Abstract
AIM: To determine the role of ciprofloxacin in reducing cholangitis in cholestatic patients with adequate biliary drainage after endoscopic retrograde cholangiopancreatography (ERCP).
METHODS: A randomized, controlled trial was performed in 48 cholestatic patients at Rajavithi Hospital (Tertiary Referral Center for ERCP: 600 cases per year). All the 48 patients received 200 mg ciprofloxacin intravenous injection for 30 min before starting any procedures, and then were randomly divided in two groups. Twenty-two patients in study group continually received ciprofloxacin until 48 h after ERCP. Causes of biliary obstruction, bacteriology of bile and blood (in cholangitis) and clinical cholangitis were recorded.
RESULTS: Forty-eight patients were enrolled and divided into continuous ciprofloxacin treatment group (n = 22) and discontinuous ciprofloxacin treatment group (n = 26). During ERCP, stones were found in 22 patients, malignant diseases in 24 patients and other pathologic lesions in 5 patients. One (4.5%) of the 22 patients who received ciprofloxacin and 2 (6.3%) of the 26 patients who discontinued ciprofloxacin after ERCP developed cholangitis (relative risk = 0.71; 95% CI = 0.14-3.65; p = 0.88). Bacterobilia was found in 27 (56.3%) out of 48 patients. E. coli and Streptococcus viridans were the most common organisms.
CONCLUSION: Continual use of ciprofloxacin in patients with cholestasis after adequate biliary drainage procedures plays no role in reducing cholangitis.
Keywords: Antibiotic, Cholestasis, Cholangitis, Endoscopic retrograde cholangiopancreatography, Biliary drainage
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is widely used in diagnosis and treatment of biliary and pancreatic diseases[1,2]. Acute cholangitis and septicemia remain serious complications related to this technique[3]. The incidence of cholangitis is 0.8%-19%, and its mortality rate is 10%[4].
The role of prophylactic antibiotics after ERCP has been recently assessed in several placebo-controlled randomized trials[5-9]. This procedure plays an essential part in high risk patients with prosthetic valve and history of endocarditis, obstructed bile duct, pancreatic cystic lesion and inadequate common bile duct drainage[3,10]. To date, no antibiotic regimen has emerged. Most of the bacterial florae in bile of patients with cholangitis and asymptomatic bacterobilia are Gram-negative organisms such as E. coli, Klebsella spp. and Gram-positive organisms such as Enterococcus fecalis[5-6,11-13]. The choice of an antibiotic regimen should be able to cover the Gram-negative bacteria and effectively penetrates the obstructed biliary tree. Ciprofloxacin attains a concentration in bile of approximately 20% of the mean peak level in serum, even in an obstructed biliary system. This drug level is still 10 times higher than MIC of the Gram-negative bacteria and is the most effective antibiotic against the Gram-negative bacteria, as indicated in the standard guidelines[3,5,7,10,12-13].
Ciprofloxacin is highly effective against cholestasis. However, no previous clinical study has reported the role of continuous use of ciprofloxacin in cholestatic patients after ERCP. Although many professional gastrointestinal societies recommend prophylactic use of antibiotics in the treatment of obstructive jaundice patients after ERCP, no guideline has been established after adequate biliary drainage.
The aim of this study was to evaluate the effect of ciprofloxacin in reducing the incidence of cholangitis after adequate endoscopic biliary drainage.
MATERIALS AND METHODS
Subjects
This study was a randomized, controlled trial approved by the Ethics Committee of Rajavithi Hospital. Cholestatic patients in Rajavithi Hospital (Tertiary Referral Center for ERCP), Bangkok, Thailand, admitted between June 2005 and May 2006 were included in this study. Inclusion criteria included age of 18 years or older, cholestasis (at least two from 4 criteria of total bilirubin > 2 mg/dL, serum alkaline phosphatase more than twice of normal value, alanine transaminase > 40 mmol/L and ultrasound or CT-scan showing dilated bile duct). All the patients underwent ERCP and gave their written informed consent to participate in the study. Patients were excluded from the study if they refused to participate in the study, or had endocarditis or vulvular heart disease, history of allergy to fluoroquinolone group, cholangitis or sepsis, use of antibiotics within 7 d before ERCP.
Methods
All cholestatic patients received 200 mg ciprofloxacin intravenous injection about 30 min before ERCP. Bile was aspirated before contrast medium was injected and sent to cultivation. Those who did not have biliary drainage after ERCP were excluded from this study, and ciprofloxacin was given until infection subsided. All patients who had adequate biliary drainage (defined as total relief of bile duct obstruction by stone extraction, sphincterotomy or stent replacement), were randomly divided into two groups: continuous ciprofloxacin treatment group and discontinuous ciprofloxacin treatment group. Ciprofloxacin (200 mg) intravenous injection every 12 h was given to the continuous ciprofloxacin treatment group for 48 h after ERCP, and discontinued if no infection signs and symptoms were found. The discontinuous ciprofloxacin treatment group was not given any ciprofloxacin after an adequate biliary drainage. If cholangitis occurred in any group, 200 mg ciprofloxacin intravenous injection every 12 h or other proper antibiotics (following blood or bile culture and sensitivity) were given until the infection subsided (no fever and WBC < 10 × 109/m³) (Figure 1).
Figure 1.
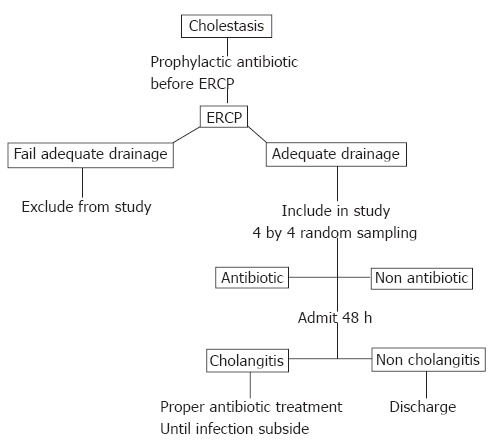
Flow chart of cholestasis management.
Vital signs were monitored every 8 h, liver function test and CBC count were performed once a day. Hemoculture was done if body temperature was above 38°C. Monitoring was continued until the doctor decided to discontinue it based on the following criteria, including stable vital signs, body temperature below 38°C, improvement in cholestasis and jaundice, and absence of abdominal pain.
ERCP was performed in all patients by the same endoscopist (Dr. Thawee Ratanachu-ek) under TJF-160 endoscope. After successful cannulation of the common bile duct, bile samples were obtained for bacteriological culture, and the definitive treatment for obstruction was performed. After each endoscopic procedure, endoscopes were manually washed by trained nurses according to the manufacturer’s instructions.
Statistical analysis
All statiscal analyses were performed using the Yates’ modified chi-square test and Student’s t test, and expressed as mean ± SD.
RESULTS
During June 2005 and May 2006, 48 patients after ERCP were enrolled in the study. All the patients received prophylactic ciprofloxacin, and divided into two groups: continuous ciprofloxacin treatment group (n = 22) and discontinuous ciprofloxacin treatment group (n = 26). The clinical characteristics and demographics in both groups were similar (Table 1). Stones were the most common cause of cholestasis in both groups. Diagnosis and intervention during ERCP are presented in Table 1.
Table 1.
Clinical characteristics of 48 patients undergoing ERCP
| Characteristic |
Ciprofloxacin
(n = 22) |
None-ciprofloxacin
(n = 26) |
||
| n | % | n | % | |
| Sex (M:F) | 13:9 | 59.1:40.9 | 11:15 | 42.3:57.7 |
| Age (mean ± SD yr) | 58.68 ± 13.7 | 61.08 ± 11.9 | ||
| Causes | ||||
| Bile duct stone | 7 | 31.8 | 15 | 57.7 |
| Cancer | 13 | 59.1 | 11 | 42.3 |
| Cholangiocarcinoma | 6 | 27.3 | 6 | 23.1 |
| Periampullary cancer | 6 | 27.3 | 5 | 19.2 |
| Hepatocellular cancer | 1 | 4.5 | 0 | 0 |
| Others | 2 | 9.1 | 3 | 11.54 |
| Bile duct stricture | 1 | 4.5 | 1 | 3.8 |
| Pseudocyst | 1 | 4.5 | 0 | 0 |
| Choledochal cyst | 0 | 0 | 1 | 3.8 |
| Chronicpancreatitis | 0 | 0 | 1 | 3.8 |
| Interventions | ||||
| Stone extraction | 5 | 22.7 | 13 | 50 |
| Sphincterotomy | 13 | 59.1 | 14 | 53.8 |
| Stent placement | 16 | 72.7 | 14 | 53.8 |
Cholangitis was found in one (4.5%) out of 22 patients in the continuous ciprofloxacin treatment group and 2 (7.7%) out of 26 patients in the discontinuous ciprofloxacin treatment group. No sepsis and death occurred in this study.
The incidence of cholangitis was also similar in ciprofloxacin-treated patients after ERCP and controls (the relative risk = 0.71, 95% CI = 0.14 to 3.65). The total incidence of bacterobilia was 56.3% (27/48). The incidence of bacterobilia was 59.1% (13/22) in the continuous ciprofloxacin treatment group and 53.8% (14/26) in the control group (relative risk = 1.12, 95% CI = 0.60 to 2.11) (Table 2).
Table 2.
Incidence of cholangitis and bacteremia in two study groups
| Complication |
Ciprofloxacin post-ERCP |
Relative risk | 95% CI | |
| Yes (n = 22) | No (n = 26) | |||
| Cholangitis | 1 (4.5%) | 2 (7.7%) | 0.71 | 0.14-3.65 |
| Bacteremia | 13 (59.1%) | 14 (53.8%) | 1.12 | 0.60-2.11 |
Gram-negative organisms were the most commonly found in the bile cultures. The bacteriology of bile cultures from both groups is illustrated in Table 3.
Table 3.
Bacterial species in cultures of bile from 48 patients
| Bacterial culture | Antibiotic (22) n (%) | No antibiotic (26) n (%) | P |
| Negative | 9 (40.9) | 12 (46.2) | 0.72 |
| Positive | 13 (59.1) | 14 (53.8) | 0.72 |
| Gram negative | |||
| E. coli | 4 (30.8) | 3 (21.4) | 0.81 |
| Klebsella spp. | 2 (15.4) | 2 (14.3) | 0.73 |
| Pseudomonas spp. | 2 (15.4) | 1 (7.1) | 0.88 |
| Gram positive | 3 (13.6) | 9 (34.6) | 0.09 |
| Streptococcus viridans | 3 | 4 (28.6) | |
| Streptococcus bovis | 0 | 1 (7.1) | |
| Enterococcus fecalis | 0 | 3 (21.4) | |
| Staphylococcus spp. | 0 | 1 (7.1) | |
| Non C. albican | 1 (7.7) | 0 |
Three (11.1%) out of 27 patients with bacterobilia who developed cholangitis were found to have Gram-negative organisms in their blood culture. One female patient in the continuous ciprofloxacin treatment group had hepatocellular cancer, and a stent was inserted to the right anterior segment of the liver. She was febrile on the second day after ERCP, indicating that she had leukocytosis (WBC count was 10.8 × 109/m³). She received 2 g ceftriazone intravenous injection daily for 11 d until infection subsided.
Two patients developed cholangitis in the discontinuous ciprofloxacin treatment group. Both received proper antibiotics until infection subsided. The results of bile cultures and hemocultures are shown in Table 4.
Table 4.
Bile and hemoculture in three cholangitis patients
| Group |
Bacteriology |
|
| Bile culture | Hemoculture | |
| Antibiotic group | Streptococcus-viridans | Negative |
| No antibiotic group | ||
| First patient | E. coli | Negative |
| Second patient | Pseudomonas spp. | Negative |
DISCUSSION
Bacteremia and cholangitis are the important complications of ERCP, and their incidence varies from 0.8%-19%[4]. It is known that prophylactic antibiotic treatment before ERCP plays a crucial role in the treatment of high risk patients with bile duct obstruction, pancreatic pseudocyst and inadequate cholestatic drainage[3,10-11]. However, the efficacy of such extensive use of antibiotics after ERCP has not been previously demonstrated, thus leading to the design of our study.
All the patients had mild or moderate cholangitis. This might be due to a benefit of the routine use of prophylactic ciprofloxacin in cholestatic patients before ERCP. Loperfido et al[14] found that small center, jaundice and male sex were risk factors for developing cholangitis after ERCP. We had only one male patient out of 3 cholangitis patients who had obstructive jaundice in our hospital (Tertiary Referral Center for ERCP: 600 cases per year).
In our study, cholangitis incidence after ERCP was lower than that in previous studies[8,9,15]. This might be attributed to the low natural incidence of the disease[4], standard sterile technique used in our hospital, and all procedures done by the same endoscopist, and our technique in collecting the bile before injecting contrast media. All these suggest that bacteriologic study and release of biliary tract pressure can reduce the incidence of pressure-induced sepsis.
E. coli, Streptococcus viridans and Klebsiella spp. are the most frequently isolated organisms from bile. Rung R et al[13] have found a similar incidence of bacterobilia (56.3%). Ciprofloxacin was chosen in our study because of its efficacy against most Gram-negative organisms (E. coli and Klebsiella spp.) which are the pathogens most frequently found in biliary tract infection[5-6,12-13,16-17] and its penetration into the obstructive biliary tree[6].
We found 12 Gram-positive bacteria in bile cultures (Streptococcus viridans and Enterococcus spp.) of the 27 bacterobilia specimens (56.3%), which is consistent with previous studies on bacteriology of bile in patients with obstructive jaundice, showing that Streptococcus viridans and Enterococcus spp. are the most common Gram-positive bacteria in bile cultures[18-23]. Clark et al[24] have found the same results and recommended ampicillin/sulbactam for all biliary obstructions with positive bile Enterococci. We conclude that once Gram positive bacteria are detected, perioperative use of appropriate antibiotics is mandatory to reduce postoperative septic complications.
The results of our trial showed no difference in the incidences of cholangitis between the two groups (4.5% in the continuous ciprofloxacin treatment group and 7.7% in discontinuous ciprofloxacin treatment group, relative risk = 0.71, 95% CI = 0.14 to 3.65, P = 0.88).
Alveyn CG et al[25] used 750 mg of ciprofloxacin taken orally in 47 jaundice patients about 90 min before ERCP to prevent sepsis and we used the same sample size (48 patients). Our suggestion is that if a larger sample size is employed, this outcome may be strongly enforced.
In conclusion, extensive use of antibiotics after adequate drainage by ERCP in cholestatic patients does not substantially reduce the incidence of cholangitis. Continuous use of antibiotics is unnecessary in these patients. The results of this study may contribute to decreasing the drawbacks of the widespread use of antibiotics including cost, adverse effects, and emergency of resistant strains of bacteria.
Footnotes
S- Editor Wang GP L- Editor Wang XL E- Editor Bai SH
References
- 1.Carr-Locke DL. Overview of the role of ERCP in the management of diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2002;56:S157–S160. doi: 10.1067/mge.2002.129023. [DOI] [PubMed] [Google Scholar]
- 2.ASGE guidelines for clinical application. The role of ERCP in diseases of the biliary tract and pancreas. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1999;50:915–920. doi: 10.1016/s0016-5107(99)70195-1. [DOI] [PubMed] [Google Scholar]
- 3.Rey JR, Axon A, Budzynska A, Kruse A, Nowak A. Guidelines of the European Society of Gastrointestinal Endoscopy (E.S.G.E.) antibiotic prophylaxis for gastrointestinal endoscopy. European Society of Gastrointestinal Endoscopy. Endoscopy. 1998;30:318–324. [PubMed] [Google Scholar]
- 4.Bilbao MK, Dotter CT, Lee TG, Katon RM. Complications of endoscopic retrograde cholangiopancreatography (ERCP). A study of 10,000 cases. Gastroenterology. 1976;70:314–320. [PubMed] [Google Scholar]
- 5.Sung JJ, Lyon DJ, Suen R, Chung SC, Co AL, Cheng AF, Leung JW, Li AK. Intravenous ciprofloxacin as treatment for patients with acute suppurative cholangitis: a randomized, controlled clinical trial. J Antimicrob Chemother. 1995;35:855–864. doi: 10.1093/jac/35.6.855. [DOI] [PubMed] [Google Scholar]
- 6.van den Hazel SJ, Speelman P, Tytgat GN, Dankert J, van Leeuwen DJ. Role of antibiotics in the treatment and prevention of acute and recurrent cholangitis. Clin Infect Dis. 1994;19:279–286. doi: 10.1093/clinids/19.2.279. [DOI] [PubMed] [Google Scholar]
- 7.Mehal WZ, Culshaw KD, Tillotson GS, Chapman RW. Antibiotic prophylaxis for ERCP: a randomized clinical trial comparing ciprofloxacin and cefuroxime in 200 patients at high risk of cholangitis. Eur J Gastroenterol Hepatol. 1995;7:841–845. [PubMed] [Google Scholar]
- 8.Niederau C, Pohlmann U, Lübke H, Thomas L. Prophylactic antibiotic treatment in therapeutic or complicated diagnostic ERCP: results of a randomized controlled clinical study. Gastrointest Endosc. 1994;40:533–537. doi: 10.1016/s0016-5107(94)70247-0. [DOI] [PubMed] [Google Scholar]
- 9.Byl B, Devière J, Struelens MJ, Roucloux I, De Coninck A, Thys JP, Cremer M. Antibiotic prophylaxis for infectious complications after therapeutic endoscopic retrograde cholangiopancreatography: a randomized, double-blind, placebo-controlled study. Clin Infect Dis. 1995;20:1236–1240. doi: 10.1093/clinids/20.5.1236. [DOI] [PubMed] [Google Scholar]
- 10.Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Waring JP, Fanelli RD, Wheeler-Harbough J, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58:475–482. doi: 10.1067/s0016-5107(03)01883-2. [DOI] [PubMed] [Google Scholar]
- 11.American Society for Gastrointestinal Endoscopy. Antibiotic prophylaxis for gastrointestinal endoscopy. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1995;42:630–635. doi: 10.1016/s0016-5107(95)70032-3. [DOI] [PubMed] [Google Scholar]
- 12.McGrath K, Baillie J. Cholangitis. Current treatment options in gastroenterology. Gastroenterology. 1999;2:323–336. [Google Scholar]
- 13.Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest Endosc. 2002;56:885–889. doi: 10.1067/mge.2002.129604. [DOI] [PubMed] [Google Scholar]
- 14.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 15.Leung JW, Venezuela RR. Cholangiosepsis: endoscopic drainage and antibiotic therapy. Endoscopy. 1991;23:220–223. doi: 10.1055/s-2007-1010662. [DOI] [PubMed] [Google Scholar]
- 16.Brandes JW, Scheffer B, Lorenz-Meyer H, Körst HA, Littmann KP. ERCP: Complications and prophylaxis a controlled study. Endoscopy. 1981;13:27–30. doi: 10.1055/s-2007-1021637. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein R, Yassin K, Suissa A, Lavy A, Eidelman S. Failure of cefonicid prophylaxis for infectious complications related to endoscopic retrograde cholangiopancreatography. Clin Infect Dis. 1996;23:378–379. doi: 10.1093/clinids/23.2.378. [DOI] [PubMed] [Google Scholar]
- 18.Siegman-Igra Y, Schwartz D, Konforti N. Polymicrobial bacteremia. Med Microbiol Immunol. 1988;177:169–179. doi: 10.1007/BF00232896. [DOI] [PubMed] [Google Scholar]
- 19.Hochwald SN, Burke EC, Jarnagin WR, Fong Y, Blumgart LH. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. doi: 10.1001/archsurg.134.3.261. [DOI] [PubMed] [Google Scholar]
- 20.Nomura T, Shirai Y, Hatakeyama K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci. 2000;45:2183–2186. doi: 10.1023/a:1026640603312. [DOI] [PubMed] [Google Scholar]
- 21.Sheen-Chen S, Chen W, Eng H, Sheen C, Chou F, Cheng Y, Lee T. Bacteriology and antimicrobial choice in hepatolithiasis. Am J Infect Control. 2000;28:298–301. doi: 10.1067/mic.2000.107071. [DOI] [PubMed] [Google Scholar]
- 22.Flores C, Maguilnik I, Hadlich E, Goldani LZ. Microbiology of choledochal bile in patients with choledocholithiasis admitted to a tertiary hospital. J Gastroenterol Hepatol. 2003;18:333–336. doi: 10.1046/j.1440-1746.2003.02971.x. [DOI] [PubMed] [Google Scholar]
- 23.Ryan JM, Ryan BM, Smith TP. Antibiotic prophylaxis in interventional radiology. J Vasc Interv Radiol. 2004;15:547–556. doi: 10.1097/01.rvi.000024942.58200.5e. [DOI] [PubMed] [Google Scholar]
- 24.Clark CD, Picus D, Dunagan WC. Bloodstream infections after interventional procedures in the biliary tract. Radiology. 1994;191:495–499. doi: 10.1148/radiology.191.2.8153328. [DOI] [PubMed] [Google Scholar]
- 25.Alveyn CG, Robertson DA, Wright R, Lowes JA, Tillotson G. Prevention of sepsis following endoscopic retrograde cholangiopancreatography. J Hosp Infect. 1991;19 Suppl C:65–70. doi: 10.1016/0195-6701(91)90169-9. [DOI] [PubMed] [Google Scholar]


