Abstract
AIM: To compare the response of standard hepatitis B virus (HBV) vaccination between patients with chronic hepatitis C virus (HCV) infection and healthy individuals.
METHODS: This is a prospective case-control study. A total of 38 patients with chronic HCV infection and 40 healthy controls were included. Vaccination was performed by injection of 20 μg recombinant HBsAg into the deltoid muscle at mo 0, 1 and 6. Anti-HBs concentration was determined 3 mo after the last dose and compared between the two groups. The response pattern was characterized as (1) high-response when the anti-HBs antibody titer was > 100 IU/L, (2) low-response when the titer was 10-100 IU/L and (3) no-response when the titer was < 10 IU/L.
RESULTS: In the patient group, there were 10/38 (26.3%) non-responders, 8/38 (21.1%) low-responders and 20/38 (52.6%) high-responders. The corresponding values in the control group were 2/40 (5.0%), 7/40 (17.5%) and 31/40 (77.5%), respectively. The response pattern was statistically different between the two groups. In multivariate analysis, smoking was a significant confounder, while HCV infection lost its significant correlation with lower antibody response.
CONCLUSION: Patients with chronic HCV infection tend to respond weakly to HBV vaccination compared to healthy individuals, though this correlation is not independent according to multivariate analysis.
Keywords: Immunogenicity, Hepatitis B, Vaccine, Hepatitis C, Antibody response
INTRODUCTION
One major transmission route for both hepatitis C virus (HCV) and hepatitis B virus (HBV) is the parenteral route, and the sources of infection include administration of blood or blood products[1,2], intravenous drug use[3,4] and needle-stick accidents[5,6]. According to the analysis of the Third National Health and Nutrition Survey, more than 25% of HCV-positive patients in the United States had hepatitis B markers, a proportion nearly six times that in the HCV-negative group[3]. However, the actual prevalence of HBV infection in patients with HCV infection is probably underestimated[7,8]. Although it has been shown that superinfection of either HBV or HCV may suppress the other’s replicative levels, coinfection with both viruses has synergistic effects with regard to histological lesions, progression to cirrhosis and cancer development[9-13]. As such it has been recommended by the National Institutes of Health (NIH) that individuals with HCV be vaccinated against HBV infection to prevent such an outcome[14].
HBV vaccination at standard doses (20 μg for adults at mo 0, 1, and 6) results in an effective antibody response in 90% to 98% of healthy individuals[15,16]. However, reduced immunogenicity of the vaccine has been established in persons with chronic liver disease, patients receiving hemodialysis, patients with HIV infection and those awaiting transplantation[17-21]. To the best of our knowledge, only a few studies, with inconsistent results, have compared the immunogenicity of standard HBV vaccination in chronic hepatitis C patients with that in healthy individuals through a case-control study[22-24]. Therefore, we found it valuable to compare the response of standard HBV vaccination between patients with chronic HCV infection and healthy individuals in a prospective case-control study.
MATERIALS AND METHODS
Subjects
Between April 2005 and August 2006, 38 patients with chronic hepatitis C infection (patients group) and 40 healthy individuals (control group) referred to our clinic were enrolled in this case-control study. Totally there were 50 males and 28 females with a mean age of 37.6 ± 12.8 years. All participants gave written informed consent and the study protocol was approved by the Ethical Committee of Tehran University of Medical Sciences. Inclusion criteria for the patient group were: age > 18 years, HCV infection diagnosed by positive HCV serological markers assessed by ELISA (Abbott Laboratories, North Chicago, IL, USA) and confirmed by the presence of serum HCV RNA detected by PCR, chronic infection diagnosed by serum alanine aminotransferase (ALT) levels of at least twice the upper normal values (> 90 IU/L) for at least two times within a period longer than 6 mo and/or a liver biopsy showing evidence of chronic hepatitis. The control group was selected from healthy adults older than 18 years. Exclusion criteria were: pregnancy, lactation, known bleeding diathesis, current intravenous drug use, alcohol consumption > 30 g/d, history of cancer or transplantation, receiving immunosuppressive medications (excluding interferon), previous hepatitis B vaccination, history of allergy to vaccine components, current or previous hepatitis B infection (positive HBs Ag, anti-HBc Ab/anti-HBs Ab by ELISA), laboratory or clinical evidence of other chronic liver diseases including cirrhosis of any etiology, presence of HIV infection, chronic renal failure (serum creatinine > 2.5) or hemodialysis.
The following variables were recorded for all participants: age (year), sex, body mass (kg), height (m), smoking, alcohol use, history of intravenous drug use. Body mass index (BMI) was calculated by dividing mass (kg) by squared height (m2). Liver biopsy during two years before vaccination showing the stage and grade of liver involvement according to Ishak et al[25] and HCV genotype (determined by PCR-RFLP) was available for 31 and 34 patients, respectively. Baseline ALT (IU/L) was determined by commercial kits for all patients.
Methods
Vaccination was performed by injection of 20 μg recombinant HBsAg (Euvax B, LG Chem, Korea) into the deltoid muscle at mo 0, 1 and 6. Anti-HBs concentration was determined 3 mo after the last dose and expressed as IU/L. The response pattern was characterized as (1) high-response when anti-HBs antibody titer was > 100 IU/L, (2) low-response when the titer was 10-100 IU/L and (3) no-response when the titer was < 10 IU/L. Patients were monitored after each vaccine dose for the occurrences of local (pain, induration, flush) and general (headache, fatigue, fever) side effects.
Statistical analysis
The results are presented as mean ± SD. χ2 test with Fisher’s exact test was used to compare qualitative variables between the groups. Student’s t test and analysis of variance (ANOVA) were used to compare the quantitative variables between two and multiple groups, respectively. The independent predictive factors of vaccine response were identified by multivariate analysis using multiple logistic regression. Statistical analysis was conducted with SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). Throughout analysis, P < 0.05 was considered statistically significant.
RESULTS
Characteristics of patients and controls
Patients were significantly older than healthy subjects (P = 0.017). Also, patients were more frequently males (P = 0.010), smokers (P < 0.001) and previous intravenous drug users (P = 0.001) (Table 1).
Table 1.
Characteristics of patients and controls (mean ± SD)
| Characteristic | Patient group (n = 38) | Control group (n = 40) | P |
| Age (yr) | 41.1 ± 10.3 a | 34.2 ± 14.2 | 0.017 |
| Male/Female | 30/8 b | 20/20 | 0.010 |
| BMI (kg/m2) | 24.8 ± 4.7 | 24.3 ± 4.9 | 0.691 |
| Smoking n (%) | 25 (65.8) b | 3 (7.5) | < 0.001 |
| Alcohol n (%) | 6 (15.8) | 1 (2.5) | 0.054 |
| History of iv drug use n (%) | 9 (23.7) b | 0 (0) | 0.001 |
P < 0.05,
P < 0.01 vs control group.
Antibody response
A total of 28/38 (73.7%) chronic hepatitis C patients responded to the vaccination course (anti-HBs > 10 IU/L) compared with 38/40 (95.0%) controls (P = 0.012). In the patient group, there were 10/38 (26.3%) non-responders, 8/38 (21.1%) low-responders and 20/38 (52.6%) high-responders. The corresponding values in the control group were 2/40 (5.0%), 7/40 (17.5%) and 31/40 (77.5%), respectively (Figure 1). The response pattern was statistically different between the two groups (P = 0.021). The frequency of non-responders was significantly higher in the patient group (P = 0.024 after Bonferroni’s correction). However, the frequency of low-responders (P = 0.778) and high-responders (P = 0.064) was not significantly different between the two groups. Since the patient and control groups were not matched in age, sex, smoking and history of iv drug use, binary logistic regression was performed to adjust for these parameters. Response (anti-HBs > 10 IU/L) was considered as the dependent variable, while hepatitis C infection, age, sex, smoking and history of iv drug use were included as covariates. None of hepatitis C infection (P = 0.448), age (P = 0.078), sex (P = 0.480) and history of iv drug use (P = 0.127) were independently correlated with lower antibody response in the patient group. However, smoking was a significant confounder (P = 0.024; odds ratio: 25.64; 95% confidence interval: 1.54-500).
Figure 1.
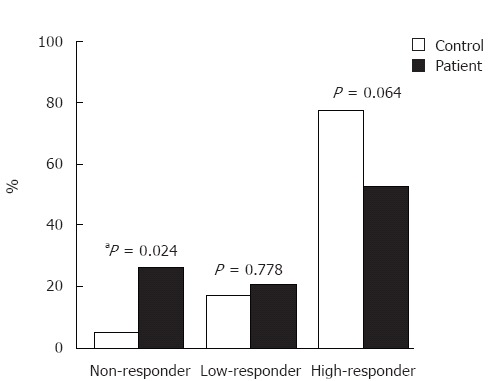
Response to HBV vaccination in patient and control groups. Non-responder (anti-HBs < 10 IU/L); Low-responder (10 IU/L < anti-HBs < 100 IU/L); High-responder (anti-HBs > 100 IU/L). aP < 0.05 vs control group.
Characteristics of hepatitis C patients according to vaccine response
The comparison of characteristics of patients between responders and non-responders to HBV vaccine showed no significant difference regarding age, sex ratio, BMI, smoking, alcohol use, history of iv drug use, baseline ALT level, stage and grade of liver disease and genotype (1a vs others, Table 2). When patients were categorized into three groups of non-, low- and high-responders, none of the variables except genotype (P = 0.038) had a significant correlation with response category anymore (Table 3). Genotype 1a was more frequently observed in non-responders. However, when Bonferroni’s correction was applied for subgroup analysis, this correlation was no longer significant. In summary, no correlation was found between the variables studied and response in either type of analysis.
Table 2.
Characteristics of hepatitis C patients according to vaccine response (mean ± SD)
| Characteristics | Non-responder (n = 10) | Responder (n =28) | P |
| Age (yr) | 41.5 ± 11.3 | 41.0 ± 10.2 | 0.897 |
| Male/Female | 8/2 | 22/6 | 1.000 |
| BMI (kg/m2) | 26.6 ± 6.0 | 24.2 ± 4.1 | 0.192 |
| Smoking n (%) | 9 (90.0) | 16 (57.1) | 0.118 |
| Alcohol n (%) | 2 (20.0) | 4 (14.3) | 0.644 |
| Hx of iv drug use n (%) | 2 (20.0) | 7 (25.0) | 1.000 |
| ALT (IU/L) | 177.1 ± 293.9 | 78.3 ± 165.3 | 0.199 |
| Liver disease | |||
| HAI grade (0-18)1 | 5.4 ± 2.1 | 5.6 ± 1.9 | 0.773 |
| HAI stage (0-6)1 | 2.3 ± 1.1 | 2.6 ± 1.6 | 0.669 |
| HCV genotype n (%) | 8 | 26 | |
| 1a | 6 (75.0) | 14 (53.8) | 0.422 |
| Other than 1a | 2 (25.0) | 12 (46.2) |
According to Knodell’s histological activity index (HAI) as modified by Ishak et al[25]. Non-responder (anti-HBs < 10 IU/L); Responder (anti-HBs > 10 IU/L). Hx:history. .
Table 3.
Characteristics of hepatitis C patients according to vaccine response
| Characteristic | Non-responder (n = 10) | Low-responder (n = 8) | High-responder (n = 20) | P |
| Age (yr) | 41.5 ± 11.3 | 46.1 ± 9.0 | 39.0 ± 10.1 | 0.394 |
| Male/Female | 8/2 | 5/3 | 17/3 | 0.417 |
| BMI (kg/m2) | 26.6 ± 6.0 | 24.9 ± 3.0 | 23.8 ± 4.6 | 0.903 |
| Smoking n (%) | 9 (90.0) | 4 (50.0) | 12 (60.0) | 0.150 |
| Alcohol n (%) | 2 (20.0) | 0 (0) | 4 (20.0) | 0.387 |
| Hx of iv drug use n (%) | 2 (20.0) | 2 (25.0) | 5 (25.0) | 0.950 |
| ALT (IU/L) | 177.1 ± 293.9 | 149.8 ± 304.6 | 49.8 ± 40.3 | 0.711 |
| Liver disease | ||||
| HAI grade (0-18) | 5.4 ± 2.1 | 5.4 ± 2.8 | 5.7 ± 1.3 | 0.607 |
| HAI stage (0-6) | 2.3 ± 1.1 | 2.4 ± 1.8 | 2.7 ± 1.5 | 0.986 |
| HCV genotype n (%) | 8 | 8 | 18 | |
| 1a | 6 (75.0)a | 7 (87.5)a | 7 (38.9)a | 0.038 |
| Other than 1a | 2 (25.0) | 1 (12.5) | 11 (61.1) |
Non-responder (anti-HBs < 10 IU/L); Low-responder (10 IU/L < anti-HBs < 100 IU/L); High-responder (anti-HBs > 100 IU/L).
P < 0.05 comparison between three groups.
Side effects
No severe side effects following vaccination were observed in chronic hepatitis C patients. Local adverse effects (erythema, pain at injection point, induration) following vaccination were observed in 4/38 (10.5%) patients. Systemic side effects such as flulike syndrome, headache, fever and fatigue occurred several days following vaccine injections in 1/38 (2.6%) patients.
DISCUSSION
In the present study, the antibody response to standard HBV vaccination with a dose of 20 μg at mo 0, 1 and 6 in individuals with non-cirrhotic chronic hepatitis C infection was evaluated and compared with healthy controls. The frequency of high-responders, low-responders and non-responders in the patient and control groups was 52.6%, 21.1%, 26.3% and 77.5%, 17.5%, 5.0%, respectively (P = 0.021). Non-responders were significantly more common in the patient group (P = 0.024). However, this correlation was not significant in multivariate analysis anymore when age, sex, smoking and history of iv drug use were controlled as potential confounders. In our patient group, there was no correlation between antibody response and variables such as age, sex, BMI, smoking, alcohol use, history of iv drug use, baseline ALT levels, stage and grade of liver disease and genotype.
Several studies have compared the immunogenicity of hepatitis B vaccination with different protocols between healthy individuals and hepatitis C patients. Some of them, like our study, have failed to demonstrate a significant correlation between chronic hepatitis C and antibody response[22,26-28]. However, some authors have shown that responses were weaker in patients than in controls[23,24,29,30]. Hence the results still remain controversial. Among the above mentioned studies, only three have used the same vaccination protocol as ours[22-24]. Lee et al[22] compared the immunogenicity of HBV vaccination between 26 hepatitis C patients and 35 controls. The groups were similar in age, but the control group was significantly younger than the patient group. One month after the last dose, 88.5% of patients and 91.4% of controls responded to vaccination with anti-HBs > 10 IU/L. The difference was not statistically significant[22].
In another study of 48 patients and 11 controls, Chlabicz et al[23] showed that 72.9% of patients and 90.9% of controls responded (anti-HBs > 10 IU/L) to HBV vaccination one month after the last dose. The groups were similar in age, sex, BMI and smoking frequency but the difference in antibody response was not significant either. One year after the last dose, there was a significant reduction in antibody response among patients so that only 34.1% of them remained responders. The corresponding value in the control group was 90% at the same time (P < 0.05).
Finally, Mattos et al[24] reported in their study of 85 patients and 46 healthy adults that 55.3% of patients and 97.8% of controls responded (anti-HBs > 10 IU/L) to HBV vaccination one month after the third vaccine dose. Non-responders were significantly more common in the patient group (P < 0.001). The patient and control groups were matched in sex, BMI, alcohol use and smoking, but the patient group was significantly older than the control group. In multivariate regression to control for age as a potential confounder, HCV positivity remained significantly correlated with the lower antibody response (P = 0.0013). The patient group in this study included both cirrhotic and non-cirrhotic individuals. Considering that cirrhosis is associated with a lower antibody response[26,31], inhomogeneity of the patient group might have had a role in the significant correlation in this study[24].
We only considered non-cirrhotic patients in our study. The results of multivariate regression showed that hepatitis C infection did not play an independent role in decreasing antibody response. Rather, smoking was a significant confounder (P = 0.024; odds ratio: 25.64; 95% confidence interval: 1.54-500). This finding is in agreement with previous studies[32,33]. When patients were categorized into two (responder and non-responder) or three groups (non-, low- and high-responder), no variables studied had a significant correlation with antibody response. Previous studies, as well as ours, failed to show a significant correlation between antibody response and age, sex, BMI, smoking, alcohol use, iv drug use, baseline ALT level or liver histology (grade, stage)[24,29,30]. Mattos et al[24] showed that the percentage of non-responders was significantly higher in patients with genotype 1 (P = 0.04). Considering that they had three subgroups of patients according to their response, subgroup analysis with a Bonferroni’s correction (which changes the P value to Pc = 0.08) seemed to be necessary to make a justifiable conclusion. We did not achieve a significant correlation, which was supported by Leroy et al[29].
No clinically significant adverse effect was seen in our patients. Local side effects following vaccination were observed in 10.5%, while systemic side effects such as flulike syndrome, headache, fever and fatigue occurred in 2.6% of patients. These rates are almost similar to those reported in other studies[22,26,30]. In conclusion, patients with chronic hepatitis C infection tend to respond weakly to HBV vaccination compared to healthy individuals, though this correlation is not independent according to multivariate analysis.
COMMENTS
Background
Several studies have compared the immunogenicity of hepatitis B vaccination between healthy individuals and hepatitis C patients. Some of them have failed to demonstrate a significant correlation between chronic hepatitis C and antibody response. However, some authors have shown that responses were weaker in patients than in controls.
Research frontiers
Coinfection with both hepatitis B and C viruses has synergistic effects with regard to histological lesions, progression to cirrhosis and cancer development. Therefore, it may be very beneficial to prevent HBV superinfection in hepatitis C patients.
Innovations and breakthroughs
Considering the various vaccination protocols used in HCV patients and also controversial results, we found it valuable to compare the response of standard HBV vaccination between patients with chronic HCV infection and healthy individuals in a prospective case-control study. The results showed that hepatitis C infection does not decrease the immune response to HBV vaccination.
Applications
Based on the results of this study, in the absence of factors known to weaken the immune response, hepatitis C patients do not seem to need additional doses of HBV vaccine or antibody titration after standard HBV vaccination. However, these considerations should be taken into account when vaccinating a hepatitis C patient in general, i.e. one with commonly coexisting immunity-related risk factors.
Terminology
Multivariate analysis: When compared groups are not similar in some possibly important features such as sex or age, performing a multivariate analysis using multiple logistic regression can adjust groups for such potential confounders.
Bonferroni’s correction: A method to adjust the level of significance when multiple comparisons are made.
Peer review
If we accept that HBV vaccine may be useful in HCV patients, the basic steps of research should be: to evaluate the rate of response in HCV carriers and to verify whether this rate is acceptable in terms of cost benefit. The patients and controls were significantly different in age, sex, smoking frequency and intravenous drug use.
Footnotes
S- Editor Wang GP L- Editor Zhu LH E- Editor Liu WF
References
- 1.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Murphy EL, Bryzman SM, Glynn SA, Ameti DI, Thomson RA, Williams AE, Nass CC, Ownby HE, Schreiber GB, Kong F, et al. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology Donor Study (REDS) Hepatology. 2000;31:756–762. doi: 10.1002/hep.510310329. [DOI] [PubMed] [Google Scholar]
- 5.Balasekaran R, Bulterys M, Jamal MM, Quinn PG, Johnston DE, Skipper B, Chaturvedi S, Arora S. A case-control study of risk factors for sporadic hepatitis C virus infection in the southwestern United States. Am J Gastroenterol. 1999;94:1341–1346. doi: 10.1111/j.1572-0241.1999.01084.x. [DOI] [PubMed] [Google Scholar]
- 6.Kiyosawa K, Sodeyama T, Tanaka E, Nakano Y, Furuta S, Nishioka K, Purcell RH, Alter HJ. Hepatitis C in hospital employees with needlestick injuries. Ann Intern Med. 1991;115:367–369. doi: 10.7326/0003-4819-115-5-367. [DOI] [PubMed] [Google Scholar]
- 7.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 8.Villa E, Grottola A, Buttafoco P, Trande P, Merighi A, Fratti N, Seium Y, Cioni G, Manenti F. Evidence for hepatitis B virus infection in patients with chronic hepatitis C with and without serological markers of hepatitis B. Dig Dis Sci. 1995;40:8–13. doi: 10.1007/BF02063934. [DOI] [PubMed] [Google Scholar]
- 9.Crespo J, Lozano JL, Carte B, de las Heras B, de la Cruz F, Pons-Romero F. Viral replication in patients with concomitant hepatitis B and C virus infections. Eur J Clin Microbiol Infect Dis. 1997;16:445–451. doi: 10.1007/BF02471908. [DOI] [PubMed] [Google Scholar]
- 10.Ohkawa K, Hayashi N, Yuki N, Masuzawa M, Kato M, Yamamoto K, Hosotsubo H, Deguchi M, Katayama K, Kasahara A. Long-term follow-up of hepatitis B virus and hepatitis C virus replicative levels in chronic hepatitis patients coinfected with both viruses. J Med Virol. 1995;46:258–264. doi: 10.1002/jmv.1890460316. [DOI] [PubMed] [Google Scholar]
- 11.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, Seigneurin JM, Buffet C, Dhumeaux D. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 12.Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95:2978–2980. doi: 10.1111/j.1572-0241.2000.02337.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaklamani E, Trichopoulos D, Tzonou A, Zavitsanos X, Koumantaki Y, Hatzakis A, Hsieh CC, Hatziyannis S. Hepatitis B and C viruses and their interaction in the origin of hepatocellular carcinoma. JAMA. 1991;265:1974–1976. [PubMed] [Google Scholar]
- 14.NIH consensus development conference targets prevention and management of hepatitis C. Am Fam Physician. 1997;56:959–961. [PubMed] [Google Scholar]
- 15.Dienstag JL, Werner BG, Polk BF, Snydman DR, Craven DE, Platt R, Crumpacker CS, Ouellet-Hellstrom R, Grady GF. Hepatitis B vaccine in health care personnel: safety, immunogenicity, and indicators of efficacy. Ann Intern Med. 1984;101:34–40. doi: 10.7326/0003-4819-101-1-34. [DOI] [PubMed] [Google Scholar]
- 16.Rahman F, Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 17.Keeffe EB, Krause DS. Hepatitis B vaccination of patients with chronic liver disease. Liver Transpl Surg. 1998;4:437–439. doi: 10.1002/lt.500040515. [DOI] [PubMed] [Google Scholar]
- 18.Stevens CE, Szmuness W, Goodman AI, Weseley SA, Fotino M. Hepatitis B vaccine: immune responses in haemodialysis patients. Lancet. 1980;2:1211–1213. doi: 10.1016/s0140-6736(80)92477-0. [DOI] [PubMed] [Google Scholar]
- 19.Collier AC, Corey L, Murphy VL, Handsfield HH. Antibody to human immunodeficiency virus (HIV) and suboptimal response to hepatitis B vaccination. Ann Intern Med. 1988;109:101–105. doi: 10.7326/0003-4819-109-2-101. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson IM, Jaffers G, Dienstag JL, Tolkoff-Rubin NE, Cosimi AB, Delmonico F, Watkins E, Hinkle C, O'Rourke S, Russell PS. Immunogenicity of hepatitis B vaccine in renal transplant recipients. Transplantation. 1985;39:393–395. doi: 10.1097/00007890-198504000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Van Thiel DH, el-Ashmawy L, Love K, Gavaler JS, Starzl TE. Response to hepatitis B vaccination by liver transplant candidates. Dig Dis Sci. 1992;37:1245–1249. doi: 10.1007/BF01296567. [DOI] [PubMed] [Google Scholar]
- 22.Lee SD, Chan CY, Yu MI, Lu RH, Chang FY, Lo KJ. Hepatitis B vaccination in patients with chronic hepatitis C. J Med Virol. 1999;59:463–468. [PubMed] [Google Scholar]
- 23.Chlabicz S, Grzeszczuk A, Łapiński TW. Hepatitis B vaccine immunogenicity in patients with chronic HCV infection at one year follow-up: the effect of interferon-alpha therapy. Med Sci Monit. 2002;8:CR379–CR383. [PubMed] [Google Scholar]
- 24.Mattos AA, Gomes EB, Tovo CV, Alexandre CO, Remião JO. Hepatitis B vaccine efficacy in patients with chronic liver disease by hepatitis C virus. Arq Gastroenterol. 2004;41:180–184. doi: 10.1590/s0004-28032004000300008. [DOI] [PubMed] [Google Scholar]
- 25.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 26.De Maria N, Idilman R, Colantoni A, Van Thiel DH. Increased effective immunogenicity to high-dose and short-interval hepatitis B virus vaccination in individuals with chronic hepatitis without cirrhosis. J Viral Hepat. 2001;8:372–376. doi: 10.1046/j.1365-2893.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- 27.De Maria N, Idilman R, Colantoni A, Harig JM, Van Thiel DH. Antibody response to hepatitis B virus vaccination in individuals with hepatitis C virus infection. Hepatology. 2000;32:444–445. doi: 10.1053/jhep.2000.9873. [DOI] [PubMed] [Google Scholar]
- 28.Kamel M, el Manialawi M, Miller FD. Recombinant hepatitis B vaccine immunogenicity in presence of hepatitis C virus seropositivity. Lancet. 1994;343:552. doi: 10.1016/s0140-6736(94)91510-5. [DOI] [PubMed] [Google Scholar]
- 29.Leroy V, Bourliere M, Durand M, Abergel A, Tran A, Baud M, Botta-Fridlund D, Gerolami A, Ouzan D, Halfon P, et al. The antibody response to hepatitis B virus vaccination is negatively influenced by the hepatitis C virus viral load in patients with chronic hepatitis C: a case-control study. Eur J Gastroenterol Hepatol. 2002;14:485–489. doi: 10.1097/00042737-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Wiedmann M, Liebert UG, Oesen U, Porst H, Wiese M, Schroeder S, Halm U, Mössner J, Berr F. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology. 2000;31:230–234. doi: 10.1002/hep.510310134. [DOI] [PubMed] [Google Scholar]
- 31.Domínguez M, Bárcena R, García M, López-Sanroman A, Nuño J. Vaccination against hepatitis B virus in cirrhotic patients on liver transplant waiting list. Liver Transpl. 2000;6:440–442. doi: 10.1053/jlts.2000.8313. [DOI] [PubMed] [Google Scholar]
- 32.Hollinger FB. Factors influencing the immune response to hepatitis B vaccine, booster dose guidelines, and vaccine protocol recommendations. Am J Med. 1989;87:36S–40S. doi: 10.1016/0002-9343(89)90530-5. [DOI] [PubMed] [Google Scholar]
- 33.Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med. 1997;336:196–204. doi: 10.1056/NEJM199701163360307. [DOI] [PubMed] [Google Scholar]


