Abstract
AIM: To investigate the effect of ginkgo biloba extract (EGb 761) on lung injury induced by intestinal ischemia/reperfusion (II/R).
METHODS: The rat model of II/R injury was produced by clamping the superior mesenteric artery for 60 min followed by reperfusion for 180 min. The rats were randomly allocated into sham, II/R, and EGb +II/R groups. In EGb +II/R group, EGb 761 (100 mg/kg per day) was given via a gastric tube for 7 consecutive days prior to surgery. Rats in II/R and sham groups were treated with equal volumes of the vehicle of EGb 761. Lung injury was assessed by light microscopy, wet-to-dry lung weight ratio (W/D) and pulmonary permeability index (PPI). The levels of malondialdehyde (MDA) and nitrite/nitrate (NO2-/NO3-), as well as the activities of superoxide dismutase (SOD) and myeloperoxidase (MPO) were examined. Western blot was used to determine the expression of inducible nitric oxide synthase (iNOS).
RESULTS: EGb 761 markedly improved mean arterial pressure and attenuated lung injury, manifested by the improvement of histological changes and significant decreases of pulmonary W/D and PPI (p < 0.05 or 0.01). Moreover, EGb 761 markedly increased SOD activity, reduced MDA levels and MPO activity, and suppressed NO generation accompanied by down-regulation of iNOS expression (p < 0.05 or 0.01).
CONCLUSION: The results indicate that EGb 761 has a protective effect on lung injury induced by II/R, which may be related to its antioxidant property and suppressions of neutrophil accumulation and iNOS-induced NO generation. EGb 761 seems to be an effective therapeutic agent for critically ill patients with respiratory failure related to II/R.
Keywords: Ginkgo biloba Extract, Intestine, Reperfusion injury, Lung, Adult respiratory distress syndrome, Vascular permeability, Nitric oxide, Lipid peroxidation
INTRODUCTION
Intestinal ischemia/reperfusion (II/R) injury is a grave condition resulting from acute mesenteric ischemia, hemorrhagic, traumatic or septic shock, or severe burns and some surgical procedures including small bowel transplantation and abdominal aortic surgery[1]. It is well-known that II/R not only causes injury of the intestine itself, but also involves severe destruction of remote organs and even multiple organ dysfunction[2,3]. Of these remote organ injuries, lung injury has been well-characterized as an acute inflammation with sequestration of leukocytes and their enzymatic products in lung tissue, increased microvascular permeability, perivascular and interstitial edema, and pulmonary edema[4]. These pulmonary processes incited by remote II/R injury frequently lead to the clinical picture of acute respiratory distress syndrome[5].
The mechanisms of lung injury induced by II/R are very complex. It is well-established that lipid peroxidation is one of the major factors causing lung injury[6,7]. In addition, evidence showed that overproduction of nitric oxide (NO) generated by inducible nitric oxide synthase (iNOS) not only aggravates oxidative damage[8,9], but leads to pulmonary microvascular dysfunction as well[10]. Thus, the therapeutical strategy by removing free radicals and reducing NO overproduction should be potential effective strategies for the protection against lung injury following II/R.
Extracts from the leaves of ginkgo biloba have been widely used therapeutically in China and Western countries for years. Standard ginkgo biloba extract, EGb 761, contains 22%-27% flavonoids and 5%-7% terpenoids, which are the most important active substances in the extract[11]. Today, EGb 761 is widely prescribed for treatment of disorders such as Alzheimer’s disease and neuronal hypoxia, both of which have etiologies associated with oxidative stress[11-13]. In the cardiovascular system, it can protect the heart against ischaemia/reperfusion damage[14] and alleviate vascular endothelial cell injury[15]. Thus, currently, EGb 761 is also widely used in treating cardiovascular diseases[16].
EGB 761 has a broad spectrum of pharmacological activities. However, most of the investigations were focused on cardio-cerebral vascular diseases. Recently, several studies showed that EGb 761 decreases malondialdehyde (MDA) and myeloperoxidase (MPO, an indicator of tissue neutrophil accumulation) levels[17] and protects against histological damage in intestinal mucosa after II/R[18]. However, there is no report about the effect of EGb 761 on lung injury induced by II/R.
Based on the above findings, we postulate that EGb 761 can exert a protective effect on II/R-induced lung injury. Thus, the present study was undertaken to confirm the above hypothesis and elucidate the mechanisms related to pulmonary lipid peroxidation, neutrophil sequestration and nitric oxide (NO) production regulated by induced nitric oxide synthase (iNOS) expression.
MATERIALS AND METHODS
Animal preparation
The current study was approved by the Animal Care Committee of Sun Yat-sen University and performed in accordance with the guidelines for the use of experimental animals by the Ministry of Health. Twenty-four adult pathogen-free male Wistar rats weighing between 230-302 g were housed in individual cages in a cohorted temperature-controlled room with alternating 12 h light/dark cycles, and acclimated for a week before the study. Food was removed 8 h prior to the study, and all rats had free access to water.
Establishment of intestinal ischemia/reperfusion rat models
All rats were anesthetized with pentobarbital (30 mg/kg body weight, intraperitoneally). A polyethylene catheter (PE-10) was inserted into the left carotid artery. The catheter was connected to MacLab digital data acquisition system (PowerLab/4SP ADI Instruments, Ugo Basile Comerio, VA, Italy) via a pressure transducer (TSD104A Biopac Systems, 2 Biological Instruments, Besozzo, VA, Italy) for monitoring mean arterial pressure (MAP). Periodically, the cannula was flushed with normal saline (100 μL) to maintain recording fidelity. The rat model was established according to our previous method[19]. The small intestine was exteriorized by midline laparotomy and the superior mesenteric artery (SMA) was occluded by microvascular clip. After 60 min of ischemia, the SMA was reperfused for 180 min. Ischemia was determined by the existence of pulseless or pale color of the small intestine. The return of pulse and restoration of pink color were assumed to be due to the reperfusion of the intestine.
Experimental protocol
The rats were randomly allocated into one of 3 equal groups (n = 8): Sham, II/R and EGb + II/R. The surgical sham group underwent full surgical preparation including the isolation of SMA without the occlusion. In EGb + II/R group, EGb 761 (100 mg/kg per day) was given via gastric tube for 7 consecutive days prior to surgery. EGb 761 was dissolved in normal saline at the concentration of 100 mg/mL. Rats in II/R group and sham group were treated with equal volumes of the vehicle (normal saline solution) of EGb 761. EGb 761 was supplied by Zhejiang Kangenbei Pharmaceutical Company, China (No. 21003). It contains 24% ginkgo-flavonole glycosides and 6% terpenoids.
Sample collection of blood and lung tissues
After 3-h reperfusion, blood samples were taken from carotid artery. A median sternotomy was performed, and the left main bronchus and right lower lobe bronchus were clamped. The trachea was cannulated and the right upper and middle lobes were lavaged three times with 2 mL of saline containing 0.07 mmol/L EDTA. The collected blood and bronchoalveolar lavage (BAL) fluids were centrifuged at 3000 r/min for 15 min, and the supernatant was stored at -80°C for subsequent measurement of protein content. The right lower lung lobe was divided into two parts for histology examination and the assessment of pulmonary edema. The left upper and lower lung lobes were used for biochemical and Western blotting analyses, respectively.
Lung histology examination
Part of the right lower lung lobe was harvested and fixed in 10% formalin. After embedded in paraffin, sections of 8 mm were stained with hematoxylin and eosin for light microscopy.
Assessment of pulmonary vascular permeability and pulmonary edema
Pulmonary permeability index (PPI) served as indicators of high pulmonary vascular permeability. PPI was assessed by the ratio of protein concentration in BAL fluid to that in plasma. The protein concentrations of blood and BAL fluid were detected by Coomassie brilliant blue method according to the manufacturer’s instructions (Nanjing Jiancheng Corp., China). The severity of pulmonary edema was estimated by wet-to-dry lung weight ratio (W/D). After the wet weight of the lungs was measured, the lungs were completely dried in a vacuum oven (DP22; Yamato Scientific, Tokyo, Japan) at 95°C for 48 h to remove any gravimetrically detectable water.
Detection of myeloperoxidase activity in lung tissue
Myeloperoxidase (MPO) activity was detected according to the method described by Barry et al[20]. After weighing, the lung assay sample was homogenised in 5 mL of 0.5% hexadecyltrimethyl ammonium bromide (Sigma, U.K.) in 50 mmol potassium phosphate buffer (pH 6). The homogenate was freeze-thawed twice, and then centrifuged at 13 000 g for 5 min. The resulting supernatant was assayed spectrophotometrically for MPO activity by incubating 0.1 mL of the supernatant with 2.9 mL of solution B. Solution B was prepared by dissolving 2.9 mL of O-dionisidine hydrochloride (Sigma, U.K.) in 90 mL of distilled water and addition of 10 mL of 50 mmol potassium phosphate buffer (pH 6) and hydrogen peroxide (final concentration 0.0005%). The change in absorbance with time at 460 nm was then recorded continuously (Philips PU/VIS specvasculature trophotometer). One unit of MPO was defined as that degrading 1 μmol peroxide per minute at 25°C. Results were expressed as units per gram of lung tissue.
Detection of oxidative stress in lung tissue
Lung tissues were homogenized on ice in normal saline. The homogenates were centrifuged at 4000 r/min at 4°C for 10 min. MDA levels in the supernatants were determined by measurement of thiobarbituric acid-reactive substance levels using MDA assay kit (Nanjing Jiancheng Corp., China) according to the manufacturer’s instructions. The results were calculated as nmol per 100 mg of protein (nmol/100 mg). SOD activity in the supernatants was evaluated by inhibition of nitroblue tetrazolium (NBT) reduction by O2- generated by the xanthine/xanthine oxidase system in accordance with the manufacturer’s instructions (Nanjing Jiancheng Corp., China). The results were expressed as U/100 mg protein.
Detection of nitrite/nitrate in lung tissue
Lung tissues (100 mg) were weighed and made into 10% homogenates with 0.9 mL normal saline. After centrifugation for 10 min at 10 000 r/min, the supernatant was placed in boiling water for 3 min and then centrifuged for 5 min at 10 000 g. The supernatant (0.1 mL) was taken for the detection of nitrite/nitrate (NO2-/NO3-) production, an indicator of NO synthesis, with an NO assay kit (Nanjing Jiancheng Corp., China) following the manufacturer’s instructions. Results were calculated as micromoles per 100 grams of protein (μmol/100 mg protein).
Western blotting analysis for inducible nitric oxide synthase in lung tissue
The left lower lung lobe was homogenized with PBS (pH 7.2) and centrifuged at 4°C, 18 000 r/min for 10 min. After precipitation, the unsolubilized fraction was discarded. The protein concentration in the supernatant was determined by Coomassie blue dyebinding assay (Nanjing Jiancheng Corp. China). Aliquots (30 mg) of proteins from each sample were electrophoresed on a 120 g/L SDS-polyacrylamide gel for 4 h at 100 V. The protein samples were transferred onto a nitrocellulose membrane (Amersham, USA). The membrane was then probed with polyclonal rabbit anti-rat inducible nitric oxide synthase (iNOS) antibody (1:50 dilution, Santa Cruz Co., USA) for 2 h at 37°C. After 3 washes with TPBS, blots were visualized with the use of an amplified HRP kit (Wuhan Boshide Corp, China). The presence of iNOS was indicated by the presence of brown color.
Statistical analysis
Statistical analysis was performed using SPSS (version 10.1; SPSS for Windows, Chicago, IL) software. Data were expressed as mean ± SD. One-way analysis of variance was used for multiple comparisons and least significant difference test (LSD-t) was used for intra-group comparison. P < 0.05 was considered statistically significant.
RESULTS
Changes in MAP
There was no death of any rats during the experiment. There were no significant differences in the weight of the rats and the temperature in the laboratory among the groups. Figure 1 illustrates the time course of MAP in the three experimental groups. A rapid drop in MAP was recorded immediately after the release of the arterial occlusion and the beginning of reperfusion of the ischemic bowel. EGb 761 significantly improved MAP. There was a significant difference in MAP at all time points after reperfusion between II/R and EGb + II/R groups (P < 0.01).
Figure 1.

Effects of EGb 761 (arrow indicates the starting point of reperfusion) on mean arterial pressure (MAP) after II/R in anaesthetized rats. A rapid drop in MAP was recorded immediately after the beginning of reperfusion of the ischemic bowel. Data are the means of 8 rats (SD not shown). EGb 761 markedly increased MAP. There were significant differences in MAP at all time points after reperfusion between II/R and EGb + II/R groups (P < 0.01).
Pathological changes of lung tissue
The histological structure of alveolar and mesenchymal cells was normal in the lungs of sham group (Figure 2A), while the lung tissues from II/R group were significantly damaged with pulmonary edema, hemorrhage and inflammatory cell infiltration (Figure 2B). Pretreatment with EGb 761 could attenuate significantly the lung injury as shown by light microscopy (Figure 2C).
Figure 2.
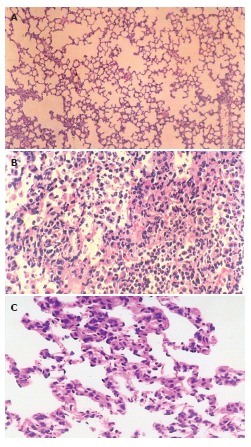
Light microscopic observation of the lung tissues after II/R with pretreatment of EGb 761 in rats. A: The normal lung tissue structure was found in sham group (× 100); B: Lung edema, hemorrhage and inflammatory cell sequestration were found in II/R group (× 200); C: Decreased morphological changes induced by II/R were found in EGb +II/R group (× 200).
Changes in W/D and PPI in lung tissues
Compared with the sham group, the lung W/D and PPI in II/R group were increased significantly (P < 0.05, P < 0.01). Compared with the II/R group, the lung W/D and PPI in EGb + II/R group were significantly decreased (P < 0.05 or 0.01) (Table 1).
Table 1.
Changes in W/D and PPI in lung tissues (mean ± SD)
| n | Sham | II/R | EGb +II/R | |
| PPI (× 10-3) | 8 | 1.08 ± 0.42 | 4.02 ± 0.82b | 2.23 ± 0.45ac |
| W/D (%) | 8 | 13.75 ± 5.18 | 29.62 ± 3.39a | 16.34 ± 6.45d |
P < 0.05,
P < 0.01 vs sham group;
P < 0.05,
P < 0.01 vs II/R group. W/D: Wet-to-dry lung weight ratio, PPI: pulmonary permeability index.
Changes in MPO activity in lung tissues
MPO activity in II/R group was significantly higher than that in the sham group (P < 0.01). Compared with II/R group, MPO activity in EGb +II/R group was markedly reduced (P < 0.01), but still higher than that in the sham group (P < 0.05) (Table 2).
Table 2.
Changes in the activities of SOD and MPO, the levels of MDA and NO2-/NO3- in lung tissues (mean ± SD)
| n | Sham | II/R | EGb + II/R | |
| MDA (nmol/100 mg) | 8 | 41.34 ± 6.45 | 68.52 ± 8.69b | 44.56 ± 6.13d |
| SOD activity (U/100 mg) | 8 | 101.8 ± 10.32 | 61.09 ± 6.52b | 84.36 ± 8.25ac |
| NO2-/NO3- (μmol/100 mg) | 8 | 40.36 ± 9.68 | 78.54 ± 12.36b | 44.13 ± 8.56d |
| MPO activity (U/g) | 8 | 3.26 ± 0.78 | 6.93 ± 0.79b | 4.14 ± 0.76ad |
P < 0.05,
P < 0.01 vs sham group;
P < 0.05,
P <0.01 vs II/R group. MPO: Myeloperoxidase; NO2-/NO3-: nitrite/nitrate; MDA: malondialdehyde; SOD: superoxide dismutase.
Changes in lung MDA levels and SOD activity in lung tissues
MDA levels in II/R group were significantly higher than that in the sham group (P < 0.01). Compared with II/R group, MDA levels in EGb+II/R group were markedly decreased (P < 0.01). SOD activity in II/R group was markedly lower than that of the sham group (P < 0.01). It was increased significantly in EGb +II/R group (P < 0.01), but still lower than that of the sham group (P < 0.05) (Table 2).
Changes in lung NO2-/NO3- levels in lung tissues
Compared with the sham group, lung NO2-/NO3- levels in II/R group were increased significantly (P < 0.01). Compared with the II/R group, NO2-/NO3- levels in EGb + II/R group were decreased significantly (P < 0.01) (Table 2).
Changes in iNOS expression in lung tissues
Western blotting showed that very weak positive signals were found in the lung tissues of the sham group. Significant increases of iNOS protein expression were seen in the II/R group. There were still notable positive signals in EGb + II/R group, but it was weaker for the II/R group (Figure 3).
Figure 3.
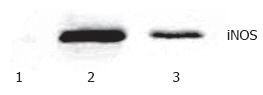
Western blotting analysis of iNOS in rat lung. 1. Sham group; 2. II/R group; 3. EGb + II/R group. The intensity of the bands was greater in II/R group compared with EGb +II/R group. A prominent band of iNOS protein was demonstrated at approximately 70 Kd.
DISCUSSION
Currently, EGb 761 is commonly used in treating cardiovascular diseases and cerebral vascular diseases in many countries. As far as we know, the present study is the first to investigate its effects on II/R-induced lung injury. The results showed that EGb 761 can markedly improve MAP and attenuate lung injury, manifested by the improvement of the histological damage and significant decreases of pulmonary W/D and PPI (variables related to lung injury). These findings suggest that the administration of EGb 761 may be a potential effective therapeutical approach for the prevention from II/R-induced lung injury.
The mechanisms of lung injury after II/R are complex and poorly understood. It is thought that the damage of intestinal mucosal barrier following II/R causes the dislocation of bacteria or endogenous endotoxins, leading to systemic inflammatory reactions[20-22]. The neutrophil and their enzymatic products are sequestrated in lung tissues, which causes increased microvascular permeability, perivascular and interstitial edema, and pulmonary edema[4,22,23].
MPO is a haem-containing enzyme located within the azurophil granules of neutrophils, and its activity is known as an indicator of tissue neutrophil accumulation[24]. The present study showed that EGb 761 markedly reduced MPO activity, suggesting that its protective effect on II/R-induced lung injury might be related to the suppression of neutrophil accumulation. Our findings are well supported by previous studies, in which EGb 761 suppressed neutrophil sequestration in hepatic and renal tissues evidenced by decreased MPO activity[25,26]. Previous studies also demonstrated that EGb 761 increases peripheral and cerebral blood flow and improves microcirculation, and reduces capillary permeability[27,28], indicating the effect of EGb 761 on neutrophil accumulation. However, the related mechanisms need further investigation.
It is well documented that lipid peroxidation due to II/R is one of the main causes for lung injury[6,7]. MDA is the direct product of lipid peroxidation. Therefore, the extent of lipid peroxidation can be assessed by measuring MDA levels in tissues[29]. SOD is the major enzyme for scavenging oxygen free radicals, and its activity can reflect its functional status[30]. Previous studies have shown that EGb 761 can interact as a free radical scavenger and an inhibitor of lipid peroxidation with all, or nearly all, reactive oxygen species[27]. In the present study, EGb 761 was demonstrated to inhibit MDA production and increase SOD activity, suggesting that the inhibition of lipid peroxidation may be one of the mechanisms attributable to the protective effects of EGb 761 on II/R-induced lung injury.
Several recent observations implicate that NO may be an important participant in the pulmonary response to II/R[31]. It has been suggested that NO, produced from endothelial constitutive nitric oxide synthase (ecNOS), may be an important protective molecule at the onset of II/R. In this regard, inhibitors of endogenous NO production greatly exacerbate the increase in epithelial permeability and cardiovascular dysfunction in the reperfused post-ischemia intestine[31,32]. Excessive NO production has been attributed to the second NOS (inducible NOS, iNOS) that is not present under normal conditions but can be induced in response to systemic inflammatory states, including II/R. The induction of iNOS has been implicated in the pathogenesis of II/R and it was reported that the inhibitions of iNOS activity and NO production could attenuate II/R injury[10,33]. In the present study, we further studied the contribution of iNOS to II/R-induced lung injury. The results showed that 60 min of intestinal ischemia followed by 180 min of reperfusion significantly upregulated the lung iNOS expression, accompanied by marked elevation of pulmonary nitrate/nitrite (stable metabolites of NO) levels. This is consistent with the findings of Virlos et al[10], who demonstrated that pulmonary iNOS activity in rats subjected to II/R was significantly increased and of Zhou et al[8], who showed that systemic inflammatory response and lung injury occur following II/R with an overproduction of NO accompanied by the increases of iNOS expression and the formation of peroxynitrite in the lungs. The mechanisms of the cytotoxic actions of excessive NO production have not been fully understood. It has been suggested that the superoxide ions react with NO to produce peroxynitrite, which then causes accentuated lipid peroxidation, proteic and DNA modifications resulting in cellular damages[34]. Taken together, the iNOS-NO-peroxynitrite dependent pathway may be one of the mechanisms of II/R-induced lung injury.
The effect of EGb 761 on NO generation in lung tissue following II/R was studied for the first time in the present study. The results showed that EGb 761 significantly reduced the generation of NO accompanied by the down-regulation of iNOS expression. Varga et al[35] showed that EGb 761 directly acts as an NO scavenger and concomitantly inhibits the expression of iNOS mRNA in myocardial tissues, thus improving the recovery of postischemic cardiac function after myocardial ischemia/reperfusion. In addition, EGb 761 inhibits NO production in lipopolysaccharide/gamma interferon (LPS/IFN-γ)-activated macrophages by concomitantly scavenging NO and inhibiting iNOS mRNA and enzyme activity[36,37]. Although the experimental models employed in previous studies are different from the present study, these findings can, at least in part, support our current conclusion that the protective effect of EGb 761 on lung injury may be attributable to its suppression on the iNOS-NO dependent pathway.
There is little information on the mechanisms of the effect of EGb 761 on the iNOS-NO dependent pathway. A most recent study showed the preventive effect of EGb on the lipopolysaccharide-induced expressions of iNOS via the suppression of nuclear factor-kappaB (NF-κB) in RAW 264.7 cells. Thus, the suppression of NF-κB might be the potential mechanism underlying the findings that EGb 761 reduces NO production and concomitantly inhibits iNOS expression in lung tissues following II/R[38].
There are some limitations in the present study. First, EGb 761 is a specific and complex product prepared from ginkgo leaves. EGb 761 used in this study contained 24% ginkgo-flavonole glycosides and 6% terpenoids. Which components produce the protective effect or work more in protecting against II/R-induced lung injury in the present study remains to be elucidated. Second, we did not employ selective inhibitor of iNOS to strengthen the present conclusion, because we focused on investigating the protective effect of EGb 761 on lung injury and thus only preliminarily studied the related mechanisms.
In conclusion, the present study indicates that EGb 761 has a protective effect on lung injury induced by II/R, which may be related to its antioxidative property and the suppressions of neutrophil accumulation and iNOS-induced NO generation. EGb 761 appears to be an effective therapeutic agent for some critically ill patients with respiratory failure related to II/R, although its mechanisms remain to be elucidated.
ACKNOWLEDGMENTS
We thank Dr. You-Kai Zhu and Dr. Ming-Qi Zhao for their help with experimental techniques.
COMMENTS
Background
EGb 761 is widely used in treating cardio-cerebral vascular diseases mainly due to its action of anti-oxidative damage; however, there is no report about its effect on lung injury induced by intestinal ischemia/reperfusion. The present study was undertaken to confirm the above hypothesis and elucidate the mechanisms related to pulmonary lipid peroxidation, neutrophil sequestration and nitric oxide (NO) production regulated by induced nitric oxide synthase (iNOS) expression.
Research frontiers
EGb 761 has been reported to be effective for the disorders such as Alzheimer’s disease and neuronal hypoxia and to protect the heart against ischaemia/reperfusion damage and to alleviate vascular endothelial cell injury, which all have etiologies associated with oxidative stress.
Innovations and breakthroughs
Previous studies showed the protective effect of EGb 761 on cardio-cerebral ischemia/reperfusion injury and intestinal mucosa injury following intestinal ischemia/reperfusion (II/R). The present study indicates that EGb 761 has a protective effect on lung injury induced by II/R and the mechanism may be related to its antioxidative property and the suppressions of neutrophil accumulation and iNOS-induced NO generation.
Applications
Remote lung injury induced by intestinal ischemia reperfusion (II/R) due to acute mesenteric ischemia, hemorrhagic, traumatic or septic shock, or severe burns and some surgical procedures including small bowel transplantation and abdominal aortic surgery often leads to the clinical picture of acute respiratory distress syndrome. EGb 761 has been demonstrated to have the protective effect on lung injury and thus appears to be an effective therapeutic agent for some critically ill patients with respiratory failure related to II/R.
Terminology
Excessive production of nitric oxide (NO) is attributed to the upregulation of inducible nitric oxide synthase (iNOS) expression that is not present under normal conditions, but can be induced in response to systemic inflammatory states, including II/R. NO can react with superoxide ions to produce peroxynitrite, which then causes accentuated lipid peroxidation, proteic and DNA modifications, resulting in cellular damages. This is described as an iNOS-NO-peroxynitrite dependent pathway.
Peer review
This manuscript is very interesting. The title accurately reflects the major contents of the article. The results provide sufficient experimental evidences from which conclusions are drawn. The conclusions are scientifically reliable and valuable.
Footnotes
Supported by grants from the Administration of Traditional Chinese Medicine of Guangdong Province, China, No. 1040066; Natural Science Foundation of Guangdong Province, China, No. 05300758; National Natural Science Foundation of China, No. 30672021
S- Editor Liu Y L- Editor Zhu LH E- Editor Liu WF
References
- 1.Homer-Vanniasinkam S, Crinnion JN, Gough MJ. Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg. 1997;14:195–203. doi: 10.1016/s1078-5884(97)80191-8. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–209. doi: 10.1097/00024382-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Turnage RH, Guice KS, Oldham KT. Pulmonary microvascular injury following intestinal reperfusion. New Horiz. 1994;2:463–475. [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 6.Rossman JE, Caty MG, Zheng S, Karamanoukian HL, Thusu K, Azizkhan RG, Dandona P. Mucosal protection from intestinal ischemia-reperfusion reduces oxidant injury to the lung. J Surg Res. 1997;73:41–46. doi: 10.1006/jsre.1997.5196. [DOI] [PubMed] [Google Scholar]
- 7.Giakoustidis AE, Giakoustidis DE, Iliadis S, Papageorgiou G, Koliakou K, Kontos N, Taitzoglou I, Botsoglou E, Papanikolaou V, Atmatzidis K, et al. Attenuation of intestinal ischemia/reperfusion induced liver and lung injury by intraperitoneal administration of (-)-epigallocatechin-3-gallate. Free Radic Res. 2006;40:103–110. doi: 10.1080/10715760500133479. [DOI] [PubMed] [Google Scholar]
- 8.Zhou JL, Jin GH, Yi YL, Zhang JL, Huang XL. Role of nitric oxide and peroxynitrite anion in lung injury induced by intestinal ischemia-reperfusion in rats. World J Gastroenterol. 2003;9:1318–1322. doi: 10.3748/wjg.v9.i6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pararajasingam R, Weight SC, Bell PR, Nicholson ML, Sayers RD. Pulmonary nitric oxide metabolism following infrarenal aortic cross-clamp-induced ischaemia-reperfusion injury. Eur J Vasc Endovasc Surg. 2000;19:47–51. doi: 10.1053/ejvs.1999.0930. [DOI] [PubMed] [Google Scholar]
- 10.Turnage RH, Wright JK, Iglesias J, LaNoue JL, Nguyen H, Kim L, Myers S. Intestinal reperfusion-induced pulmonary edema is related to increased pulmonary inducible nitric oxide synthase activity. Surgery. 1998;124:457–462; discussion 462-463. [PubMed] [Google Scholar]
- 11.Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y. Alzheimer's disease, the nematode Caenorhabditis elegans, and ginkgo biloba leaf extract. Life Sci. 2006;78:2066–2072. doi: 10.1016/j.lfs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekaran K, Mehrabian Z, Spinnewyn B, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of the Ginkgo biloba extract (EGb 761), in gerbil global brain ischemia. Brain Res. 2001;922:282–292. doi: 10.1016/s0006-8993(01)03188-2. [DOI] [PubMed] [Google Scholar]
- 14.Yuan LP, Chen ZW, Li F, Dong LY, Chen FH. Protective effect of total flavones of rhododendra on ischemic myocardial injury in rabbits. Am J Chin Med. 2006;34:483–492. doi: 10.1142/S0192415X06004016. [DOI] [PubMed] [Google Scholar]
- 15.Cheung F, Siow YL, Chen WZ, O K. Inhibitory effect of Ginkgo biloba extract on the expression of inducible nitric oxide synthase in endothelial cells. Biochem Pharmacol. 1999;58:1665–1673. doi: 10.1016/s0006-2952(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 16.Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–678. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 17.Pehlivan M, Dalbeler Y, Hazinedaroglu S, Arikan Y, Erkek AB, Günal O, Türkçapar N, Türkçapar AG. An assessment of the effect of Ginkgo Biloba EGb 761 on ischemia reperfusion injury of intestine. Hepatogastroenterology. 2002;49:201–204. [PubMed] [Google Scholar]
- 18.Onen A, Deveci E, Inalöz SS, Isik B, Kilinc M. Histopathological assessment of the prophylactic effect of gingko-biloba extract on intestinal ischemia-reperfusion injury. Acta Gastroenterol Belg. 1999;62:386–389. [PubMed] [Google Scholar]
- 19.Liu KX, Wu WK, He W, Sun HL. Study on sini decoction in treatment of intestinal ischemia-reperfusion injury in rats: mechanism relating to oxygen radical and bcl-2 protein. Zhongguo Zhongyao Zazhi. 2006;31:329–332, 348. [PubMed] [Google Scholar]
- 20.Turnage RH, Guice KS, Oldham KT. Endotoxemia and remote organ injury following intestinal reperfusion. J Surg Res. 1994;56:571–578. doi: 10.1006/jsre.1994.1091. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y, Yu Y, Chen J. The effect of intestinal ischemia/reperfusion on increased sensitivity to endotoxin and its potential mechanism. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1999;15:301–304. [PubMed] [Google Scholar]
- 22.Olanders K, Sun Z, Börjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86–92. doi: 10.1097/00024382-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Ishii H, Ishibashi M, Takayama M, Nishida T, Yoshida M. The role of cytokine-induced neutrophil chemoattractant-1 in neutrophil-mediated remote lung injury after intestinal ischaemia/reperfusion in rats. Respirology. 2000;5:325–331. [PubMed] [Google Scholar]
- 24.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med. 2000;29:403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 25.Sener G, Kabasakal L, Yüksel M, Gedik N, Alican Y. Hepatic fibrosis in biliary-obstructed rats is prevented by Ginkgo biloba treatment. World J Gastroenterol. 2005;11:5444–5449. doi: 10.3748/wjg.v11.i35.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulec M, Iraz M, Yilmaz HR, Ozyurt H, Temel I. The effects of ginkgo biloba extract on tissue adenosine deaminase, xanthine oxidase, myeloperoxidase, malondialdehyde, and nitric oxide in cisplatin-induced nephrotoxicity. Toxicol Ind Health. 2006;22:125–130. doi: 10.1191/0748233705th255oa. [DOI] [PubMed] [Google Scholar]
- 27.Clostre F. Ginkgo biloba extract (EGb 761). State of knowledge in the dawn of the year 2000. Ann Pharm Fr. 1999;57 Suppl 1:S18–S88. [PubMed] [Google Scholar]
- 28.Lagrue G, Behar A, Kazandjian M, Rahbar K. Idiopathic cyclic edema. The role of capillary hyperpermeability and its correction by Ginkgo biloba extract. Presse Med. 1986;15:1550–1553. [PubMed] [Google Scholar]
- 29.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11 Suppl 5:48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- 30.Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Aston K, Riley DP, Salvemini D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br J Pharmacol. 2001;132:19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanna A, Rossman JE, Fung HL, Caty MG. Attenuated nitric oxide synthase activity and protein expression accompany intestinal ischemia/reperfusion injury in rats. Biochem Biophys Res Commun. 2000;269:160–164. doi: 10.1006/bbrc.2000.2266. [DOI] [PubMed] [Google Scholar]
- 32.Virlos IT, Inglott FS, Williamson RC, Mathie RT. Differential expression of pulmonary nitric oxide synthase isoforms after intestinal ischemia-reperfusion. Hepatogastroenterology. 2003;50:31–36. [PubMed] [Google Scholar]
- 33.Suzuki Y, Deitch EA, Mishima S, Lu Q, Xu D. Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia-reperfusion injury. Crit Care Med. 2000;28:3692–3696. doi: 10.1097/00003246-200011000-00026. [DOI] [PubMed] [Google Scholar]
- 34.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 35.Varga E, Bodi A, Ferdinandy P, Droy-Lefaix MT, Blasig IE, Tosaki A. The protective effect of EGb 761 in isolated ischemic/reperfused rat hearts: a link between cardiac function and nitric oxide production. J Cardiovasc Pharmacol. 1999;34:711–717. doi: 10.1097/00005344-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Kobuchi H, Packer L. Bio-normalizer modulates interferon-gamma-induced nitric oxide production in the mouse macrophage cell line RAW 264.7. Biochem Mol Biol Int. 1997;43:141–152. doi: 10.1080/15216549700203911. [DOI] [PubMed] [Google Scholar]
- 37.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 38.Park YM, Won JH, Yun KJ, Ryu JH, Han YN, Choi SK, Lee KT. Preventive effect of Ginkgo biloba extract (GBB) on the lipopolysaccharide-induced expressions of inducible nitric oxide synthase and cyclooxygenase-2 via suppression of nuclear factor-kappaB in RAW 264.7 cells. Biol Pharm Bull. 2006;29:985–990. doi: 10.1248/bpb.29.985. [DOI] [PubMed] [Google Scholar]


