite/nitrate (NOx), soluble Fas (sFas) antigen, tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) in patients with compensated and acute decompensated cirrhosis and to evaluate mediators causing acute decompensation in liver cirrhosis.
METHODS: This prospective study was conducted in the medical intensive care unit of an academic tertiary center. Fifty-five patients with acute decompensation (gastrointestinal hemorrhage, encephalopathy, hydropic decompensation) and twenty-five patients with compensated liver cirrhosis were included. Blood samples were taken for analyses of sFas, Nox, IL-6, TNF-α. Liver enzymes and kidney functions were also tested.
RESULTS: In patients with acute decompensation, plasma sFas levels were higher than in non-decompensated patients (15 305 ± 4646 vs 12 458 ± 4322 pg/mL, P < 0.05). This was also true for the subgroup of patients with alcoholic liver cirrhosis (P < 0.05). The other mediators were not different and none of the parameters predicted survival, except for ALT (alanine-aminotransferase). In patients with portal-hypertension-induced acute hemorrhage, NOx levels were significantly lower than in patients with other forms of decompensation (70.8 ± 48.3 vs 112.9 ± 74.9 pg/mL, P < 0.05). When NOx levels were normalized to creatinine levels, the difference disappeared. IL-6, TNF-α and sFas were not different between bleeders and non-bleeders. In decompensated patients sFas, IL-6 and NOx levels correlated positively with creatinine levels, while IL-6 levels were dependent on Child class.
CONCLUSION: In acute decompensated cirrhotic patients sFas is increased, suggesting a role of apoptosis in this process and patients with acute bleeding have lower NOx levels. However, in this acute complex clinical situation, kidney function seems to have a predominant influence on mediator levels.
Keywords: Variceal hemorrhage, Liver cirrhosis, Cytokine, Nitrite/nitrate, Soluble Fas
INTRODUCTION
Liver cirrhosis with portal hypertension is characterized by several systemic and splanchnic hemodynamic changes, such as splanchnic and systemic vasodilation, with increased cardiac output and a compensatory renal vasoconstriction[1]. A high level of nitric oxide (NOx), a short-lived soluble vasodilating molecule, has been proposed as one of the major endogenous vasodilators in portal hypertension[2,3]. Increased serum NOx levels in patients with cirrhosis have been reported repeatedly[4-10]. It is also thought to be the cause of some of the complications associated with end stage liver disease. Certain complications of cirrhosis such as the hepatopulmonary syndrome may be in part mediated by local overproduction of nitric oxide[11]. In contrast, exogenous application of nitrates is helpful in primary prophylaxis of variceal hemorrhage[12] and the endothelial dysfunction in cirrhosis results in impaired release of endothelial relaxing factors including NO[13]. Therefore, we speculated that changes in NOx levels could lead to acute decompensation of liver cirrhosis. In addition to NOx, other mediators such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) have also been associated with the hemodynamic alterations in liver cirrhosis[14-18] and might therefore influence events of acute decompensation.
Experimental studies have also suggested that apoptosis via the soluble Fas (sFas)/Fas-ligand (FasL) signalling system may play an important role in the development of liver failure[19]. High levels of sFas have also been described in certain chronic liver diseases[20,21]. However, the effects on acute decompensation of chronic liver diseases are not yet known. The aim of our study was to evaluate serum levels of NOx, cytokines and sFas in patients with portal-hypertension-associated gastrointestinal hemorrhage and to compare these with levels in patients with other forms of acute decompensation or compensated cirrhosis.
MATERIALS AND METHODS
Patients
A total of 80 consecutive patients with liver cirrhosis with (n = 55) and without (n = 25) decompensation entered this prospective study at the Department of Medicine, University Hospital Heidelberg between October 1999 and June 2000. Patients with decompensation had to be admitted immediately to the Intensive Care Unit of the medical department. Patients with additional signs of infection were excluded from this study to eliminate the influence of the infection per se on serum levels of NOx, cytokines and sFas[22]. The study was approved by the ethical committee of the University of Heidelberg. After signing informed consent patients were enrolled.
Criteria for decompensation in patients with histologically proven cirrhosis were: hydropic decompensation with ascites and peripheral edema (hydropic decompensation), encephalopathy or portal-hypertension-associated gastrointestinal hemorrhage. Patients were categorized according to their initial symptoms, and no patient was in more than one category. The severity of liver disease was evaluated according to Pugh's modification of Child's criteria.
Since cytokine levels are influenced by accompanying infections, patients with additional signs of infection were excluded. Spontaneous bacterial peritonitis (SBP) was defined as infection of the ascetic fluid, in the absence of any intra-abdominal source of infection, with a neutrophil count higher than 250 cells/mm3 ascitic fluid and/or a positive culture. Urinary tract infection was considered when the polymorphonuclear cell count was higher than 105/mm3 and/or a positive culture of urine. Systemic infection was diagnosed when two positive blood cultures were found. A pneumonia was diagnosed based on a positive sputum culture and/or typical chest X-ray.
On admission blood samples for cytokines, NOx and soluble Fas were taken. These parameters were measured together with routine laboratory tests on admission so as to exclude influences of therapies and disease outcomes on cytokine levels. The clinical and biochemical features of the patients are shown in Table 1. Five ml blood was obtained on admission in S-monovettes (Sarstedt, Germany). Serum was immediately separated by centrifugation (3000 r/min), divided into aliquot portions and stored at -25°C until assayed (within 2 mo of sampling).
Table 1.
Patient characteristics on admission
| Characteristics | Decompensated cirrhosis (n = 55) | Compensated cirrhosis (n = 25) |
| Age (yr) | 56.1 ± 14.2 | 55.6 ± 13.7 |
| Gender (M/F) | 34/21 | 15/10 |
| Etiology of cirrhosis | ||
| Alcoholic | 31 (56%) | 14 (56%) |
| Viral | 8 (15%) | 2 (8%) |
| Others | 16 (29%) | 9 (36%) |
| Child Pugh class | ||
| A/B/C | 7/24/24 | 19/6/0 |
Methods
IL-6 and TNF-α were measured in duplicates using commercially available EIA assays (CYTImmune Sciences Inc. College Park, MD USA). TNF-α assay sensitivity was 4.8 pg/mL, the intra-assay coefficient of variation was ± 8.3%, and the inter-assay coefficient of variation was ± 10.8%. For IL-6 the assay sensitivity was 3.4 pg/mL, the intra-assay coefficient of variation was ± 8.1%, and the inter-assay coefficient of variation was ± 10.4%.
SFas in serum was quantified by a colorimetric ELISA kit (Quantikine, R&D Systems GmbH Wiesbaden, Germany) according to the manufacturers’ instructions. Serum samples (100 μL) were poured into antibody-coated wells using multichannel pipettes, then incubated for 2 h at room temperatures. After washing four times 200 μL peroxidase-conjugated-polyclonal antibody solution was added to each well and incubated for 2 h at a room temperature. After washing, the substrate solution (200 μL) was added to each well and incubated for 30 min. The stop solution (50 μL) was then added to the wells and the absorbance of each well was measured at a wavelength of 450 nm (Anthos AR 2001). SFas concentrations were calculated by comparison of the optical densities (mean of duplicate patient samples) to those of the standard run on the same plate. For sFas the assay sensitivity was 3.4 pg/mL, the intra-assay coefficient of variation was ± 3.8%, and the inter-ssay coefficient of variation was ± 4.5%.
NOx was determined from total nitrite levels. Total nitrite measurements were performed on stored samples from all patients, using commercially available kits (Nitric Oxide Assay, R&D Systems GmbH Wiesbaden, Germany). The assay involved the conversion of nitrate to nitrite by nitrate reductase. The detection of nitrite is determined as a colored azo-dye product of the Griess reaction that absorbs visible light. The concentration of NO was indirectly measured by determining both nitrate and nitrite levels in the same sample. Before analyses samples were thawed at a room temperature and ultrafiltered through a 10 000 Molecular Weight cutoff filter (Microcon YM-10, Millipore Corporation, USA) to eliminate proteins. Supernatants were placed in microtiter plates in duplicates. The steps used in the assay followed manufacturers' guidelines. After development, plates were read on a microtiter plate scanner (Anthos AR 2001 [550 nm]) and the endogenous nitrite and total nitrite concentrations were calculated by normalization of the optical densities (mean of duplicate samples) of patient samples to those of the standard on the same plate.
The nitrate concentration was determined by subtracting the endogenous nitrite concentration from the total nitrite concentration. The analytic sensitivity of the nitrite assay is typically less than 0.22 μmol/L and of the nitrate reduction assay 0.54 μmol/L. Liver enzymes and creatinine levels were measured using standard commercial photometric assays. Because NOx levels correlate with kidney function (serum creatinine), they were divided by creatinine levels[16].
Statistical analysis
The data were evaluated using descriptive statistical methods (mean ± SD, ranges). For comparison of two independent variables the non-parametric Mann-Whitney U-test was used. P < 0.05 was considered significant. Correlations were assessed by Spearman's test. Statistical calculations were performed using SPSS Version 10.0 for Windows (SPSS Inc. Chicago, USA).
RESULTS
A total of 80 patients were consecutively analyzed. There were more men than women (Table 1). Alcohol was the predominant reason for cirrhosis in both groups (56%). Liver cirrhosis, as assessed by the Child-Pugh score, showed higher stages in the decompensated group.
Decompensated vs compensated cirrhosis
Serum levels of soluble Fas, NOx, TNF-α and IL-6 of patients with and without hepatic decompensation are summarized in Table 2. Among these only sFas was different (P < 0.05) between the groups with greater values in patients with decompensation. Further differences between the groups were aspartate-aminotransferase (AST) and alanine-aminotransferase (ALT) levels. In decompensated patients the values averaged 99.7 ± 273.1 U/L and 46.3 ± 153.8 U/L, respectively. The corresponding values in compensated patients were 25.2 ± 15.6 and 20.5 ± 13.9 U/L (P < 0.05). Kidney function, as determined by serum levels of creatinine, was impaired in patients with decompensation (1.3 ± 0.9 vs 0.8 ± 0.2 mg/dL, P < 0.01).
Table 2.
Serum levels of soluble Fas (sFas), nitrite/nitrate (NOx), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) in patients with acute decompensation (hemorrhage, encephalopathy, and hydropic decompensation) of liver cirrhosis (mean ± SD)
| Decompensated cirrhosis (n = 55) | Compensated cirrhosis (n = 25) | |
| SFas (pg/mL) | 15 305 ± 4646 | 12 458 ± 4322a |
| NOx (μmol/L) | 96.9 ± 68.7 | 64.2 ± 40.2 |
| TNF-α (pg/mL) | 90.9 ± 65.2 | 73.5 ± 46.5 |
| IL-6 (pg/mL) | 84.8 ± 154.3 | 180.9 ± 662.2 |
P < 0.05 vs decompensated cirrhosis.
Survival
Within the follow up period of two months, 7 patients with decompensated livers died, while none of the compensated patients died. In comparison to the decompensated survivors, neither sFas, NOx, and TNF-α nor IL-6 levels were different. Kidney function was also comparable. However, ALT levels were significantly higher in patients who died during the observation period (73.6 ± 74.8 vs 42.0 ± 162.8 U/L, P < 0.01).
Comparison of decompensated patients with and without bleeding
Portal hypertension related gastrointestinal hemorrhage was the reason for admission in 21 patients. IL-6, TNF-α and sFas levels were not significantly different between patients with and without bleeding. However, NOx levels were significantly lower in patients with bleeding (70.8 ± 48.3 vs 112.9 ± 74.9 pg/mL, P < 0.05). At the beginning kidney function, as assessed by creatinine levels, was poorer in patients with bleeding compared to patients without bleeding (0.9 ± 0.4 vs 1.5 ± 1.0 mg/dL, P < 0.05). NOx levels are dependent on kidney function and were therefore normalized to creatinine levels. This eliminated the difference. In the bleeding group, the ratio was 0.88 ± 0.62 vs 1.13 ± 0.95 in decompensated patients without bleeding (P = NS). In addition, transaminase levels were not different between the patient groups.
Correlation between mediators, transaminases (ALT, AST) and creatinine levels in patients with decompensated cirrhosis
Serum sFas levels and NOx correlated positively with creatinine levels (P < 0.05). Serum TNF-α and IL-6 levels showed no correlations and none of the mediators correlated with each other, whereas ALT correlated with AST (P < 0.001).
Etiology of cirrhosis
In patients with decompensated livers, alcohol was the reason for cirrhosis in 31 cases and viral infection was the cause in 8 patients. In alcoholic cirrhosis NOx levels were higher (104.2 ± 57.5 μmol/L), while in viral hepatitis NOx levels were significantly lower (63.9 ± 60.7 μmol/L, P < 0.05). In patients with alcoholic decompensated cirrhosis sFas levels were higher (15711 ± 4406 pg/mL) than in patients with compensated alcoholic cirrhosis (14 882 + 4588 pg/mL, P < 0.05). This again indicates a potential role of apoptosis in deteriorating liver function in these patients. The TNF-α and IL-6 levels were similar in both groups.
Dependency on liver function
Several reports have found a correlation between liver function, as assessed by the Child-Pugh score, and cytokine levels. For sFas, TNF-α and NOx no dependency on liver function was observed. However, there was a clear dependency of IL-6 levels on liver function. With deteriorating liver function, IL-6 levels increased significantly (P < 0.05) (Figure 1).
Figure 1.
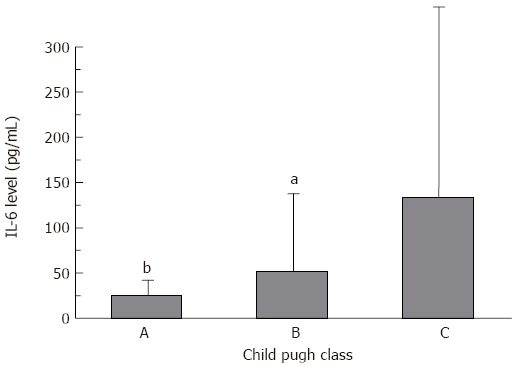
Dependency of IL-6 on Child Pugh class in patients with acutely decompensated liver cirrhosis (mean ± SD). aP < 0.05, bP < 0.01 vs class C.
DISCUSSION
Acute decompensation of liver cirrhosis is a life threatening complication of a chronic disease. The reasons why acute decompensation occurs are not well understood, but bacterial infections are thought to be involved in some patients[23]. To exclude this possibility and to evaluate the role of different mediators only patients without any signs of infection were enrolled in this study. Our results showed that in a large cohort of decompensated cirrhotic patients decompensation was accompanied by increased sFas levels. This was independent of the kind of decompensation, i.e., bleeding or hydropic decompensation and was also independent of Child stage. SFas had no relation to outcomes or transaminase levels, but correlated with kidney function.
The data showed that clinical progression and decompensation of liver disease was accompanied by an increase in sFas levels. These results are consistent with other studies demonstrating that the clinical progression of HBV from acute to chronic to malignancy is reflected by a profile of steadily increasing sFas levels[21]. Similar results were reported in patients with chronic hepatitis C[24]. We first describe such a correlation in patients with acute decompensation of chronic liver disease, especially in patients with alcoholic cirrhosis. Evidence from in vitro studies suggests that sFas can interfere with immune cell-mediated Fas/FasL apoptosis induction[25,26]. The results therefore indicate that such interference plays a progressively enhanced role during the evolution of the disease and the occurrence of acute decompensation.
In accordance with other studies we also found a dependency of sFas levels on kidney function[27]. In patients with chronic renal failure and in patients undergoing dialysis elevated sFas levels have been described. Therefore, the elevation of sFas levels in our patients might also be mediated by the degree of kidney failure. This additional factor was not mentioned in several other studies where kidney function was not evaluated.
Among many potential mediators, NOx has emerged as the main candidate implicated in the pathogenesis of the hemodynamic alterations in liver cirrhosis. In chronic liver diseases NOx is either produced as a result of high systemic endotoxin levels or shares stress of endothelial cells. In our study, NOx levels tended to be greater in patients with decompensated liver cirrhosis, however, this did not reach statistical significance. Among the decompensated patients, those with portal hypertension associated bleeding (i.e., variceal bleeding, portal hypertensive gastropathy and colonopathy) had significantly lower NOx levels than patients with other forms of decompensation (i. e., encephalopathy, hydropic decompensation).
NOx levels are determined not only by NO production but also by the degree of kidney dysfunction, the fasting state and exogenous nitrate sources[28]. There is an inverse correlation of kidney function and NOx serum levels[16]. In our cirrhotic patients with bleeding, kidney function at entry was better, as assessed by measuring serum creatinine levels, than in patients with other forms of decompensation. We therefore normalized NOx levels to serum creatinine levels and found no difference in NOx levels in decompensated patients with and without bleeding[16], thus changes in NOx levels were not responsible for the acute bleeding episode. Kidney function was evaluated by creatinine levels. This is sufficient, since alterations in kidney functions reflected by changes of creatinine concentrations are accompanied by changes in NOx levels[29]. NOx levels were determined within 24 h after admission of the patients. Since NOx serum levels have a half-life period of 5 h and serum samples from all patients were obtained in similar fashion, this should not have influenced our results[30].
In our study NOx levels were determined regardless of the fasting state. However, it has to be kept in mind that a gastrointestinal bleeding leads to a high intestinal protein load and induces encephalopathy in severe cirrhotic patients. Thus, our bleeding patients had an endogenous protein load, which could have even higher plasma NOx levels.
NOx is not the only mediator involved in the hyperdynamic circulation in cirrhosis. IL-6 and TNF-α are also thought to be involved[14,31,32] and both are able to increase NOx levels. In our study there were no differences at all between the groups and also patients with bleeding had no difference in these cytokine levels compared to patients with other forms of decompensation. Moreover, we could not find a dependency of TNF-α, sFas and NOx levels on liver function as assessed by the Child classification. Only IL-6 levels showed a dependency on liver function. This is in contrast to some studies, where such a relation could be observed[10]. Other studies were inconsistent[16]. However, serum levels were not corrected for kidney function[10]. It has been suggested that some of the elevations seen in these mediators are a consequence of liver cirrhosis and its hemodynamic alterations, rather than the cause of them. In fact, IL-6 is cleared mainly in the liver[33], and consequently the elevated IL-6 levels might be secondary to liver dysfunction.
In summary, we show that IL-6 and TNF-α are not altered in acute decompensated patients. SFas and NOx levels are different, but dependent of and correlated with kidney function. None of the mediators alone is able to induce acute decompensation. However, in this acute complex clinical situation, kidney function seems to have predominant influence on mediator levels.
Footnotes
S- Editor Wang GP L- Editor Zhu LH E- Editor Bi L
References
- 1.Liu H, Gaskari SA, Lee SS. Cardiac and vascular changes in cirrhosis: pathogenic mechanisms. World J Gastroenterol. 2006;12:837–842. doi: 10.3748/wjg.v12.i6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776–778. doi: 10.1016/0140-6736(91)91384-7. [DOI] [PubMed] [Google Scholar]
- 3.Sogni P, Moreau R, Gadano A, Lebrec D. The role of nitric oxide in the hyperdynamic circulatory syndrome associated with portal hypertension. J Hepatol. 1995;23:218–224. doi: 10.1016/0168-8278(95)80339-4. [DOI] [PubMed] [Google Scholar]
- 4.Moriyama A, Masumoto A, Nanri H, Tabaru A, Unoki H, Imoto I, Ikeda M, Otsuki M. High plasma concentrations of nitrite/nitrate in patients with hepatocellular carcinoma. Am J Gastroenterol. 1997;92:1520–1523. [PubMed] [Google Scholar]
- 5.Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139–1143. [PubMed] [Google Scholar]
- 6.Yokoyama M, Shijo H, Ota K, Kubara K, Kokawa H, Kim T, Akiyoshi N, Okumura M, Inoue K. Systemic hemodynamics and serum nitrate levels in patients undergoing endoscopic variceal ligation. Hepatology. 1996;24:47–52. doi: 10.1053/jhep.1996.v24.pm0008707281. [DOI] [PubMed] [Google Scholar]
- 7.Bories PN, Campillo B, Azaou L, Scherman E. Long-lasting NO overproduction in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1997;25:1328–1333. doi: 10.1002/hep.510250604. [DOI] [PubMed] [Google Scholar]
- 8.Genesca J, Segura R, Gonzalez A, Catalan R, Marti R, Torregrosa M, Cereto F, Martinez M, Esteban R, Guardia J. Nitric oxide may contribute to nocturnal hemodynamic changes in cirrhotic patients. Am J Gastroenterol. 2000;95:1539–1544. doi: 10.1111/j.1572-0241.2000.02092.x. [DOI] [PubMed] [Google Scholar]
- 9.Barak N, Zemel R, Ben-Ari Z, Braun M, Tur-Kaspa R. Nitric oxide metabolites in decompensated liver cirrhosis. Dig Dis Sci. 1999;44:1338–1341. doi: 10.1023/a:1026631230611. [DOI] [PubMed] [Google Scholar]
- 10.Arkenau HT, Stichtenoth DO, Frölich JC, Manns MP, Böker KH. Elevated nitric oxide levels in patients with chronic liver disease and cirrhosis correlate with disease stage and parameters of hyperdynamic circulation. Z Gastroenterol. 2002;40:907–913. doi: 10.1055/s-2002-35413. [DOI] [PubMed] [Google Scholar]
- 11.Gschossmann JM, Essig M, Reichen J, Scheurer U, Gerken G. The hepato-pulmonary syndrome--where do we stand in the year 2006? Z Gastroenterol. 2006;44:249–256. doi: 10.1055/s-2005-858995. [DOI] [PubMed] [Google Scholar]
- 12.Lowe RC, Grace ND. Primary prophylaxis of variceal hemorrhage. Clin Liver Dis. 2001;5:665–676. doi: 10.1016/s1089-3261(05)70187-2. [DOI] [PubMed] [Google Scholar]
- 13.Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–931. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 14.Byl B, Roucloux I, Crusiaux A, Dupont E, Devière J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492–1497. doi: 10.1016/0016-5085(93)90361-f. [DOI] [PubMed] [Google Scholar]
- 15.Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 16.Genesca J, Gonzalez A, Segura R, Catalan R, Marti R, Varela E, Cadelina G, Martinez M, Lopez-Talavera JC, Esteban R, et al. Interleukin-6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol. 1999;94:169–177. doi: 10.1111/j.1572-0241.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Talavera JC, Merrill WW, Groszmann RJ. Tumor necrosis factor alpha: a major contributor to the hyperdynamic circulation in prehepatic portal-hypertensive rats. Gastroenterology. 1995;108:761–767. doi: 10.1016/0016-5085(95)90449-2. [DOI] [PubMed] [Google Scholar]
- 18.Lee FY, Lu RH, Tsai YT, Lin HC, Hou MC, Li CP, Liao TM, Lin LF, Wang SS, Lee SD. Plasma interleukin-6 levels in patients with cirrhosis. Relationship to endotoxemia, tumor necrosis factor-alpha, and hyperdynamic circulation. Scand J Gastroenterol. 1996;31:500–505. doi: 10.3109/00365529609006772. [DOI] [PubMed] [Google Scholar]
- 19.Nakae H, Narita K, Endo S. Soluble Fas and soluble Fas ligand levels in patients with acute hepatic failure. J Crit Care. 2001;16:59–63. doi: 10.1053/jcrc.2001.25470. [DOI] [PubMed] [Google Scholar]
- 20.Jodo S, Kobayashi S, Nakajima Y, Matsunaga T, Nakayama N, Ogura N, Kayagaki N, Okumura K, Koike T. Elevated serum levels of soluble Fas/APO-1 (CD95) in patients with hepatocellular carcinoma. Clin Exp Immunol. 1998;112:166–171. doi: 10.1046/j.1365-2249.1998.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song le H, Binh VQ, Duy DN, Bock TC, Kremsner PG, Luty AJ, Mavoungou E. Variations in the serum concentrations of soluble Fas and soluble Fas ligand in Vietnamese patients infected with hepatitis B virus. J Med Virol. 2004;73:244–249. doi: 10.1002/jmv.20082. [DOI] [PubMed] [Google Scholar]
- 22.Connert S, Stremmel W, Elsing C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z Gastroenterol. 2003;41:165–170. doi: 10.1055/s-2003-37314. [DOI] [PubMed] [Google Scholar]
- 23.Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139–142. doi: 10.1016/S0140-6736(98)06020-6. [DOI] [PubMed] [Google Scholar]
- 24.Raghuraman S, Abraham P, Daniel HD, Ramakrishna BS, Sridharan G. Characterization of soluble FAS, FAS ligand and tumour necrosis factor-alpha in patients with chronic HCV infection. J Clin Virol. 2005;34:63–70. doi: 10.1016/j.jcv.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 26.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perianayagam MC, Murray SL, Balakrishnan VS, Guo D, King AJ, Pereira BJ, Jaber BL. Serum soluble Fas (CD95) and Fas ligand profiles in chronic kidney failure. J Lab Clin Med. 2000;136:320–327. doi: 10.1067/mlc.2000.109318. [DOI] [PubMed] [Google Scholar]
- 28.Heller J, Kristeleit H, Brensing KA, Woitas RP, Spengler U, Sauerbruch T. Nitrite and nitrate levels in patients with cirrhosis of the liver: influence of kidney function and fasting state. Scand J Gastroenterol. 1999;34:297–302. doi: 10.1080/00365529950173726. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie IM, Ekangaki A, Young JD, Garrard CS. Effect of renal function on serum nitrogen oxide concentrations. Clin Chem. 1996;42:440–444. [PubMed] [Google Scholar]
- 30.Ward FW, Coates ME, Walker R. Influence of dietary protein and gut microflora on endogenous synthesis of nitrate and N-nitrosamines in the rat. Food Chem Toxicol. 1989;27:445–449. doi: 10.1016/0278-6915(89)90030-6. [DOI] [PubMed] [Google Scholar]
- 31.Odeh M, Sabo E, Srugo I, Oliven A. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 2004;24:110–116. doi: 10.1111/j.1478-3231.2004.0894.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagano T, Yamamoto K, Matsumoto S, Okamoto R, Tagashira M, Ibuki N, Matsumura S, Yabushita K, Okano N, Tsuji T. Cytokine profile in the liver of primary biliary cirrhosis. J Clin Immunol. 1999;19:422–427. doi: 10.1023/a:1020511002025. [DOI] [PubMed] [Google Scholar]
- 33.Castell JV, Geiger T, Gross V, Andus T, Walter E, Hirano T, Kishimoto T, Heinrich PC. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988;177:357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]


