Abstract
AIM: To prospectively present our initial experience with totally laparoscopic transhiatal esophagogastrectomies for benign diseases of the cardia and distal esophagus.
METHODS: Laparoscopic gastric mobilization and tubularization combined with transhiatal esophageal dissection and intrathoracic esophagogastric anastomosis accomplished by a circular stapler was done in 3 patients. There were 2 females and 1 male patient with a mean age of 73 ± 5 years.
RESULTS: Two patients were operated on due to benign stromal tumor of the cardia and one patient had severe oesophageal peptic stenosis. Mean blood loss was 47 ± 15 mL and mean operating time was 130 ± 10 min. There were no cases that required conversion to laparotomy. All patients were extubated immediately after surgery. Soft diet intake and ambulation times were 5.1 ± 0.4 d and 2.6 ± 0.6 d, respectively. There were no intraoperative and postoperative complications and there were no perioperative deaths. The average length of hospital stay was 9.3 ± 3 d. All procedures were curative and all resected margins were tumor free. The mean number of retrieved lymph nodes was 18 ± 8.
CONCLUSION: Laparoscopic transhiatal esophago-gastrectomy for benign lesions has good effects and proves feasible and safe.
Keywords: Esophagogastrectomy, Transhiatal resection, Cardial tumor, Laparoscopy, Stromal tumor
INTRODUCTION
In recent years, the incidence of adenocarcinoma of the lower esophagus and cardia has increased[1]. Surgery remains the treatment of choice for these cancers, since it provides definitive treatment and long-term survival for some patients and offers splendid palliation for many others. The most common surgeries for resectable lesions are total gastrectomy with distal esophagectomy, Ivor Lewis esophagectomy and the blunt transhiatal procedure[2,3]. These traditional approaches are frequently associated with significant morbidity and mortality rates ranging from 5% to 10%[4-6]. With the development of minimally invasive surgery during the last decade, attempts were made to use alternative minimally invasive methods for esophageal dissection, which avoid an open thoracotomy incision and therefore reduce the associated morbidity[7-11]. However, combined methods using thoracoscopic dissection with conventional abdominal approaches have not achieved a significant reduction in respiratory morbidity, mostly due to the upper midline abdominal incision[12,13]. Better outcomes were achieved by the use of laparoscopic gastric mobilization combined with transhiatal or thoracoscopic esophageal mobilization and cervical or thoracic anastomoses[14,15].
The present study reports on our initial experience with laparoscopic gastric mobilization and transhiatal oesophageal dissection with intrathoracic esophagogastric anastomosis without abdominal, cervical or thoracic incisions for benign diseases of the cardia and distal esophagus prospectively.
MATERIALS AND METHODS
Patients
The clinical records of patients who underwent laparoscopic gastroesophagectomy in the Department of Abdominal Surgery of the Institute of Laparoscopic Surgery (ILS, Bordeaux) were collected prospectively. All patients underwent preoperative workup including upper gastrointestinal barium swallow, endoscopy with biopsies, endoscopic ultrasonography and dynamic CT scanning of the chest, abdomen and pelvis, in order to establish the diagnosis and determine the extent and staging of the disease. Twenty-eight patients with a malignancy underwent combined laparoscopic and thoracoscopic Ivor Lewis esophagectomy and were excluded from this study. For precise laparoscopic resection in one case of a small intraluminal lesion, we used preoperative endoscopic location and applied metal clips identified by intraoperative X-ray control. Prior to the operations, all cases were reviewed at a meeting attended by staff surgeons, oncologists, gastroenterologists and pathologists. Patients were informed which procedure was expected and the possibility of conversion was discussed. All patients were put on antithrombotic prophylaxis by low-weight heparin and given elastic stockings. Postoperatively, all patients were given total parenteral nutrition for the first 5 d. A hydrosoluble contrast swallow was performed on the 5th postoperative day and if normal, enteral feeding was started.
Studied data
The patients’ demographic data, surgeries, post-operative courses and outpatient follow-up were studied. The following data were collected prospectively: age, sex, preoperative work-up, types and locations of the tumor, duration of surgery, blood loss, intraoperative complications, pathological findings and nodal status, postoperative complications, hospital stay, recurrence and distant events. Variables are presented as mean and standard deviation.
Surgical techniques
The patient was placed in a 25o reverse-Trendelenburg position with split legs; the operating surgeon stood between the legs, the assistant surgeon stood on the left side of the patient and the camera holding assistant was on the right side. A total of five operating trocars were used: a 0o videoscope was introduced through a 10 mm supraumbilical trocar, two 12 mm working ports were placed to the right and left of the midline, and two 5 mm exposure trocars were in sub-xiphoid and left lateral position (Figure 1). Laparoscopic explorations were done by creating a pneumoperitoneum with CO2 to a maximum pressure of 12 mmHg. After exploration of the peritoneal cavity the greater curvature was mobilized by dissection of the greater omentum from the transverse colon using scissors and Ligasure® (Valleylab, Tyco Healthcare Group Lp, Boulder, CO 80 301-3299, UK) or harmonic scalpel (Ethicon Endo-surgery, Cincinnati, OH. USA). The left gastric vessels were exposed and clipped and divided at their roots while the lymph nodes were dissected. The greater curvature mobilization was continued in a distal to proximal direction while the right gastric and gastroepiploic arteries were preserved. A Kocher maneuver was performed followed by gastric tubularization: After definition of the distal margin of the specimen, an endoscopic linear stapler (Ethicon Endo-surgery, Cincinnati, OH. USA) was used to divide the small curvature and create a tube of the greater curvature that would later allow anastomosis with the esophagus. The right diaphragmatic crus was dissected to expose the lower mediastinum. The fundus and abdominal esophagus were mobilized by division of the gastrophrenic peritoneal reflection and separation of the gastroesophageal junction from the left and right crus. The hiatus was entered and the mediastinal esophagus was dissected. The dissection limits were between the left and right parietal pleuras, the pericardium and left pulmonary vein anteriorly and the aorta posteriorly. The anterior and posterior vagal nerves were identified and divided and finally the esophagus was transected 2 to 3 cm above the lesion with free margins.
Figure 1.
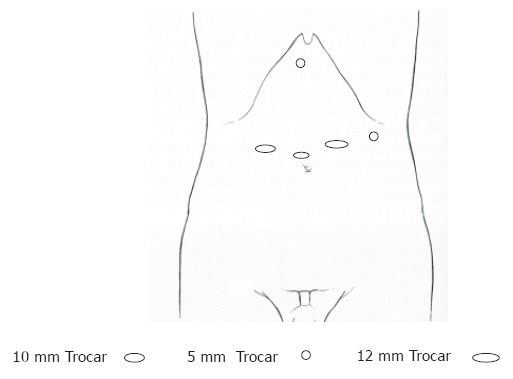
Trocar placement for transhiatal esophagogastrectomy.
For reconstruction a 25 mm circular stapler (Ethicon Endo-surgery, Cincinnati, OH, USA) was used to perform intracorporeal esophagogastric anastomosis. The anvil was prepared by tying a thread with a needle to the tip and then inserted into the peritoneal cavity through a port-site. The anvil was introduced through the lateral esophageal wall 1.5 cm proximal to the transection line, the needle exited through the esophageal wall and then the esophageal stump was closed by a linear endo GIA stapler. The circular stapler was introduced by enlarging one of the left sided trocar sites, entered into the still attached lesser curvature, then passed into the gastric tube. After the stapler was connected to the anvil, the eso-gastric anastomosis was created, and the stapler removed. The tissue doughnuts were carefully checked. The gastric tube was transected with a linear stapler proximal to the anastomosis, making sure to leave at least 1 cm of tissue so as not to create ischemia. The resected specimen was placed inside a bag (Endo Catch II, Auto Suture European Services Center, S.A., 78990 Elancourt, France) and was removed through enlargement of one of the port wounds.
RESULTS
Patients’ characteristics
Between April 2002 and January 2005, we performed laparoscopic gastroesophagectomy in 3 patients. There were 2 female and 1 male patients with a mean age of 73 ± 5 years. The presenting syndromes were abdominal pain and dyspepsia in the first patient, upper gastrointestinal bleeding in the second patient, and dysphagia and weight loss in the last patient. Patients’ preoperative risk was evaluated according to the American Society of Anesthesiologists physical status score (ASA) (Table 1).
Table 1.
Patient demographics and presenting symptoms
| No. | Age (yr) | Gender | ASA | Symptoms | Pathologic site | Pathology |
| 1 | 67 | M | 1 | Abdominal pain and dyspepsia | Gastroesophageal junction | Gastrointestinal stromal tumor |
| 2 | 79 | F | 2 | Upper gastrointestinal bleeding | Gastroesophageal junction | Gastrointestinal stromal tumor |
| 3 | 73 | F | 2 | Dysphagia and weight loss | Distal esophagus | Severe fibrosis |
ASA: American Society of Anesthesiologists physical status score.
Perioperative data
The perioperative data were as follows: Mean blood loss was 47 ± 15 mL and mean operating time was 130 ± 10 min. There were no cases that required conversion to laparotomy. All patients had their tracheal tube removed immediately after surgery. Mean stay time in Intensive Care Unit was 2.3 ± 0.5 d. Resumation of soft diet intake and ambulation times were 5.1 ± 0.4 d and 2.6 ± 0.6 d, respectively. There were no intraoperative or postoperative complications and there were no perioperative deaths. The average length of hospital stay was 9.3 ± 3 d.
Pathologic findings
Two patients were operated on for benign lesions, which were gastrointestinal stromal tumors of the gastroesophageal junction. One patient had severe fibrosis causing severe stenosis of the distal esophagus due to prolonged gastroesophageal reflux.
All procedures were curative and all resected margins were disease or tumor free. The mean tumor size of the surgical specimens was 3.1 ± 1 cm and the mean number of retrieved lymph nodes was 18 ± 8. The patients were cured of lesions and symptoms during a follow-up of 25 ± 12 mo.
DISCUSSION
In spite of modern surgical techniques and improved perioperative care, conventional surgical approaches for benign and malignant lesions of the esophagus and cardia have not significantly lowered the postoperative morbidity and mortality rates. The mortality rates from esophagectomy ranged from 8% in high volume-centers to as high as 23% in low-volume centers[16]. Respiratory complications associated with thoracotomy and prolonged deflation of the right lung during the operation, as well as infections due to anastomotic leaks are the major causes of perioperative morbidity in these surgeries. The high morbidity rates lead to increased cost, prolonged hospital stay, and occasionally, to mortality. Therefore, patients with esophageal cancer, in particular, older patients and those with co-morbid conditions may not be referred for operation at all.
During the 1970s Orringer introduced the technique of transhiatal esophagectomy, which avoids thoracotomy[3]. However, the use of an open approach has not clearly demonstrated reduction of the risk of postoperative respiratory complications or postoperative mortality. In addition, part of the dissection is “blind” with the consequent risk of bleeding, particularly from the azygus vein, and damage to the trachea and bronchi.
With the advent of minimally invasive surgical techniques, various minimally invasive surgical approaches to esophagectomy were introduced. The initial one was thoracoscopic esophagectomy combined with a laparotomy[13,17-19]. In contrast to expectations, no clear benefits were shown and in some of the early series it was clear that postoperative pulmonary complications were common following this approach[13]. Many other groups who performed thoracoscopic esophagectomy noted respiratory complications. Cuschieri noted pulmonary consolidation in 12% of 26 patients[7]. Gossot and colleagues had a 17% incidence of atelectasis requiring prolonged ventilation[20], Collard and co-workers described a 17% incidence of pneumonitis[21], and Dexter and associates reported that 3 of 13 respiratory complications were fatal[9]. A study from Hong Kong comparing thoracoscopy and open thoracotomy found no significant difference in cardiopulmonary complications[17].
Considering that postoperative pulmonary complications are mainly caused by the prolonged deflation of the right lung during the operation and that midline abdominal and thoracic incisions compromise respiratory ability, we tried to avoid laparotomy and thoracotomy using the laparoscopic transhiatal approach. This approach could reduce postoperative morbidities and speed recovery. We performed laparoscopic gastric mobilization and transhiatal esophageal dissection with intrathoracic esophagogastric anastomosis to treat benign cardial and distal esophageal tumors. Laparoscopic transhiatal esophagogastrectomy was described recently by Costi and colleagues, who found that this approach minimizes postoperative complications and gives good results. The technique described by Costi et al[22] is quite similar to ours while the main difference is the esophago-gastric anastomosis: Costi et al[22] tied the stapler’s anvil to the end of an oro-gastric tube which was inserted orally down to the esophageal stump.
Our preliminary results showed that laparoscopic transhiatal esophagogastrectomy without abdominal or thoracic incisions were feasible and safe. There was no need to convert to open surgery, the estimated blood loss was minimal and there were no intraoperative complications. The operative time was shorter than in the other approaches, which necessitated patient’s position change and two working fields. There were no intraoperative ventilation difficulties, all patients were extubated immediately after surgery and there were no postoperative pulmonary complications. The patients were ambulated early and the postoperative course was uneventful in all cases. The mean hospital stay was 11 d (Hospital stay in France, unlike US or other countries, is influenced not only by medical, but also cultural and patient related factors.). We used a narrow gastric tube without pyloroplasty, to avoid the potential problems associated with dumping as supported by previous studies[23]. One concern usually raised regarding the treatment of malignant diseases by minimally invasive approaches is whether these approaches provide an adequate cancer resection, allowing free tumor margins and extensive lymph node dissection, while being minimally invasive. Previous studies concluded that the use of laparoscopic assisted transhiatal dissection for distal esophageal cancer allows enhanced tumor and nodal clearance compared with the standard transhiatal approach[24]. A randomized study comparing an extended thoracic approach and transhiatal approach in 220 patients with adenocarcinoma of the esophagus found no significant difference in survival between the 2 groups[25]. There are no randomized studies comparing the laparoscopic and open transhiatal approach for malignant lesions. Despite that all resected margins in our study were tumor free and the mean number of retrieved lymph nodes was 18 ± 8, which is comparable with the thoracoscopic and open surgery series, our strategy for malignant lesions of the cardia and distal esophagus is to perform combined laparoscopic and thoracoscopic Ivor Lewis esophagectomy with en bloc mediastinal lymhadenectomy.
In conclusion, our preliminary results demonstrate that laparoscopic transhiatal esophagogastrectomy for benign lesions of the cardia and distal esophagus has good effects and is feasible and safe. Further studies with a larger number of patients and longer follow up are needed to establish this approach.
ACKNOWLEDGMENTS
We thank Dr. Mitkal Sharon for valuable help in proofreading this paper.
Footnotes
S- Editor Wang GP L- Editor Zhu LH E- Editor Bai SH
References
- 1.Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- 2.Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–31. doi: 10.1002/bjs.18003413304. [DOI] [PubMed] [Google Scholar]
- 3.Orringer MB. Transhiatal esophagectomy without thoracotomy for carcinoma of the thoracic esophagus. Ann Surg. 1984;200:282–288. doi: 10.1097/00000658-198409000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 5.Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg. 1980;67:381–390. doi: 10.1002/bjs.1800670602. [DOI] [PubMed] [Google Scholar]
- 6.Watson A. Operable esophageal cancer: current results from the West. World J Surg. 1994;18:361–366. doi: 10.1007/BF00316815. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri A. Thoracoscopic subtotal oesophagectomy. Endosc Surg Allied Technol. 1994;2:21–25. [PubMed] [Google Scholar]
- 8.Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37:7–11. [PubMed] [Google Scholar]
- 9.Dexter SP, Martin IG, McMahon MJ. Radical thoracoscopic esophagectomy for cancer. Surg Endosc. 1996;10:147–151. doi: 10.1007/BF00188361. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd DM, Vipond M, Robertson GS, Hanning C, Veitch PS. Thoracoscopic oesophago-gastrectomy--a new technique for intra-thoracic stapling. Endosc Surg Allied Technol. 1994;2:26–31. [PubMed] [Google Scholar]
- 11.Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc. 1999;13:293–297. doi: 10.1007/s004649900969. [DOI] [PubMed] [Google Scholar]
- 12.Gossot D, Cattan P, Fritsch S, Halimi B, Sarfati E, Celerier M. Can the morbidity of esophagectomy be reduced by the thoracoscopic approach? Surg Endosc. 1995;9:1113–1115. doi: 10.1007/BF00188998. [DOI] [PubMed] [Google Scholar]
- 13.McAnena OJ, Rogers J, Williams NS. Right thoracoscopically assisted oesophagectomy for cancer. Br J Surg. 1994;81:236–238. doi: 10.1002/bjs.1800810225. [DOI] [PubMed] [Google Scholar]
- 14.DePaula AL, Hashiba K, Ferreira EA, de Paula RA, Grecco E. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc. 1995;5:1–5. [PubMed] [Google Scholar]
- 15.Jagot P, Sauvanet A, Berthoux L, Belghiti J. Laparoscopic mobilization of the stomach for oesophageal replacement. Br J Surg. 1996;83:540–542. doi: 10.1002/bjs.1800830435. [DOI] [PubMed] [Google Scholar]
- 16.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 17.Law S, Fok M, Chu KM, Wong J. Thoracoscopic esophagectomy for esophageal cancer. Surgery. 1997;122:8–14. doi: 10.1016/s0039-6060(97)90257-9. [DOI] [PubMed] [Google Scholar]
- 18.Peracchia A, Rosati R, Fumagalli U, Bona S, Chella B. Thoracoscopic esophagectomy: are there benefits? Semin Surg Oncol. 1997;13:259–262. doi: 10.1002/(sici)1098-2388(199707/08)13:4<259::aid-ssu7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Kawahara K, Maekawa T, Okabayashi K, Hideshima T, Shiraishi T, Yoshinaga Y, Shirakusa T. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc. 1999;13:218–223. doi: 10.1007/s004649900948. [DOI] [PubMed] [Google Scholar]
- 20.Gossot D, Fourquier P, Celerier M. Thoracoscopic esophagectomy: technique and initial results. Ann Thorac Surg. 1993;56:667–670. doi: 10.1016/0003-4975(93)90947-g. [DOI] [PubMed] [Google Scholar]
- 21.Collard JM, Lengele B, Otte JB, Kestens PJ. En bloc and standard esophagectomies by thoracoscopy. Ann Thorac Surg. 1993;56:675–679. doi: 10.1016/0003-4975(93)90949-i. [DOI] [PubMed] [Google Scholar]
- 22.Costi R, Himpens J, Bruyns J, Cadière GB. Totally laparoscopic transhiatal esophago-gastrectomy without thoracic or cervical access. The least invasive surgery for adenocarcinoma of the cardia? Surg Endosc. 2004;18:629–632. doi: 10.1007/s00464-003-9053-5. [DOI] [PubMed] [Google Scholar]
- 23.Bemelman WA, Taat CW, Slors JF, van Lanschot JJ, Obertop H. Delayed postoperative emptying after esophageal resection is dependent on the size of the gastric substitute. J Am Coll Surg. 1995;180:461–464. [PubMed] [Google Scholar]
- 24.Sadanaga N, Kuwano H, Watanabe M, Ikebe M, Mori M, Maekawa S, Hashizume M, Kitano S, Sugimachi K. Laparoscopy-assisted surgery: a new technique for transhiatal esophageal dissection. Am J Surg. 1994;168:355–357. doi: 10.1016/s0002-9610(05)80165-5. [DOI] [PubMed] [Google Scholar]
- 25.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]


