Abstract
AIM: To evaluate the time dependence of intra-arterial 5-fluorouracil (5-FU) therapy for advanced hepatocellular carcinoma (aHCC).
METHODS: Thirty-seven adult Japanese patients who had aHCC and liver cirrhosis were treated with combined intra-arterial 5-FU, cisplatin (CDDP), and leucovorin (LV). The Japan Integrated Staging score (JIS score) of each patient was 3 or more. The patients were divided into two groups, after which the 15 patients in group S were treated with 6-h infusion chemotherapy (LV at 12 mg/h, CDDP at 10 mg/h, and 5-FU at 250 mg/m2 per 4 h) and the 22 patients in group L were treated with 24-h infusion chemotherapy (LV at 12 mg/h, CDDP at 10 mg/h, and 5-FU at 250 mg/m2 per 22 h). Continuous infusion chemotherapy was performed via the proper hepatic artery every 5 d for 4 wk using an implanted drug reservoir.
RESULTS: The percentages of patients with a partial response after 4 wk of chemotherapy were 6.7% in group S and 31.8% in group L. The survival of group L was significantly better than that of group S, with the median survival time being 496 d in group L and 226 d in group S (p < 0.05).
CONCLUSION: Continuous 24-h intra-arterial infusion is more effective for aHCC and can markedly prolong survival time as compared to 6-h infusion.
Keywords: 5-fluorouracil, Cisplatin, Advanced hepato-cellular carcinoma, Liver cirrhosis, Intra-arterial chemotherapy
INTRODUCTION
The majority of patients with advanced hepatocellular carcinoma (aHCC) survive no longer than 6 mo from the day of initial diagnosis[1]. It was reported that improvement of implanted drug delivery systems has made it possible to administer repeated hepatic arterial infusion of anticancer agents to patients with aHCC and that hepatic arterial infusion therapy not only improved survival but also the quality of life (QOL)[2]. Continuous local arterial infusion of 5-fluorouracil (5-FU) and cisplatin (CDDP) using an infuser pump and an implanted reservoir has been shown to prolong the survival of patients with severe advanced HCC[2-4]. Leucovorin is a biochemical modulator of 5-FU[5-7]. A randomized study showed that the regimen using CDDP, 5-FU, and leucovorin (LV) was significantly better than that of the low-dose CDDP and 5-FU alone[8]. However, there were differences of the response rate and the regimen used in these studies, such as 250 mg of 5-FU for 5 or 24 h. For staging of HCC, Cancer of the Liver Italian Program (CLIP) score[9] has been reported to be very useful[10-12]. However, it was also reported that the stratification ability and prognostic power of the Japan Integrated Staging (JIS) score[13] were superior to those of the CLIP score for staging of HCC[14]. Accordingly, this study was performed to evaluate the time dependence of intra-arterial 5-FU therapy for aHCC by using the JIS score.
MATERIALS AND METHODS
Patients
Thirty-seven adult Japanese patients who had aHCC and liver cirrhosis due to HCV infection (C-LC) were treated with combined intra-arterial 5-FU, CDDP, and LV at Omori Hospital, Japan between 2000 and 2005. According to the computed tomography (CT) findings, the tumors were inoperable, with a JIS score of 3 or more in all patients.
Chemotherapy regimen
The patients were divided into two groups. Group S comprised 15 patients (10 men and 5 women) who were treated with 6-h infusion chemotherapy (LV at 12 mg/h, CDDP at 10 mg/h, and 5-FU at 250 mg/m2 per 4 h), while group L included 22 patients (15 men and 7 women) who were treated with 24-h infusion chemotherapy (LV at 12 mg/h, CDDP at 10 mg/h, and 5-FU at 250 mg/m2 per 22 h). Doses of the chemotherapy were according to a previous report[8]. Continuous infusion chemotherapy was performed via the proper hepatic artery every 5 d for 4 wk using a catheter connected to a subcutaneously implanted drug delivery system. Subsequently, the same chemotherapy was continued for as long as possible.
System placement technique
In all patients, an intra-arterial catheter was inserted via the femoral artery and was attached to a subcutaneously implanted reservoir[15]. In principle, the gastroduodenal artery and the right gastric artery were occluded with steel coils to prevent gastroduodenal injury from anticancer agents. Written informed consent was obtained from each patient or family members after the possible complications of reservoir implantation and arterial infusion chemotherapy had been fully explained.
Evaluation of therapeutic effect
On CT scans obtained after 4 wk of treatment, the size of the intrahepatic tumors was measured as the product of the two longest perpendicular diameters of the largest tumor. CT images were acquired according to the same method as performed for pretreatment workup. The response criteria defined by the Liver Cancer Study Group of Japan were used. A complete response (CR) was defined as disappearance of the tumor and no evidence of new lesions for at least 4 wk, while a partial response (PR) was defined as reduction of the product of the two longest diameters by more than 50%. An increase of the product by more than 25% was defined as progressive disease (PD), and the changes in between PD and PR were defined as stable disease (SD). The response was also evaluated by measuring serum alpha-fetoprotein (AFP), AFP-L3, and PIVKA-II levels in the patients with elevated levels of these markers. The survival period was defined as the interval between the start of treatment and death.
Statistical analysis
The Mann-Whitney test was used to compare the patient characteristics between the two groups. Survival was evaluated by the Kaplan-Meier method, and the significance of differences in survival was determined by the log rank test. P value less than 0.05 was considered statistically significant.
RESULTS
The group S comprised a total of 10 men and 5 women aged 54 to 79 years (mean ± SD, 68.3 ± 7.4 years), while the group L comprised 15 men and 7 women aged 52 to 76 years old (mean ± SD, 66.6 ± 7.8 years). There was no significant difference between the both groups. The Child-Pugh class was A for 6 patients in group S and 11 patients in group L, while it was B for 7 and 9 patients, respectively, and C for 2 patients in each group. Two patients had stage III, 12 stage IVA, and one patient stage IVB disease in group S, while the respective numbers were 1, 14, and 7 in group L. Seven patients had a JIS score of 3, seven patients had a JIS score of 4, and one patient had a JIS score of 5 in group S, while the respective numbers were 12, 8, and 2 in group L. In group S, one patient had tumor thrombi in major branches of the portal vein and four patients had tumor thrombi in the first portal branch. In group L, there was also one patient with tumor invasion into the right hepatic vein and four patients with tumor thrombi in the first portal branch (Table 1).
Table 1.
Clinical characteristic of the 37 patients with advanced HCC and HCV cirrhosis
| Mean age | Group S: 68.3 yr |
| Group L: 66.6 yr | |
| Gender | Group S: 10 males, 15 females |
| Group L: 15 males, 7 females | |
| Child-Pugh classification | Group S A: 6, B: 7, C: 2 |
| Group L A: 11, B: 9, C: 2 | |
| Stage | Group S III: 2, IVA: 12, IVB: 1 |
| (Vp3: 4, Vp4: 1, vv2: 0) | |
| Group L III: 1, IVA: 14, IVB: 7 | |
| (Vp3: 3, Vp4: 0, vv2: 1) | |
| JIS score | Group S 3: 7, 4: 7, 5: 1 |
| Group L 3: 12, 4: 8, 5: 2 |
Response
Table 2 summarizes the response to treatment and the survival of the aHCC patients, all of whom had a JIS score ≥ 3. In group S, one of the 15 (6.7%) patients achieved PR, but no patient achieved CR. Also, 11 of the 15 (73.3%) patients showed PD and 3 (20.0%) patients had SD. In group L, seven of the 22 (31.8%) patients achieved PR, although none of the patients achieved CR. Six of the 22 (27.3%) patients showed PD, but 9 (40.9%) patients had SD. The response rate in group L was significantly better than that in group S (P < 0.05, Mann-Whitney test).
Table 2.
Objective response and survival in the both group (%)
| Group |
JIS score |
|||
| 3 | 4 | 5 | ||
| 1-yr | Group S | 14.3 | 0.0 | 0.0 |
| Group L | 50.0 | 16.7 | 16.7 | |
| 2-yr | Group S | 0.0 | 0.0 | 0.0 |
| Group L | 16.7 | 0.0 | 0.0 | |
| 3-yr | Group S | 0.0 | 0.0 | 0.0 |
| Group L | 16.7 | 0.0 | 0.0 | |
Objective response rate: Group S 6.7% (1/15 cases) CR: PR: SD: PD = 0: 1: 3: 11; Group L 31.8% (7/22 cases) CR: PR: SD: PD = 0: 7: 9: 6.
Survival
In group S, the 1-year survival rates for the patients with a JIS score of 3, 4, and 5 were 14.3%, 0.0%, and 0.0%, respectively. There were no survivors for 2 years or more. In group L, the 1-year survival rates for the patients with a JIS score of 3, 4, and 5 were 50.0%, 16.7%, and 16.7%, respectively, while the 2-year survival rates for those patients were 16.7%, 0%, and 0%, respectively. None of the patients in either group survived for 3 years (Table 2).
The survival of group L was significantly better than that of group S, with the median survival time being 496 d in group L and 226 d in group S (P < 0.05) (Figure 1).
Figure 1.
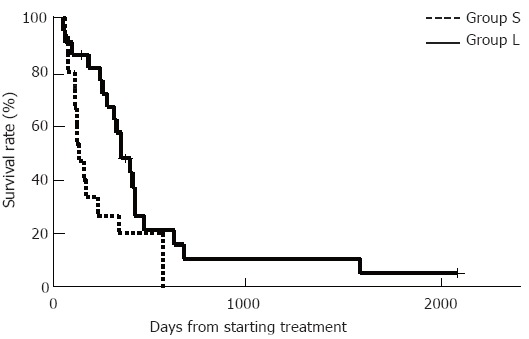
Survival curves plotted by the Kaplan-Meier method. The survival of group L was significantly better than that of group S. The median survival time was 496 d in group L versus 226 d in group S (aP < 0.05, Kaplan-Meier method and log-rank test).
Tumor markers
Figure 2 summarizes the changes of serum AFP following treatments in both groups. In group L, the serum AFP level decreased significantly after treatment compared with that before treatment. However, there was no significant change of the serum AFP level in group S. Moreover, there were no significant changes of the serum AFP-L3 and PIVKA-II levels in both groups (data not shown).
Figure 2.
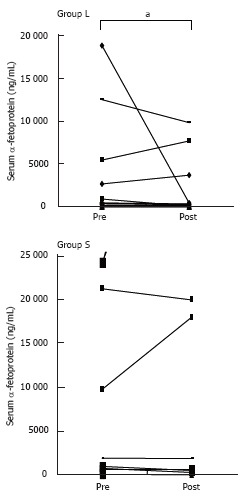
Changes of serum AFP following treatment in the both groups. In group L, the serum AFP level decreased significantly after treatment compared with before treatment (aP < 0.05, Mann-Whitney test).
Hematologic toxicity
Figure 3 summarizes the changes of the white blood cell count following treatment in both groups, showing that there were no significant differences in either of the groups. Similarly, there were no significant changes of lymphocyte or platelet count before and after treatment in either group (data not shown). However, the neutrophil and monocyte counts were significantly decreased after treatments in group L compared to group S (Figure 4).
Figure 3.
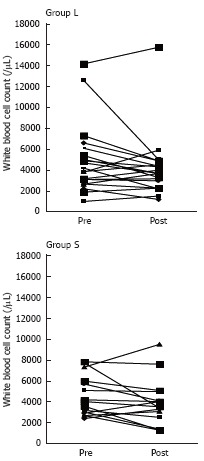
Changes of the white blood cell count following treatment in the both groups. There were no significant differences in either group.
Figure 4.
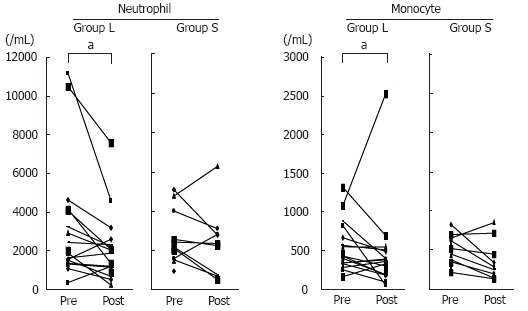
Changes of neutrophil and monocyte counts following treatment in the both groups. In group L, the neutrophil and monocyte counts were significantly decreased after treatment as compared with before treatment (aP < 0.05, Mann-Whitney test).
DISCUSSION
The majority of patients with advanced hepatocellular carcinoma (aHCC) live no longer than 6 mo from the day of diagnosis[1]. It was also reported that the average survival of patients with aHCC was 4 mo from the onset of symptoms and 2 mo from the time of admission[16]. In the present study, one of 15 (6.7%) patients in group S achieved PR, while seven of 22 (31.8%) patients in group L reached a state of PR. The survival of group L was significantly better than that of group S, with the median survival time being 496 d in group L versus 226 d in group S. Moreover, the serum AFP level decreased significantly after treatment in group L, although this change was not observed in group S. Regarding hematologic toxicity, the neutrophil and monocyte counts were significantly decreased after treatment in group L, while these changes were not observed in group S. In the present study, the severity of disease was assessed by the JIS score, making it possible to observe effect of continuous intra-arterial infusion for 6 and 24 h in C-LC patients with aHCC of similar severity. It was demonstrated that the method of continuous intra-arterial infusion for 24 h was more effective and could prolong survival compared to 6-h infusion. Both treatments were effective for aHCC in the patients with C-LC, when we excluded the patients with a JIS score ≤ 2 in this study. However, 24-h infusion caused a greater decrease of the neutrophil and monocyte counts compared to 6-h infusion.
5-FU and CDDP have been the most commonly used drugs in combination regimens because CDDP amplifies the effect of 5-FU by biochemical modulation in addition to its own action[6,17,18]. Moreover, LV has a synergistic effect in promoting the biochemical modulation of 5-FU. It has been reported that chemotherapy using 5-FU, CDDP, and LV is superior to other treatments (5-FU alone, CDDP alone, and 5-FU plus LV) with respect to controlling tumor growth, even if the concentrations of 5-FU and LV are reduced by half[8]. Therefore, we selected the combination of 5-FU, CDDP, and LV for intra-arterial infusion to treat aHCC, based on the employment of CDDP and LV as modulators of 5-FU. 5-FU has been reported to exhibit its anticancer effects via the following mechanisms: (1) Inhibition of deoxyribonucleic acid (DNA) synthesis through inactivation of thymidylate synthase (TS) by formation of a complex between methylenetetrahydrofolate (CH2FH4) and 5-fluoro-2’-deoxyuridine 5’-monophosphate (FdUMP), which is synthesized from 5-FU. (2) Interference with ribonucleic acid (RNA) metabolism by the uptake of phosphated 5-fluorouridine 5’-triphosphate into RNA[19]. It was also reported that a single dose of 5-FU is more effective for causing RNA dysfunction, while continuous infusion causes more DNA damage[20]. Another study showed that 5-FU was almost undetectable in the peripheral blood when 5-FU and low-dose CDDP were continuously infused via a central vein or via the hepatic artery in patients with adavanced or metastatic HCC[21]. These reports indicate that the method of continuous intra-arterial infusion for 24 h would cause more damage to tumor DNA in our C-LC patients with aHCC compared with 6-h infusion, although 24-h infusion had stronger hematologic toxicity than 6-h infusion. Our results might also be supported by the report that 5-FU has a time-dependent anticancer effect and shows stronger cell-killing activity in vitro when exposure is continued for a longer period[22].
In conclusion, continuous 24-h intra-arterial infusion is more effective and can prolong survival as compared with 6-h infusion in C-LC patients with aHCC, although 24-h infusion is associated with stronger hematologic toxicity.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Bai SH
References
- 1.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, Kiriyama S, Suga T, Takahashi M. The efficacy of continuous local arterial infusion of 5-fluorouracil and cisplatin through an implanted reservoir for severe advanced hepatocellular carcinoma. Oncology. 1995;52:295–299. doi: 10.1159/000227477. [DOI] [PubMed] [Google Scholar]
- 3.Murata K, Shiraki K, Kawakita T, Yamamoto N, Okano H, Nakamura M, Sakai T, Deguchi M, Ohmori S, Nakano T. Low-dose chemotherapy of cisplatin and 5-fluorouracil or doxorubicin via implanted fusion port for unresectable hepatocellular carcinoma. Anticancer Res. 2003;23:1719–1722. [PubMed] [Google Scholar]
- 4.Okuda K, Tanaka M, Shibata J, Ando E, Ogata T, Kinoshita H, Eriguchi N, Aoyagi S, Tanikawa K. Hepatic arterial infusion chemotherapy with continuous low dose administration of cisplatin and 5-fluorouracil for multiple recurrence of hepatocellular carcinoma after surgical treatment. Oncol Rep. 1999;6:587–591. doi: 10.3892/or.6.3.587. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell MJ. A phase III trial of 5-fluorouracil and leucovorin in the treatment of advanced colorectal cancer. A Mayo Clinic/North Central Cancer Treatment Group study. Cancer. 1989;63:1026–1030. doi: 10.1002/1097-0142(19890315)63:6+<1026::aid-cncr2820631307>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–1418. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- 7.Buroker TR, O'Connell MJ, Wieand HS, Krook JE, Gerstner JB, Mailliard JA, Schaefer PL, Levitt R, Kardinal CG, Gesme DH. Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol. 1994;12:14–20. doi: 10.1200/JCO.1994.12.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Masuhara M, Okita K. Novel arterial infusion chemotherapy using cisplatin, 5-fluorouracil, and leucovorin for patients with advanced hepatocellular carcinoma. Hepatol Res. 2002;23:7–17. doi: 10.1016/s1386-6346(01)00163-2. [DOI] [PubMed] [Google Scholar]
- 9.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 10.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266–2273. [PubMed] [Google Scholar]
- 11.Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–885. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529–534. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 15.Iwamiya T, Sawada S, Ohta Y. Repeated arterial infusion chemotherapy for inoperable hepatocellular carcinoma using an implantable drug delivery system. Cancer Chemother Pharmacol. 1994;33 Suppl:S134–S138. doi: 10.1007/BF00686685. [DOI] [PubMed] [Google Scholar]
- 16.Nagasue N, Yukaya H, Hamada T, Hirose S, Kanashima R, Inokuchi K. The natural history of hepatocellular carcinoma. A study of 100 untreated cases. Cancer. 1984;54:1461–1465. doi: 10.1002/1097-0142(19841001)54:7<1461::aid-cncr2820540740>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.LoRusso P, Pazdur R, Redman BG, Kinzie J, Vaitkevicius V. Low-dose continuous infusion 5-fluorouracil and cisplatin: phase II evaluation in advanced colorectal carcinoma. Am J Clin Oncol. 1989;12:486–490. doi: 10.1097/00000421-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbers E, Chaudhuri NK, Heidelberger C. Studies on fluorinated pyrimidines. VIII. Further biochemical and metabolic investigations. J Biol Chem. 1959;234:1255–1262. [PubMed] [Google Scholar]
- 20.Iba T, Kidokoro A, Fukunaga M, Sugiyama K, Fukunaga T, Aihara N. Effect and mechanism of orally administered leucovorin/5-fluorouracil on colon cancer. Gan To Kagaku Ryoho. 2003;30:2077–2081. [PubMed] [Google Scholar]
- 21.Tanioka H, Tsuji A, Morita S, Horimi T, Takamatsu M, Shirasaka T, Mizushima T, Ochi K, Kiura K, Tanimoto M. Combination chemotherapy with continuous 5-fluorouracil and low-dose cisplatin infusion for advanced hepatocellular carcinoma. Anticancer Res. 2003;23:1891–1897. [PubMed] [Google Scholar]
- 22.Drewinko B, Yang LY. Cellular basis for the inefficacy of 5-FU in human colon carcinoma. Cancer Treat Rep. 1985;69:1391–1398. [PubMed] [Google Scholar]


