Abstract
AIM: To evaluate the ability of the time-signal intensity curve (TIC) of the pancreas obtained from dynamic contrast-enhanced magnetic resonance imaging (MRI) for differentiation of focal pancreatic masses, especially pancreatic carcinoma coexisting with chronic pancreatitis and tumor-forming pancreatitis.
METHODS: Forty-eight consecutive patients who underwent surgery for a focal pancreatic mass, including pancreatic ductal carcinoma (n = 33), tumor-forming pancreatitis (n = 8), and islet cell tumor (n = 7), were reviewed. Five pancreatic carcinomas coexisted with longstanding chronic pancreatitis. The pancreatic TICs were obtained from the pancreatic mass and the pancreatic parenchyma both proximal and distal to the mass lesion in each patient, prior to surgery, and were classified into 4 types according to the time to a peak: 25 s and 1, 2, and 3 min after the bolus injection of contrast material, namely, type-I, II, III, and IV, respectively, and were then compared to the corresponding histological pancreatic conditions.
RESULTS: Pancreatic carcinomas demonstrated type-III (n = 13) or IV (n = 20) TIC. Tumor-forming pancreatitis showed type-II (n = 5) or III (n = 3) TIC. All islet cell tumors revealed type-I. The type-IV TIC was only recognized in pancreatic carcinoma, and the TIC of carcinoma always depicted the slowest rise to a peak among the 3 pancreatic TICs measured in each patient, even in patients with chronic pancreatitis.
CONCLUSION: Pancreatic TIC from dynamic MRI provides reliable information for distinguishing pancreatic carcinoma from other pancreatic masses, and may enable us to avoid unnecessary pancreatic surgery and delays in making a correct diagnosis of pancreatic carcinoma, especially, in patients with longstanding chronic pancreatitis.
Keywords: Pancreatic carcinoma, Chronic pancreatitis, Focal pancreatic mass, Tumor-forming pancreatitis, Differential diagnosis, Dynamic magnetic resonance imaging, Time-signal intensity curve
INTRODUCTION
The differential diagnosis between carcinoma and benign lesion in the pancreas is extremely important because surgical resection offers the only chance of a cure in patients with pancreatic carcinoma or, conversely, may result in unnecessary risk of morbidity and mortality for benign lesions. Recent advances in imaging techniques have enabled us to precisely detect pancreatic carcinoma, however, it still remains difficult to distinguish chronic pancreatitis from this dismal pancreatic malignancy because chronic pancreatitis occasionally presents as a focal pancreatic swelling or mass with similar clinical and radiologic features to pancreatic carcinoma[1-3]. To complicate this issue even further, chronic pancreatitis may develop into pancreatic carcinoma[4-7], and also pancreatic carcinoma may develop obstructive chronic pancreatitis secondary to pancreatic ductal obstruction[2,8,9].
Both pancreatic carcinoma and chronic pancreatitis possess a large degree of fibrosis[10-13], which is associated with a gradual progressive enhancement on contrast-enhanced computed tomography (CT) and dynamic magnetic resonance imaging (MRI)[2,3,14,15], making the distinction of these entities difficult. We recently demonstrated a time-signal intensity curve (TIC) of the pancreas obtained from dynamic contrast-enhanced MRI to be a reliable and non-invasive monitoring technique for a precise evaluation of pancreatic fibrosis[16]. In this study, we investigated the ability of the pancreatic TIC from dynamic MRI to differentiate pancreatic carcinoma from other focal pancreatic masses, especially in patients with chronic pancreatitis.
MATERIALS AND METHODS
We evaluated 48 consecutive patients who underwent surgery for focal pancreatic masses due to focal solid tumors or focal enlargement of the pancreas, between March 1999 and May 2006. The pancreatic masses with cystic components, such as cystadenocarcinoma, intraductal papillary-mucinous neoplasm, solid-pseudopapillary tumor, or pseudocyst, were excluded. The patients ranged in age from 45 to 82 years, with a mean of 65 years. There were 30 men and 18 women. From the clinical and radiologic findings, 34 patients were suspected of having pancreatic carcinoma, 7 of having focal chronic pancreatitis (so-called tumor-forming pancreatitis), and 7 of having islet cell tumor. The surgical interventions consisted of a pancreaticoduodenectomy (PD, n = 13), a pylorus-preserving pancreaticoduodenectomy (PPPD, n = 18), a duodenum-preserving pancreatic head resection (DPPHR, n = 1), a middle pancreatectomy (MP, n = 2), a distal pancreatectomy (DP, n = 13), and a hepaticojejunostomy together with biopsies of the pancreas (n = 1). The postoperative histological evaluations of the surgical specimens revealed the pancreatic masses to be pancreatic ductal carcinoma in 33, tumor-forming pancreatitis in 8, and an islet cell tumor in 7.
All 48 patients received dynamic contrast-enhanced MRI of the pancreas prior to surgery. The pancreatic MRI was conducted by using the 1.5-T superconducting system (SIGNA Horizon LXTM; GE Medical Systems, Milwaukee, WI). We used a fat-suppressed three-dimensional fast spoiled gradient re-called echo sequence with the following imaging parameters: TR/TE, 6.0-6.1/1.3-1.4 msec; flip angle, 20°; section thickness, 6-8 mm; no intersection gap; matrix, 256 × 160; 1 excitation; field of view, 32-36 cm. The dynamic series comprised 5 individual dynamic images, obtained before and 25 s and 1, 2 and 3 min after the rapid bolus injection of 0.1 mmol of meglumine gadopentetate (Magnevist®; Schering, Berlin, Germany)/kg of body weight. The contrast medium was administered intravenously at approximately 3 mL/s followed by flushing with 20 mL saline solution. The original MRI data were then loaded onto a workstation, and the regions of interest (ROI) were placed at 3 different parts of the pancreas in each patient, i.e., the pancreatic mass and the non-tumorous pancreatic parenchyma both proximal (head-sided) and distal (tail-sided) to the mass lesion. The ROI ranged in size from 0.2 to 1 cm2. The pancreatic TIC was then generated as a percentage increase in the signal intensity (SI), according to the following enhancement formula: (SIpost-SIpre)/SIpre × 100, where SIpre and SIpost represent the pre- and post-contrast SIs, respectively. 16The patterns of pancreatic TIC were classified into 4 types (Figure 1): type-I, characterized by a rapid rise to a peak (25 s after injection of contrast material) followed by a rapid decline; type-II, with a slow rise to a peak (1 min after administration of contrast material) followed by a slow decline; and type-III or IV, with an even slower rise to a peak (2 or 3 min after the administration of contrast material) followed by a slow decline or plateau.
Figure 1.
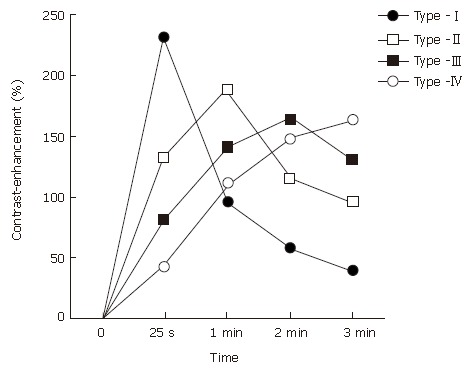
Patterns of the time-signal intensity curve (TIC) from dynamic contrast-enhanced magnetic resonance imaging of the pancreas.
A retrospective review of the preoperative pancreatic MRI study and pancreatic histology was performed, and the patterns of TIC from dynamic MRI measured at the 3 parts of the pancreas were then compared with the corresponding histological pancreatic sections in each patient.
RESULTS
The clinicopathological characteristics and the results of a pancreatic MRI study of 33 patients with pancreatic ductal carcinoma are described in Table 1.
Table 1.
Clinicopathological characteristics and pancreatic TIC profiles in patients with focal pancreatic mass due to pancreatic carcinoma
| Location of focal mass | Underlying chronic pancreatitis |
Type of pancreatic TIC of |
Histopathology of |
|||||||||
| Preoperative | operative | proximal | focal | distal | proximal | focal | distal | |||||
| Case No. | Age | Sex | diagnosis | procedure | pancreas | mass | pancreas | pancreas | mass | pancreas | ||
| 1 | 53 | M | Ph | Carcinoma | No | PD | I | III | I | Normal | IDC | Normal |
| 2 | 64 | M | Ph | Carcinoma | No | PD | I | III | I | Normal | IDC | Normal |
| 3 | 73 | F | Ph | Carcinoma | No | PD | I | III | II | Normal | IDC | TACP |
| 4 | 52 | F | Ph | Carcinoma | No | PD | I | III | II | Normal | IDC | TACP |
| 5 | 69 | M | Ph | Carcinoma | No | PPPD | I | III | II | Normal | IDC | TACP |
| 6 | 70 | F | Ph | Carcinoma | No | PPPD | I | III | II | Normal | IDC | TACP |
| 7 | 53 | M | Ph | Carcinoma | No | PPPD | I | III | II | Normal | IDC | TACP |
| 8 | 78 | F | Ph | Carcinoma | No | PPPD | I | III | II | Normal | IDC | TACP |
| 9 | 57 | M | Ph | Carcinoma | No | PPPD | I | III | II | Normal | IDC | TACP |
| 10 | 59 | F | Pb | Carcinoma | No | DP | I | III | II | Normal | IDC | TACP |
| 11 | 75 | F | Pb | Carcinoma | No | DP | I | III | II | Normal | IDC | TACP |
| 12 | 75 | F | Pb | Carcinoma | No | DP | I | III | II | Normal | IDC | TACP |
| 13 | 54 | F | Ph | Carcinoma | No | PD | I | IV | I | Normal | IDC | Normal |
| 14 | 63 | M | Ph | Carcinoma | No | PPPD | I | IV | I | Normal | IDC | Normal |
| 15 | 57 | F | Ph | Carcinoma | No | PPPD | I | IV | I | Normal | IDC | Normal |
| 16 | 67 | M | Ph | Carcinoma | No | PD | I | IV | II | Normal | IDC | TACP |
| 17 | 67 | M | Ph | Carcinoma | No | PD | I | IV | II | Normal | IDC | TACP |
| 18 | 59 | M | Ph | Carcinoma | No | PD | I | IV | II | Normal | IDC | TACP |
| 19 | 74 | M | Ph | Carcinoma | No | PD | I | IV | II | Normal | IDC | TACP |
| 20 | 69 | M | Ph | Carcinoma | No | PD | I | IV | II | Normal | IDC | TACP |
| 21 | 73 | M | Ph | Carcinoma | No | PD | I | IV | II | Normal | IDC | TACP |
| 22 | 74 | M | Ph | Carcinoma | No | PPPD | I | IV | II | Normal | IDC | TACP |
| 23 | 61 | M | Ph | Carcinoma | No | PPPD | I | IV | II | Normal | IDC | TACP |
| 24 | 64 | F | Ph | Carcinoma | No | PPPD | I | IV | II | Normal | IDC | TACP |
| 25 | 63 | M | Ph | Carcinoma | No | PPPD | I | IV | II | Normal | IDC | TACP |
| 26 | 68 | M | Ph | Carcinoma | No | PPPD | I | IV | II | Normal | IDC | TACP |
| 27 | 65 | M | Pb | Carcinoma | No | DP | I | IV | II | Normal | IDC | TACP |
| 28 | 76 | M | Pb | Carcinoma | No | DP | I | IV | II | Normal | IDC | TACP |
| 29 | 59 | M | Ph | Carcinoma | Yes | PD | II | III | II | CP | IDC | CP |
| 30 | 75 | F | Ph | Carcinoma | Yes | PPPD | II | IV | II | CP | IDC | CP |
| 31 | 57 | F | Ph | TF- pancreatitis | Yes | palliative | II | IV | II | CP | IDC | CP |
| 32 | 67 | M | Ph | TF-pancreatitis | Yes | PD | II | IV | III | CP | IDC | CP |
| 33 | 79 | F | Ph | Carcinoma | Yes | PPPD | III | IV | III | CP | IDC | CP |
TIC: time-signal intensity curve; Ph: pancreatic head; Pb: pancreatic body; TF: tumor-forming; PD: pancreaticoduodenectomy; PPPD: pylorus-preserving pancreaticoduodenectomy; DP: distal pancreatectomy; N: normal; IDC: invasive ductal carcinoma; CP: chronic pancreatitis; TACP: tumor-associated chronic pancreatitis.
Pancreatic carcinomas developed in a normal pancreas in 28 patients, whose preoperative diagnosis based on the clinical and radiologic findings was identical to the histological diagnosis. Pancreatic carcinomas demonstrated type-III (n = 12) or type-IV (n = 16) TIC. In contrast, the pancreatic parenchyma proximal to the tumor showed type-ITIC, while the distal pancreas revealed type-I(n = 5) or type-II (n = 23) TIC (Figure 2). A histological study of the distal pancreas showing type-II TIC revealed obstructive chronic pancreatitis with mild to severe fibrosis.
Figure 2.
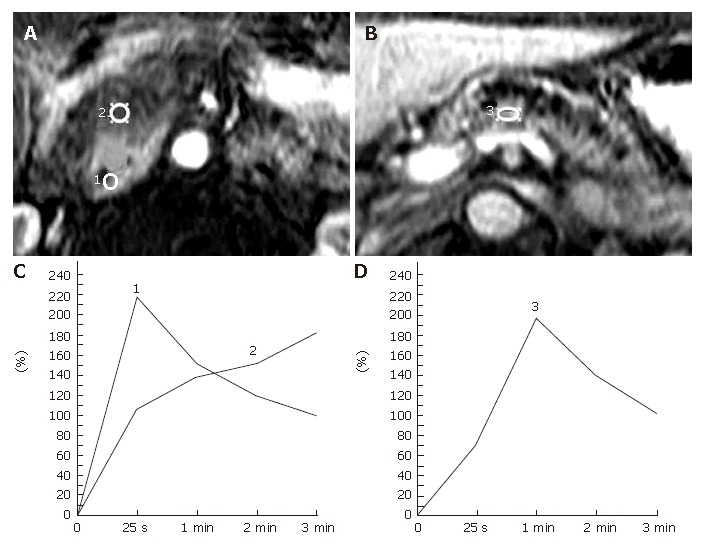
Representative pancreatic TIC profiles in patients with pancreatic ductal carcinoma developed in a normal pancreas. A, B: Dynamic contrast-enhanced MRI images of the pancreas in a 59-year-old man with carcinoma of the head of the pancreas. The ROIs are placed at the pancreatic mass (No.2 ROI) and the non-tumorous pancreatic parenchyma both proximal (No.1 ROI) and distal (No.3 ROI) to the mass lesion; C: Pancreatic TICs obtained from the no.1 and no. 2 ROIs as in (A) demonstrate type-I and type-IV, respectively; D: Pancreatic TIC obtained from the No.3 ROI as in Figure 2B shows type-II.
Five patients had pancreatic carcinoma in the background of longstanding chronic pancreatits. Two of them underwent surgery under a diagnosis of tumor-forming pancreatitis and were confirmed to be pancreatic carcinoma during the operation: one patient underwent a pancreaticoduodenectomy, while the other received a palliative operation because of the far advanced stage of the disease. The TIC profiles of 5 carcinomas coexisting with chronic pancreatitis showed type-III (n = 1) or type-IV (n = 4) TIC. Although the proximal and distal pancreas demonstrated type-II or type-III pancreatic TIC, pancreatic carcinoma displayed a distinctive TIC profile in every patient, depicting the slowest rise to a peak among the 3 pancreatic TICs measured in each individual pancreas (Figure 3).
Figure 3.
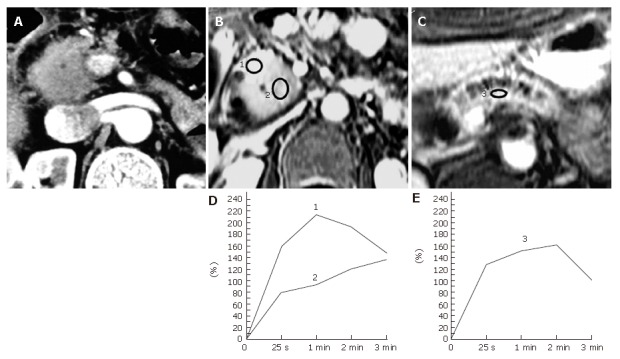
Pancreatic carcinoma occurring in a 67-year-old man with a longstanding chronic pancreatitis. A: An abdominal contrast-enhanced CT image shows a focal enlargement of the head of the pancreas. The tumor-to-parenchymal attenuation difference is obscure. The patient underwent a laparotomy under a diagnosis of tumor-forming pancreatitis presenting with obstructive jaundice and was found to have pancreas head carcinoma during the operation; B, C: Dynamic contrast-enhanced MRI images of the pancreas. The ROIs are placed at the focally enlarged pancreas head (No.2 ROI), the proximal side of the head of the pancreas (No.1 ROI) , and the body of the pancreas (No.3 ROI); D: Pancreatic TICs obtained from the No.1 and No.2 ROIs as in (B) demonstrate type-II and type-IV, respectively; E: Pancreatic TIC obtained from the No.3 ROI as in (C) shows type-III.
In 8 patients with tumor-forming pancreatitis, 6 lesions were associated with longstanding chronic pancreatitis and 2 lesions were recognized in a normal pancreas (Table 2). Preoperative diagnosis of these patients was tumor-forming pancreatitis, but in 2 patients, who underwent a pylorus-preserving pancreaticoduodenectomy together with lymphadenectomy, the possibility of carcinoma of the head of the pancreas could not be ruled out and the definitive diagnosis of chronic pancreatitis was confirmed after surgery. The focal pancreatic masses due to tumor-forming pancreatitis demonstrated the TIC of type-II (n = 5) or type-III (n = 3). Meanwhile, the TICs of the proximal and distal pancreas varied in type from type-Ito type-III. In comparing 3 pancreatic TICs measured for each patient, the TIC profile of the focal mass was identical to TICs of both the proximal and distal pancreas in 3 patients (Figure 4), or equal to at least one of these TICs in 5 patients.
Table 2.
Clinicopathological characteristics and pancreatic TIC profiles in patients with focal pancreatic mass due to chronic pancreatitis
| Location | Underlying |
Type of pancreatic TIC of |
Histopathology of |
|||||||||
| Case | of focal | Preoperative | chronic | Operative | proximal | focal | distal | proximal | focal | distal | ||
| No. | Age | Sex | mass | diagnosis | pancreatitis | procedure | pancreas | mass | pancreas | pancreas | mass | pancreas |
| 1 | 70 | M | Ph | TF-pancreatitis1 | Yes | PPPD | II | II | II | CP | CP | CP |
| 2 | 55 | M | Pb | TF-pancreatitis | Yes | MP | II | II | II | CP | CP | CP |
| 3 | 62 | M | Ph | TF-pancreatitis | Yes | PPPD | II | II | III | CP | CP | CP |
| 4 | 47 | M | Pb | TF-pancreatitis | Yes | DP | II | II | III | CP | CP | CP |
| 5 | 63 | M | Ph | TF-pancreatitis1 | Yes | PPPD | III | III | II | CP | CP | CP |
| 6 | 45 | F | Pb | TF-pancreatitis | Yes | DP | III | III | III | CP | CP | CP |
| 7 | 60 | M | Pt | TF-pancreatitis | No | DP | I | II | II | Normal | CP | CP |
| 8 | 51 | F | Pt | TF-pancreatitis | No | DP | I | III | III | Normal | CP | CP |
TIC: time-signal intensity curve; Ph: pancreatic head, Pb: pancreatic body, Pt: pancreatic tail; TF: tumor-forming; PPPD: pylorus-preserving pancreaticoduodenectomy; MP: middle pancreatectomy; DP: distal pancreatectomy; CP: chronic pancreatitis;
Suspicious of carcinoma.
Figure 4.
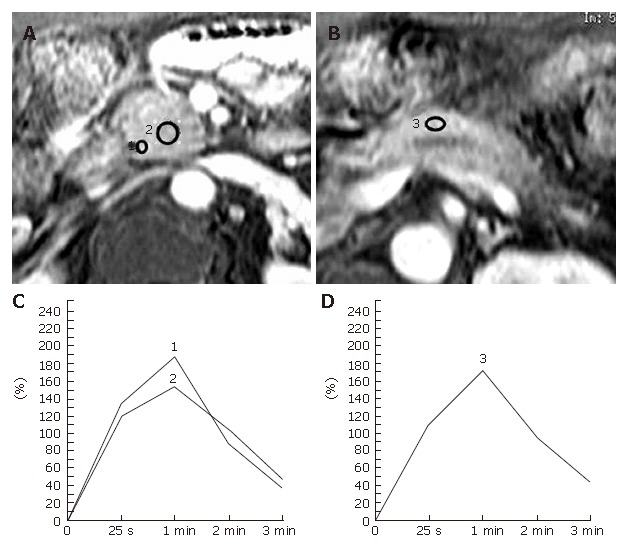
Tumor-forming pancreatitis in a 70-year-old man with a long history of alcohol abuse. The patient underwent a pylorus-preserving pancreaticoduodenectomy together with lymphadenectomy for a suspected pancreas head carcinoma associated with obstructive jaundice and was confirmed to be chronic pancreatitis after surgery. A, B: Dynamic contrast-enhanced MRI images of the pancreas. The ROIs are placed at the pancreatic mass (No.2 ROI) and the pancreatic parenchyma both proximal (nNo.1 ROI) and distal (No.3 ROI) to the mass lesion; C: Both of the pancreatic TICs obtained from the No.1 and no.2 ROIs as in Figure 4A demonstrate type-II; D: Pancreatic TIC obtained from the No.3 ROI as in Figure 4B also shows type-II.
In 7 patients with islet cell tumors, a correct diagnosis was made preoperatively on the basis of their characteristic clinical and radiologic features. The tumors demonstrated type-ITIC, and the TICs of the proximal and distal pancreas also showed type-I(Table 3). The histological study revealed the non-tumorous pancreatic parenchyma to be normal in these cases.
Table 3.
Clinicopathological characteristics and pancreatic TIC profiles in patients with focal pancreatic mass due to islet cell tumor
| Location of focal mass | Underlying chronic pancreatitis |
Type of pancreatic TIC of |
Histopathology of |
|||||||||
| Case | Preoperative | Operative | proximal | focal | distal | proximal | focal | distal | ||||
| No. | Age | Sex | diagnosis | procedure | pancreas | mass | pancreas | pancreas | mass | pancreas | ||
| 1 | 63 | M | Ph | Insulinoma | No | PPPD | I | I | I | Normal | Insulinoma | Normal |
| 2 | 68 | M | Pb | Insulinoma | No | MP | I | I | I | Normal | Insulinoma | Normal |
| 3 | 82 | M | Pb | Islet cell tumor | No | DP | I | I | I | Normal | Glucagonoma | Normal |
| 4 | 47 | F | Pb | Islet cell tumor | No | DP | I | I | I | Normal | Glucagonoma | Normal |
| 5 | 70 | M | Ph | Islet cell tumor | No | DPPHR | I | I | I | Normal | NFICT | Normal |
| 6 | 46 | F | Pb | Islet cell tumor | No | DP | I | I | I | Normal | NFICT | Normal |
| 7 | 69 | F | Pb | Islet cell tumor | No | DP | I | I | I | Normal | NFICT | Normal |
TIC: time-signal intensity curve; Ph: pancreatic head, Pb: pancreatic body; PPPD: pylorus-preserving pancreaticoduodenectomy; DPPHR: duodenum-preserving pancreatic head resection; MP: middle pancreatectomy; DP: distal pancreatectomy; NFICT: non-functioning islet cell tumor.
Overall, pancreatic ductal carcinomas demonstrated type-III (n = 13) or IV (n = 20) TIC, and the type-IV TIC was only recognized in pancreatic carcinoma among the 48 series of focal pancreatic mass. Moreover, the TIC profile of carcinoma always depicted the slowest rise to a peak among the 3 pancreatic TICs measured in each patient. Tumor-forming pancreatitis showed type-II (n = 5) or III (n = 3) TIC, and the TICs of the focal mass due to chronic pancreatitis were identical to at least one of the TICs in the proximal and distal pancreas in each patient. All islet cell tumors revealed type-ITIC.
DISCUSSION
The differentiation between pancreatic carcinoma coexisting with chronic pancreatitis and focal mass due to chronic pancreatitis continues to be a challenge. Although various diagnostic modalities have been proposed to help differentiate these 2 pancreatic entities[17-22], the accuracy of each method varies and no single non-invasive method for making a correct diagnosis has yet been suggested. The presence of a focal pancreatic mass is generally indicative of a neoplasm, and thus the patients are often subjected to major pancreatic surgery such as pancreaticoduodenectomy for presumed pancreatic malignancy that proves later to be benign in 5% to 11% of all cases[23-26]. In our series, 2 of 8 patients with tumor-forming pancreatitis underwent a pancreas head resection along with lymphadenectomy, because pancreatic carcinoma could not be ruled out. Conversely, 2 patients with preoperative diangnosis of tumor-forming pancreatitis were revealed to have pancreatic malignancy during operation. An accurate preoperative differential diagnosis is needed to avoid such unnecessary surgery and delays in making a correct diagnosis of pancreatic carcinoma.
The present study on the differentiation of focal pancreatic masses with dynamic contrast-enhanced MRI demonstrated that pancreatic ductal carcinomas exhibit a characteristic TIC profile which is different from other focal masses of the pancreas. The type-IV TIC was a unique profile indicative of pancreatic carcinoma since no other focal pancreatic masses displayed type-IV TIC. A representative TIC profile pattern of the pancreas with carcinoma was comprised of type-ITIC in the proximal pancreas, type-IV TIC in the mass lesion, and type-II TIC in the distal pancreas. Our previous study demonstrated the fibrosis ratios of pancreas with type-I, II, or III TICs as to be 3.5% (range, 1.5-10.1), 15.9% (range, 7.5-25.2), and 22.6% (range, 17.8-27.3), respectively[16]. In the present study, the type-II TIC of the distal pancreas in patients with pancreatic carcinoma also reflected the increase in fibrosis in the distal pancreas due to obstructive chronic pancreatitis. Similar findings of delayed enhancement of the pancreas distal to pancreatic carcinoma have been noted on both dual-phase CT examinations[2] and a dynamic MRI study[8].
On the other hand, there was an overlap in the TIC profile between pancreatic carcinoma and tumor-forming pancreatitis in this study, i.e., type-III TIC. The type-III TIC accounted for 39% (13/33) of pancreatic carcinoma and 38% (3/8) of tumor-forming pancreatitis. However, the series of pancreatic TICs measured in 3 parts of the individual pancreas provided helpful information for distinguishing these 2 pancreatic pathologies. The TIC profile of a mass due to carcinoma always depicted the slowest rise to a peak among the 3 pancreatic TICs, even in carcinomas occurring in patients known to have longstanding chronic pancreatitis, while the TIC profile of the focal mass due to chronic pancreatitis was identical to at least one of the proximal and distal pancreatic TICs in individual patients. Chronic pancreatitis has a risk for pancreatic carcinoma with an incidence of 2% after 10 years and 5.9% after 20 years of documented chronic pancreatitis[4], and the diagnosis of pancreatic carcinoma in this setting may therefore be difficult or even impossible[1,24,25,27,28]. Thus far, at the time of detection, the majority of patients with pancreatic carcinoma associated with chronic pancreatitis tend to be surgically unresectable. However, our findings suggest that the pancreatic TIC from dynamic MIR is a potential diagnostic tool for detecting pancreatic carcinoma in patients with longstanding chronic pancreatitis, which enable us to distinguish pancreatic carcinoma from tumor-forming pancreatitis.
The major morphologic change of chronic pancreatitis is the progressive destruction of the exocrine parenchyma with replacement by dense fibrous tissue[1-3]. However, pancreatic carcinomas also possess an abundant degree of fibrosis[10,12] since pancreatic carcinoma cells induce fibrosis by the stimulation of pancreatic stellate cells[29,30]. Fibrosis diminishes the amount of aqueous protein in the pancreatic acini and the capillary network of the pancreas that may underlie both the loss of signal intensity in the pancreas on fat-suppressed T1-weighted images and the diminished enhancement on dynamic contrast-enhanced images[31,32]. Experimental and clinical studies have demonstrated alcoholic or occlusive chronic pancreatitis and pancreatic carcinoma to be associated with tissue fibrosis, a reduced blood vessel density[33,34], and a decreased pancreatic blood flow[35-38]. In contrast, pancreatic islet cell tumors are hypervascular neoplasms[39] and it is therefore reasonable that all pancreatic islet cell tumors showed type-ITIC in this study. The number of blood vessels, the amount of aqueous protein, and the degree of fibrosis in the pancreas, along with the difference in the mass-to-pancreatic parenchymal contrast, may together play a role in the MRI contrast-enhancement of pancreatic masses. However, there is a considerable discrepancy in the reported results of the blood vessel count and the degree of fibrosis in pancreatic carcinoma, tumor-forming pancreatitis, tumor-associated chronic pancreatitis, and the normal pancreas[10,33,34,40-44]. To clarify the precise pancreatic pathology based on the pancreatic TIC from dynamic MRI, a qualitative assessment of the changes in pancreatic microcirculation during neovascularization and the obliteration of the small vessels by fibrosis or cancer cells is thus called for.
In conclusion, pancreatic TIC from dynamic MRI was found to provide reliable information for differentiating pancreatic carcinoma from a focal mass due to chronic pancreatitis and for also detecting pancreatic carcinoma associated with longstanding chronic pancreatitis. This imaging technique may therefore make it possible to eliminate the number of exploratory laparotomies as well as unnecessary major pancreatic surgery and delays in making a correct diagnosis of pancreatic carcinoma, especially in patients associated with chronic pancreatitis.
Footnotes
S- Editor Liu Y L- Editor Smith RC E- Editor Ma WH
References
- 1.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 2.Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology. 1999;212:213–218. doi: 10.1148/radiology.212.1.r99jl16213. [DOI] [PubMed] [Google Scholar]
- 3.Kim T, Murakami T, Takamura M, Hori M, Takahashi S, Nakamori S, Sakon M, Tanji Y, Wakasa K, Nakamura H. Pancreatic mass due to chronic pancreatitis: correlation of CT and MR imaging features with pathologic findings. AJR Am J Roentgenol. 2001;177:367–371. doi: 10.2214/ajr.177.2.1770367. [DOI] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 5.Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247–251. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 7.Talamini G, Falconi M, Bassi C, Sartori N, Salvia R, Caldiron E, Frulloni L, Di Francesco V, Vaona B, Bovo P, et al. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253–1260. doi: 10.1111/j.1572-0241.1999.01075.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda T, Mochizuki T, Kikuchi K, Tanaka H, Sugata S, Ikezoe J. Late-phase enhancement of the upstream portion of pancreatic adenocarcinoma on dual-phase helical CT. Abdom Imaging. 2001;26:635–639. doi: 10.1007/s00261-001-0012-0. [DOI] [PubMed] [Google Scholar]
- 9.Müller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–751. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 10.Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang ZH, Suwa H, Ishigami S, Imamura M. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas. 1995;11:357–364. doi: 10.1097/00006676-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cubilla AL, Fitzgerald PJ. Tumors of the exocrine pancreas. In: Atlas of tumor pathology., editor. 2nd ed. Volume Fascicle 19. Washington DC: Armed Forces Institute of Pathology; 1984. pp. 1–40. [Google Scholar]
- 12.Longnecker DS. Pancreas. In: Damjanov I, Linder J, editors. Anderson's Pathology. 10th ed. St. Louis: Mosby-Year Book; 1996. pp. 1891–1916. [Google Scholar]
- 13.Ritchie AC. Pancreas. In: Ritchie AC, editor. Boyd's textbook of pathology, 9th ed. Philadelphia: Lea & Febiger; 1990. pp. 1202–1234. [Google Scholar]
- 14.McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 15.Gabata T, Matsui O, Kadoya M, Yoshikawa J, Miyayama S, Takashima T, Nagakawa T, Kayahara M, Nonomura A. Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology. 1994;193:683–688. doi: 10.1148/radiology.193.3.7972808. [DOI] [PubMed] [Google Scholar]
- 16.Tajima Y, Matsuzaki S, Furui J, Isomoto I, Hayashi K, Kanematsu T. Use of the time-signal intensity curve from dynamic magnetic resonance imaging to evaluate remnant pancreatic fibrosis after pancreaticojejunostomy in patients undergoing pancreaticoduodenectomy. Br J Surg. 2004;91:595–600. doi: 10.1002/bjs.4461. [DOI] [PubMed] [Google Scholar]
- 17.Boadas J, Mora J, Urgell E, Puig P, Roca M, Cussó X, Capellà G, Lluís F, Farré A. Clinical usefulness of K-ras gene mutation detection and cytology in pancreatic juice in the diagnosis and screening of pancreatic cancer. Eur J Gastroenterol Hepatol. 2001;13:1153–1159. doi: 10.1097/00042737-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gansauge S, Gansauge F, Negri G, Galle P, Müller J, Nüssler AK, Poch B, Beger HG. The role of anti-p53-autoantibodies in pancreatic disorders. Int J Pancreatol. 1996;19:171–178. doi: 10.1007/BF02787365. [DOI] [PubMed] [Google Scholar]
- 19.Maire F, Micard S, Hammel P, Voitot H, Lévy P, Cugnenc PH, Ruszniewski P, Puig PL. Differential diagnosis between chronic pancreatitis and pancreatic cancer: value of the detection of KRAS2 mutations in circulating DNA. Br J Cancer. 2002;87:551–554. doi: 10.1038/sj.bjc.6600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Nakaoka R, Kawasaki T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–116. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 22.van Kouwen MC, Jansen JB, van Goor H, de Castro S, Oyen WJ, Drenth JP. FDG-PET is able to detect pancreatic carcinoma in chronic pancreatitis. Eur J Nucl Med Mol Imaging. 2005;32:399–404. doi: 10.1007/s00259-004-1689-4. [DOI] [PubMed] [Google Scholar]
- 23.Proca DM, Ellison EC, Hibbert D, Frankel WL. Major pancreatic resections for chronic pancreatitis. Arch Pathol Lab Med. 2001;125:1051–1054. doi: 10.5858/2001-125-1051-MPRFCP. [DOI] [PubMed] [Google Scholar]
- 24.Smith CD, Behrns KE, van Heerden JA, Sarr MG. Radical pancreatoduodenectomy for misdiagnosed pancreatic mass. Br J Surg. 1994;81:585–589. doi: 10.1002/bjs.1800810435. [DOI] [PubMed] [Google Scholar]
- 25.van Gulik TM, Reeders JW, Bosma A, Moojen TM, Smits NJ, Allema JH, Rauws EA, Offerhaus GJ, Obertop H, Gouma DJ. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417–423. doi: 10.1016/s0016-5107(97)70034-8. [DOI] [PubMed] [Google Scholar]
- 26.Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all 'chronic pancreatitis'? Am J Surg Pathol. 2003;27:110–120. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Shemesh E, Czerniak A, Nass S, Klein E. Role of endoscopic retrograde cholangiopancreatography in differentiating pancreatic cancer coexisting with chronic pancreatitis. Cancer. 1990;65:893–896. doi: 10.1002/1097-0142(19900215)65:4<893::aid-cncr2820650412>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Leung TK, Lee CM, Wang FC, Chen HC, Wang HJ. Difficulty with diagnosis of malignant pancreatic neoplasms coexisting with chronic pancreatitis. World J Gastroenterol. 2005;11:5075–5078. doi: 10.3748/wjg.v11.i32.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Semelka RC, Shoenut JP, Kroeker MA, Micflikier AB. The pancreas. In MRI of the Abdomen with CT Correlation, Semelka RC, Shoenut JP, editors. New York: Raven Press; 1993. pp. 84–98. [Google Scholar]
- 32.Semelka RC, Shoenut JP, Kroeker MA, Micflikier AB. Chronic pancreatitis: MR imaging features before and after administration of gadopentetate dimeglumine. J Magn Reson Imaging. 1993;3:79–82. doi: 10.1002/jmri.1880030114. [DOI] [PubMed] [Google Scholar]
- 33.Zhao P, Tu J, van den Oord JJ, Fevery J. Damage to duct epithelium is necessary to develop progressing lesions of chronic pancreatitis in the cat. Hepatogastroenterology. 1996;43:1620–1626. [PubMed] [Google Scholar]
- 34.De Angelis C, Valente G, Spaccapietra M, Angonese C, Del Favero G, Naccarato R, Andriulli A. Histological study of alcoholic, nonalcoholic, and obstructive chronic pancreatitis. Pancreas. 1992;7:193–196. doi: 10.1097/00006676-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Schilling MK, Redaelli C, Reber PU, Friess H, Signer C, Stoupis C, Büchler MW. Microcirculation in chronic alcoholic pancreatitis: a laser Doppler flow study. Pancreas. 1999;19:21–25. [PubMed] [Google Scholar]
- 36.Kakugawa Y, Giaid A, Yanagisawa M, Baynash AG, Melnyk P, Rosenberg L, Duguid WP. Expression of endothelin-1 in pancreatic tissue of patients with chronic pancreatitis. J Pathol. 1996;178:78–83. doi: 10.1002/(SICI)1096-9896(199601)178:1<78::AID-PATH423>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 37.Lewis MP, Lo SK, Reber PU, Patel A, Gloor B, Todd KE, Toyama MT, Sherman S, Ashley SW, Reber HA. Endoscopic measurement of pancreatic tissue perfusion in patients with chronic pancreatitis and control patients. Gastrointest Endosc. 2000;51:195–199. doi: 10.1016/s0016-5107(00)70417-2. [DOI] [PubMed] [Google Scholar]
- 38.Sofuni A, Iijima H, Moriyasu F, Nakayama D, Shimizu M, Nakamura K, Itokawa F, Itoi T. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518–525. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]
- 39.Van Hoe L, Gryspeerdt S, Marchal G, Baert AL, Mertens L. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. AJR Am J Roentgenol. 1995;165:1437–1439. doi: 10.2214/ajr.165.6.7484581. [DOI] [PubMed] [Google Scholar]
- 40.Zhongqiu W, Guangming L, Jieshou L, Xinhua Z, Ziqian C, Kui M. The comparative study of tumor angiogenesis and CT enhancement in pancreatic carcinoma. Eur J Radiol. 2004;49:274–280. doi: 10.1016/S0720-048X(03)00171-2. [DOI] [PubMed] [Google Scholar]
- 41.Rzepko R, Jaśkiewicz K, Klimkowska M, Nałecz A, Izycka-Swieszewska E. Microvascular density in chronic pancreatitis and pancreatic ductal adenocarcinoma. Folia Histochem Cytobiol. 2003;41:237–239. [PubMed] [Google Scholar]
- 42.Ueda T, Oda T, Kinoshita T, Konishi M, Nakahashi C, Takahashi S, Hasebe T, Fukao K, Ochiai A. Neovascularization in pancreatic ductal adenocarcinoma: Microvessel count analysis, comparison with non-cancerous regions and other types of carcinomas. Oncol Rep. 2002;9:239–245. [PubMed] [Google Scholar]
- 43.Cho SG, Lee DH, Lee KY, Ji H, Lee KH, Ros PR, Suh CH. Differentiation of chronic focal pancreatitis from pancreatic carcinoma by in vivo proton magnetic resonance spectroscopy. J Comput Assist Tomogr. 2005;29:163–169. doi: 10.1097/01.rct.0000153956.33296.b5. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee SK, Zoubine MN, Mullick M, Weston AP, Cherian R, Campbell DR. Tumor angiogenesis in chronic pancreatitis and pancreatic adenocarcinoma: impact of K-ras mutations. Pancreas. 2000;20:248–255. doi: 10.1097/00006676-200004000-00005. [DOI] [PubMed] [Google Scholar]


