Abstract
AIM: To examine the effects of adenosine and A1 receptor activation on reperfusion-induced small intestinal injury.
METHODS: Rats were randomized into groups with sham operation, ischemia and reperfusion, and systemic treatments with either adenosine or 2-chloro-N6-cyclopentyladenosine, A1 receptor agonist or 8-cyclopentyl-1,3-dipropylxanthine, A1 receptor antagonist, plus adenosine before ischemia. Following reperfusion, contractions of ileum segments in response to KCl, carbachol and substance P were recorded. Tissue myeloperoxidase, malondialdehyde, and reduced glutathione levels were measured.
RESULTS: Ischemia significantly decreased both contraction and reduced glutathione level which were ameliorated by adenosine and agonist administration. Treatment also decreased neutrophil infiltration and membrane lipid peroxidation. Beneficial effects of adenosine were abolished by pretreatment with A1 receptor antagonist.
CONCLUSION: The data suggest that adenosine and A1 receptor stimulation attenuate ischemic intestinal injury via decreasing oxidative stress, lowering neutrophil infiltration, and increasing reduced glutathione content.
Keywords: Adenosine, Adenosine A1 receptor, Intestinal ischemia, Pharmacological preconditioning
INTRODUCTION
Ischemia-reperfusion (I/R) injury of the intestine is a significant problem in a numerous situations such as abdominal aortic aneurysm surgery, small bowel transplantation, cardiopulmonary bypass, strangulated hernias, and neonatal necrotizing enterocolitis[1]. Decreased contractile activity, increased microvascular permeability, and dysfunction of mucosal barrier are all associated with intestinal I/R[2,3]. I/R injury of the intestine is an intricate and multifactorial pathophysiological process that involves the formation and action of oxygen free radicals (OFRs)[3-9], inflammatory cytokines, the complement system[1,3] and neutrophil infiltration[1-3,6,7,10] at the site of damage.
The purine nucleoside adenosine is one of the major local regulators of normal tissue function, acting in both an autocrine and paracrine fashion. Its regulatory function becomes pronounced especially when energy supply ceases abruptly as in the case of ischemia, and fails to meet cellular energy demand. Adenosine exerts its effects by interacting with its receptors, four of which have been cloned and characterized as adenosine A1, A2a, A2b, and A3[11-13]. The physiological role of adenosine in the gastrointestinal tract is still poorly understood, particularly with regard to colonic and ileal motor functions. It has been reported that A1 adenosine receptor (A1AR) antagonists increase defecation in rats[13] and that A1AR agonists can inhibit intestinal fluid secretion and peristalsis via adenosine A2B and A1 receptors, respectively[14].
One of the cellular events observed during ischemia is the increased consumption of ATP, leading to accumulation of adenosine with thereby elevating extracellular adenosine. The accumulation of adenosine is believed to contribute to cytoprotection in the ischemic tissue[11,12]. Furthermore, adenosine which is released during short periods of ischemia followed by reperfusion, provides cytoprotection against a subsequent sustained ischemia in heart, resulting in reduced infarct size[15-20]. This is known as the preconditioning effect of adenosine, which is mediated mostly through the activation of cardiac A1ARs before ischemia[11,12,18]. It is well documented that the early[19,20] and late[11,15,18] phases of ischemic tolerance are mediated by adenosine in myocardium. That adenosine exerts anti-ischemic actions is indicated by a number of studies using adenosine receptor agonists and antagonists[15-18] as well as animals overexpressing or lacking A1AR[21,22]. Administration of adenosine either prior to ischemia or during reperfusion has been shown to attenuate myocardial injury[23,24]. Treatment with adenosine A1AR agonist initiates preconditioning not only in heart[15-18,25] but also in tissues such as kidney[26,27] and brain[28,29], resulting in attenuation of ischemic injury. One of the underlying mechanisms suggested for adenosine receptor-mediated preconditioning in the heard is through involvement of protein kinase C (PKC) in heart[11,12,30]. Activation of PKC induces opening of ATP-sensitive K+ channels[11,12,31]. Among other effectors that most likely contribute to the cytoprotection by adenosine are mitogen activated protein (MAP) kinases[25,31-33], heat shock proteins (HSPs)[11-15,34], antioxidant enzymes[15,34] and inducible nitric oxide synthase (iNOS)[11,12]. Moreover, induction and activation of manganese superoxide dismutase (Mn-SOD) is also believed to be a significant factor in mediating myocardial adaptation in response to activation of A1AR[15].
In the phenomenon of ischemic preconditioning (IPC), a short period of ischemia protects the organs (e.g. heart) against a subsequent more substantial ischemic injury[35]. In fact, IPC has been one of the most promising strategies against reperfusion injury during the last few years. It appears to elevate the tolerance of the intestine to I/R injury. A number of experimental studies have shown that reperfusion injury in small intestine is prevented by IPC[36-40]. IPC conducted in small intestine reduces postischemic leukocyte adhesion by maintaining the bioavailability of nitric oxide[41]. Moreover, it lowers the expression of P-selectin[38], which is a downstream effector target of the adenosine-initiated, PKC dependent, signalling pathway in intestine. Although activation of PKC triggered by adenosine has been a crucial factor for initiating the beneficial actions of IPC in most tissues, the effector of the preconditioning phenomenon appears to vary among tissues. Activated-K+ channels[42], nitric oxide[39,41] and endogenous opioid peptides[43] have reported to be the other downstream effectors of IPC in intestine. Based primarily on animal experiments, the identification of the molecular mechanisms that are responsible for protection by IPC, has provided opportunities to consider several rational targets for pharmacological intervention. Consequently, a variety of drugs have been demonstrated to be able to mimic IPC when applied instead of ischemia. This is known as pharmacological preconditioning (PPC). Recently, various studies carried out in rat small intestine have demonstrated that establishing PPC by administration of either adenosine[37] or A1AR agonist[38] mimics the protective effects of IPC. Intensive investigation has been focused on explaining how adenosine accomplishes the beneficial effect of preconditioning. For instance, currently published studies suggest important anti-ischemic roles of the A1[18,25,30], A3[17,21] or A2a[44,45] adenosine receptors in heart. On the other hand, relatively little data are available on the role of the different adenosine receptors in mediating cytoprotection in intestinal tissue which is exposed to I/R. The majority of studies strongly suggest that adenosine can promote protection against I/R injury via activation of different adenosine receptors in various tissues. A substantial number of studies report that IPC has been beneficial in human heart and the liver. However, both prospective controlled studies in human and experimental studies in animals are lacking[1]. Furthermore, research based on administration of drugs that can mimic the effects of IPC is required further to explore the cellular events during I/R injury of the intestine. To date, there is no direct evidence showing possible effects of adenosine and A1AR activation on reduced contractility of intestinal smooth muscle due to I/R injury. Therefore, the present study was constructed to explore the possible effects of adenosine and A1AR activation on reperfusion injury of small intestinal tissue by evaluating contractile response and levels of thiobarbituric acid-reactive substances (TBARS, a marker of lipid peroxidation), reduced glutathione (GSH, an endogenous antioxidant), and myeloperoxidase (MPO an index of neutrophil infiltration), in terminal ileum subjected to I/R.
MATERIALS AND METHODS
Animals
Following Ethical Committee approval, forty adult male Wistar rats, weighing 200-230 g, were obtained from the Experimental Research Section of Zonguldak Karaelmas University, where animals have been reared and maintained under standard conditions, such as stable room temperature (23 ± 2°C), a 12 h light: 12 h dark cycle, and feeding with commercial rat chow and tap water ad libitum. Experimental manipulations and surgical operations were approved by the Animal Ethical Committee of the University. Maximum care and a humane approach to use of animals was of primary consideration.
Experimental groups and operative procedures
The surgical goal was to induce mesenteric ischemia in rats for 30 min followed by a 180 min reperfusion period. On the day before surgery, each animal was fasted overnight with unlimited access to water. Briefly, each animal was anesthetized by an intraperitoneal injection of 50 mg/kg sodium thiopenthal followed by a midline incision made into the peritoneal cavity. The small bowel was exteriorized gently to the left onto moist gauze, and then the superior mesenteric artery (SMA) was carefully exposed, isolated, and clamped using a microvascular clamp. Intestinal ischemia was confirmed by obvious lack of pulse in the SMA and paleness of the jejunum and ileum. The intestines were then meticulously placed back into the abdomen which was closed with two small clamps. Following 30 min of occlusion time, the clamping was gently released and the intestine inspected for proper reperfusion characterized by regular pulsation. Throughout the surgical procedure, each animal was placed under a heating lamp to maintain constant body temperature (e.g. 37°C). For the purpose of assessing the roles of adenosine and A1AR agonist, animals were randomly divided into five groups: (1) Sham-operated group, subjected to laparatomy without performing the occlusion of the SMA; (2) I/R group, subjected to the occlusion of SMA followed by reperfusion; (3) CPA-treated group (0.1 mg/kg, 5 min prior to ischemia) + I/R; (4) Adenosine-treated group (10 mg/kg, 5 min prior to ischemia) + I/R; (5) DPCPX pretreatment (1 mg/kg, 15 min prior to adenosine administration) + adenosine treatment (10 mg/kg, 5 min prior to ischemia) + I/R. In the last group, confirming the possible effect of selective A1AR agonist CPA on reperfusion injury, the selective A1AR antagonist DPCPX was administered 15 min prior to adenosine treatment. The route and volume for drug administration were the tail vein and 200 μL, respectively. To animals in both sham-control and I/R-control groups were given sterile serum physiological solution in the same volume instead. Choice of dose regimen for the drugs was based on published studies in the literature[22,37,38].
Preparation of terminal ileum
Upon completion of the I/R period, and whilst still unconcious, the animals were sacrificed by exsanguination of the abdominal aorta. Strips of terminal ileum of 10 mm length were immediately removed 10 cm oral to the ileocecal junction and transferred into a Petri dish containing Krebs solution (in mmol/L: NaCl 118, NaHCO3 24.88, KH2PO4 1.18, KCl 4.7, MgSO4 1.16, CaCl2 2.52 and glucose 11.1). Then, tissue was longitudinally suspended in a standard organ chamber, and continuously perfused with 20 mL of preoxygenated Krebs solution (pH 7.4), which was bubbled constantly with a mixture of 950 mL/L O2 and 50 mL/L CO2 gas and maintained at a temperature of 37°C. One end of the tissue strip was tied to a fixed post and the other attached to an isometric force transducer under a resting tension of 2 g. Isometric responses were monitored by external force displacement transducer (FDA-10A, Commat Iletisim Co., Ankara, Turkey) and recorded on the computer using MP 30 software (Biopac Systems Inc., Santa Barbara, CA, USA). In the organ bath, each strip was allowed to equilibrate for 1 h with intervening washes every 15 min before adding any compound. Tissue samples also obtained from small intestine approximately 10 cm proximal to the ileocecal area were frozen immediately and stored at -40°C for biochemical measurements.
Concentration-response curves
At the beginning of each experiment to observe dose-contractile response relationship, KCl was added to the organ chamber to a final concentration of 30 mmol/L. For the preparation of high K+ solutions, NaCl was exchanged for an equimolar amount of KCl to maintain the physiological osmolarity of the Krebs solution. The contraction recorded in response to KCl was considered as a reference response. Afterwards, the contractions in response to carbachol and substance P at various final concentrations ranging from 10-9 mol/L to 10-2 mol/L were recorded by pipetting these compounds into the organ bath in a cumulative fashion at equal intervals. At the end of the experiment, the response to 30 mmol/L KCl was measured again to confirm and evaluate the degree of tissue viability. The amplitude of all contractions was then normalized for each g of tissue and expressed as percentage of the initial KCl-reference response. The number of experiments, represented as “n”, indicates that each experiment was performed with a tissue sample taken from one animal. All experiments were conducted in a paired way. For the purpose of evaluating the effects of ligand, agonist, and antagonist, the maximum response (Emax) and pD2 values (e.g. the negative logarithm of the concentration for the half-maximal response, ED50) were computed by using GraphPad Prism Software 3.02 (GraphPad Prism Inc., San Diego, CA, USA)[46]. The pD2 values (apparent agonist affinity constants) were calculated from each agonist concentration–response curve by linear regression of the linear median part of the sigmoid curve and taken as a measure of the sensitivity of the tissues to each agonist.
Drugs
Adenosine, CPA, DPCPX, carbachol and substance P were purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA). They were dissolved in double distilled water, except for CPA and DPCPX which were initially prepared in dimethyl sulphoxide and then diluted in physiological saline. Adenosine, CPA, and DPCPX were prepared fresh just before usage. Carbachol and substance P were made up at different concentrations and kept frozen in aliquots. Compounds, which were used for preparing Krebs solution, were purchased from Merck (Merck KGaA, Darmstadt, Germany). All other reagents, including trichloroacetic acid (TCA), thiobarbituric acid (TBA), butylated hydroxy toluene (BHT), and dithiobisnitrobenzoate (DTNB) were obtained from Sigma.
Determination of tissue TBARS and GSH
Tissue TBARS content was measured in order to estimate the extent of lipid peroxidation in the injured terminal ileum. Samples obtained from each group were stored at -40°C until assayed. Tissue samples were washed in ice-cold Krebs solution, blotted on absorbent paper and weighed. Afterwards, each sample was minced followed by homogenization with 10 mL of 100 g/L TCA per g of tissue, using a motor-driven homogenizer (Heidolph Diax 900, Heidolph Elektro GmbH&Co.KG, Kelheim, Germany). Then, the tissue TBARS levels were measured spectrophotometrically based on a method described by Casini et al[47] and expressed as nmol/g of tissue weight. Briefly, following two consecutive centrifugations at 3000 g for 15 min, 750 μL supernatant was added to equal volume of 6.7 g/L TBA and heated to 100°C for 15 min. The absorbance of the samples was then measured spectrophotometrically at 535 nm (Smart Spectro, LaMotte Co., Chestertown, MD, USA).
The GSH content of the samples were measured by applying a modified Ellman method[48]. In brief, 2 mL of 0.3 mol/L Na2HPO4 solution was mixed with 0.5 mL of supernatant obtained by employing the homogenization procedure described above. Into the mixture, 0.2 mL of DTNB solution was added followed by reading absorbance at 412 nm. The tissue GSH levels were expressed as μmol/g of tissue weight.
Measurement of tissue MPO activity
The degree of neutrophil accumulation in the intestinal tissue samples was measured by assaying MPO activity as described by Bradley et al[49]. Briefly, upon thawing, each sample was very finely minced with surgical blade in a petri dish containing 50 mmol/L potassium phosphate buffer (PB, pH 6.0) at a volume 20 times the tissue weight (e.g. 1 mL) followed by homogenization for 5 min in ice-cold PB by means of motor driven homogenizer. The homogenate was centrifuged at 40 000 g for 15 min at 4°C. The homogenized tissue pellet was suspended in 50 mmol/L PB containing 5 g/L hexadecyltrimethylammonium bromide (HETAB) and then homogenized again. Following three freeze and thaw cycles with sonication (Bandelin Sonopuls HD2070, Bandelin Electronic GmbH&CO.KG, Berlin, Germany) between cycles, the samples were centrifuged at 40 000 g for 10 min. Aliquots of supernatant (0.1 mL) were added to 2.9 mL of reaction mixture containing 0.167 mg/mL of o-dianisidine, and 20 mmol/L H2O2 solution, which were prepared in 50 mmol/L of PB. Immediately after adding the aliquot to the mixture, the change in absorbance at 460 nm was measured for 5 min. One unit of MPO activity was defined as that degrading 1 μmol of peroxide per min at 25°C. The activity was then normalized as unit per mg of tissue (U/mg).
Statistical analysis
Values for the experiments dealing with contractility were normalized for per g of tissue followed by expressing them as percentage of KCl response. Each data point represents mean ± SE. For statistical evaluation, SPSS 11.0 statistical software package programme was used (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was applied for statistical comparison of groups, followed by analysis with Tukey-Kramer test so as to determine differences between the groups. Probability value (P) of 0.05 or less was considered statistically meaningful.
RESULTS
Ileal longitudinal muscle contractility
For longitudinal ileum muscle collected from sham control, CPA-treated, adenosine-treated, and DPCPX-adenosine-treated animals, mean contraction responses to 30 mmol/L KCl were measured as 0.59 ± 0.01 g; 0.40 ± 0.09 g; 0.50 ± 0.22 g; and 0.44 ± 0.21 g, respectively, which were statistically indistinguishable (Figure 1). In I/R control group however, the contractile response (0.26 ± 0.08 g) was significantly reduced when compared to that in sham-operated control group (P = 0.012).
Figure 1.
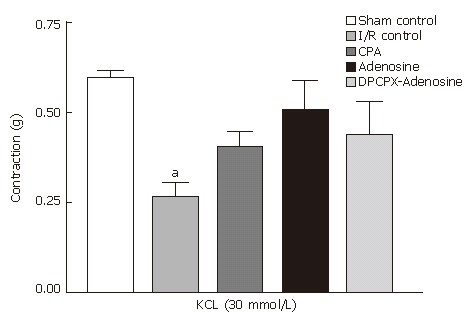
Mean contraction of longitudinal ileum muscle isolated from sham-operated control, I/R control, CPA-I/R, adenosine-I/R, and DPCPX-adenosine-I/R rats in response to 30 mmol/L KCl. Data are expressed as means ± SE (n = 8). aP < 0.05 vs sham-operated control group.
The addition of carbachol at concentrations from 10-9 mol/L to 10-2 mol/L into the organ bath resulted in a dose-dependent contractile effect on the terminal ileum segments from all groups (Figure 2), providing sigmoid curves with Emax and pD2 values (Figure 3). Emax value for carbachol was significantly lower in the I/R control group than in the sham-operated control group (157.04% ± 41.35% vs 488.66% ± 47.01%, respectively). In other words, contraction in response to carbachol was significantly reduced by induction of I/R. Statistical difference between the groups appeared to be meaningful at 10-6 mol/L (P = 0.02), reaching a maximal level at 10-3 mol/L of carbachol (P = 0.0001). The I/R-induced reduction in contractility was significantly restored by treatments with both CPA and adenosine but not by pretreatment with DPCPX. Amelioration of reduced contractions with CPA and adenosine therapies became statistically significant at millimolar doses of carbachol (P = 0.03 at 10-3 mol/L and 10-2 mol/L).
Figure 2.
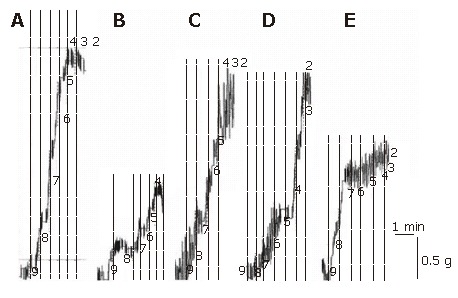
Representative traces showing responses generated by various concentrations of carbachol in longitudinal ileum muscle isolated from sham-operated control (A), I/R control (B), CPA–I/R (C), adenosine-I/R (D), and DPCPX-adenosine-I/R (E) rats.
Figure 3.
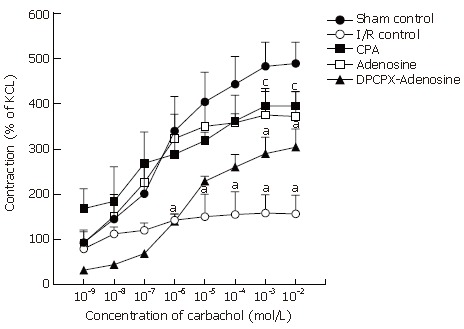
Dose-response curves of carbachol in longitudinal ileum muscle isolated from sham-operated control, I/R control, CPA-I/R, adenosine-I/R, and DPCPX-adenosine-I/R rats. Data are expressed as means ± SE (n = 8). aP < 0.05 vs sham-operated control, cP < 0.05 vs I/R control groups.
Comparison of the Emax values showed that average contraction of ileum samples in I/R group was just 32% of that in sham-operated control group, while those in CPA- and adenosine-treated groups were approximately 81% and 76%, respectively (Table 1). In the group pretreated with DPCPX, Emax was found to be approximately 60% of that in sham control group, which was statistically significant (P < 0.05). On the other hand, no statistically significant change was detected in the corresponding pD2 values in any group (Table 1).
Table 1.
Emax and pD2 values of carbachol and substance P (n = 8, means ± SE)
| Sham Control | I/R Control | CPA-I/R | ADO-I/R | DPCPX + ADO-I/R | |
| Carbachol | |||||
| Emax | 488.67 ± 47.01 | 157.05 ± 41.35a | 395.05 ± 32.62c | 372.21 ± 54.68c | 212.35 ± 40.09a |
| pD2 | 6.30 ± 0.19 | 7.02 ± 0.31 | 6.24 ± 0.40 | 6.94 ± 0.13 | 5.81 ± 0.18 |
| Substance P | |||||
| Emax | 255.94 ± 31.17 | 115.00 ± 13.36a | 148.19 ± 13.80a | 242.93 ± 46.55c | 259.61 ± 24.62c |
| pD2 | 6.51 ± 0.05 | 8.29 ± 1.08 | 7.00 ± 0.22 | 6.69 ± 0.28 | 6.51 ± 0.07 |
P < 0.05 vs sham-operated control group,
P < 0.05 vs I/R control group.
In response to various concentrations of substance P ranging from 10-9 mol/L to 10-2 mol/L, terminal ileum samples contracted in a dose-dependent fashion in all groups (Figure 4), rendering sigmoid curves with Emax and pD2 values (Figure 5). The contractile response induced by substance P was significantly and dose-dependently inhibited by induction of I/R. Statistical difference between sham-operated control rats and I/R control animals was significant at 10-6 mol/L and over doses of substance P (P < 0.05). Reduced contractility due to I/R was alleviated significantly by adenosine treatment (P < 0.05). This effect of adenosine was completely lost once DPCPX was given prior to adenosine administration. However, the exacerbating effect of DPCPX was significantly evident in response to substance P at doses lower than 10-5 mol/L (Figure 5). Above this concentration, as shown in the ascending part of the curve, responses in both adenosine- and DPCPX-adenosine-treated groups were statistically indistinguishable. Accordingly, there was a statistically significant difference between I/R control group and DPCPX-adenosine-treated group in response to 10-4 mol/L (P = 0.022), 10-3 mol/L (P = 0.004), and 10-2 mol/L (P = 0.011) of substance P. Regarding the corresponding pD2 values, no statistically significant change was detected in any group (Table 1).
Figure 4.
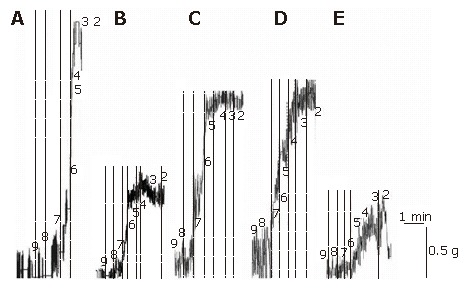
Representative traces showing responses generated by various concentrations of substance P in longitudinal ileum muscle isolated from sham-operated control (A), I/R control (B), CPA-I/R (C), adenosine-I/R (D), and DPCPX-adenosine-I/R (E) rats.
Figure 5.
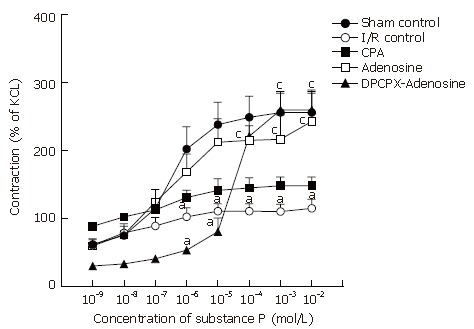
Dose-response curves of substance P in longitudinal ileum muscle isolated from sham-operated control, I/R control, CPA-I/R, adenosine-I/R, and DPCPX-adenosine-I/R rats. Data are expressed as means ± SE (n = 8). aP < 0.05 vs sham-operated control, cP < 0.05 vs I/R control groups.
TBARS level
Average TBARS content of intestinal samples from sham-operated animals was 54.18 ± 3.26 nmol/g tissue, while that from I/R control rats was 78.27 ± 7.60 nmol/g tissue (Figure 6). I/R caused approximately 1.45 fold increase in TBARS content of the tissue, which was significantly different from that measured in samples from sham-operated animals (P = 0.002). Administration of either CPA or adenosine prior to the induction of ischemia significantly reduced the elevated TBARS content to the levels observed in sham control rats. Mean values of the both groups (39.87 ± 11.02 nmol/g tissue and 56.49 ± 7.03 nmol/g tissue, respectively) were significantly different from that of the I/R control group (P = 0.001). On the other hand, in the case of DPCPX pretreatment before adenosine administration followed by I/R, the average TBARS content was 72.02 ± 4.34 nmol/g tissue, which was not significantly different from that in the I/R control group.
Figure 6.
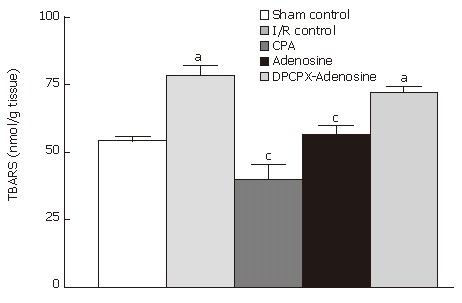
TBARS content of ileum samples from sham-operated control, I/R control, CPA-I/R, adenosine-I/R, and DPCPX-adenosine-I/R rats. Data are expressed as means ± SE (n = 8). aP < 0.05 vs sham-operated control, cP < 0.05 vs I/R control groups.
GSH level
As shown in Figure 7, the amount of GSH measured in tissues subjected to I/R (1.29 ± 0.19 μmol/g tissue) decreased approximately 58% compared to that measured in the tissues from the sham-operated group (3.08 ± 0.27 μmol/g tissue) (P < 0.001). Levels of tissue GSH were statistically indistinguishable when comparing the samples from I/R control group with those from CPA-treated group. In contrast, treatment with adenosine significantly ameliorated the decreased amount of GSH. Mean GSH content was 2.34 ± 0.31 μmol/g tissue, which was significantly different from that measured in I/R control animals (P = 0.002). However, pretreatment with DPCPX prevented this effect of adenosine, reducing GSH content to the levels observed in I/R control animals.
Figure 7.
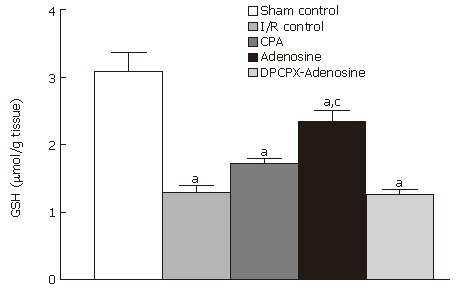
GSH content of ileum samples from sham-operated control, I/R control, CPA-I/R, adenosine-I/R, and DPCPX-adenosine-I/R rats. Data are expressed as means ± SE (n = 8). aP < 0.05 vs sham-operated control, cP < 0.05 vs I/R control groups.
MPO activity
MPO enzyme activities in the terminal ileum samples from animals subjected to sham operation, I/R, CPA treatment, adenosine treatment, and DPCPX-adenosine treatment averaged 6.09 ± 1.04 U/mg tissue, 18.19 ± 6.57 U/mg tissue, 10.80 ± 3.66 U/mg tissue, 5.58 ± 2.89 U/mg tissue, and 21.45 ± 9.61 U/mg tissue, respectively (Figure 8).
Figure 8.
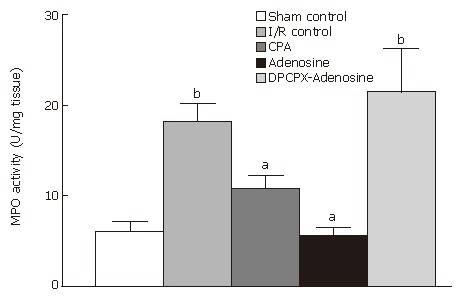
MPO level of ileum samples from sham-operated control, I/R control, CPA-I/R, adenosine-I/R, and DPCPX-adenosine-I/R animals. Results are the means ± SE of 6 to 8 animals in each group. aP < 0.05 vs I/R control, bP < 0.001 vs sham-operated control.
I/R caused approximately a 3 fold increase in MPO activity of terminal ileum tissue compared to the basal level of the activity (P < 0.0001), which was measured in tissues of sham control animals (Figure 8). As MPO activity of samples from animals treated with CPA or adenosine were significantly different from that in I/R group (P = 0.056 and P = 0.0001, respectively), it appeared that pretreatment with DPCPX completely abolished the reducing effect of adenosine on MPO activity. Clearly, no statistical difference was observed in MPO activity between I/R control animals and DPCPX-pretreated rats, while there was a significant difference between CPA-treated animals and DPCPX-pretreated animals (P = 0.023) as well as between adenosine-treated group and DPCPX-pretreated group (P < 0.0001).
DISCUSSION
The major findings of the present study can be summarized as follows: (1) I/R resulted in reduced ileal contractility in response to KCl, carbachol, and substance P as well as elevating oxidative stress and neutrophil infiltration; (2) These disturbances were significantly ameliorated by either adenosine administration or A1AR activation in the preischemic period; (3) Adenosine and A1AR-mediated protection against I/R injury seemed to be associated with decreased oxidative stress and MPO activity; (4) A1AR antagonist DPCPX diminished the injury-sparing effect of PPC with adenosine as observed in other tissues. Pharmacological blockade of A1ARs exacerbated the contractile response of small intestinal smooth muscle.
I/R results in disrupted exogenous electrical activity and contractile response of ileum[8-10]. A substantial amount of evidence indicates that the pathogenesis of I/R and I/R-induced motor alterations have been related to OFRs[1-3,6,8] and activated neutrophils[1-3,10]. Intestinal I/R sets the groundwork for an inflammatory response in the vicinity of muscularis cells, provoking the recruitment and extravasation of leukocytes into smooth muscle syncytium[2,10]. A number of experimental studies have been conducted in order to test various pharmacological agents that might reduce reperfusion injury of the intestinal mucosa[4,5,7,9,50,51] with the intention of improving life-span after acute mesenteric ischemia,.
Our results showed that intestinal I/R resulted in decreased ileal contractility in response to carbachol, subtance P, and KCl; therefore influencing both receptor-mediated induction and non-receptor-mediated induction. That the pD2 values in all groups were statistically indifferent from each other however, suggest that I/R does not alter agonist-receptor interaction. Hence, the reduced Emax value in I/R group may be dependent partly on change in the regulation of postreceptor processes (e.g. excitation-contraction coupling)[9,46]. Furthermore, the decreased contraction response also observed in non-receptor-mediated induction strongly supports this possibility.
IPC makes reference to a phenomenon in which the harmful effects of prolonged ischemia is prevented by exposure of a tissue to brief periods of ischemia[35]. In spite of the fact that it is highly complicated in nature, IPC has been successfully applied to various animal models of intestinal I/R, resulting in attenuation of the reperfusion injury[37-40,52]. However, although IPC has been shown to be beneficial in the human heart and liver, prospectively controlled studies in both humans and animals involving IPC and PPC of the intestine are inadequate. More research focused on the application of drugs that can mimic the effects of IPC is needed to analyze the cellular and molecular events during I/R injury of the intestine so as to attenuate I/R injury[1]. Both animal and human studies have revealed that adenosine is one of the major triggers for IPC. A study done by Unal et al[37] has demonstrated that administration of adenosine prior to ischemia is as effective as IPC for inducing ischemic tolerance in rats. Data gathered in the present study confirms this finding and show that the treatment with adenosine significantly restored I/R-reduced contractile response. Furthermore, the treatment also provided such beneficial effects such as elevating GSH content, lowering lipid peroxidation, and reducing neutrophil infiltration. In addition, that the A1AR antagonist DPCPX significantly blocked these protective effects of adenosine is consistent with the hypothesis that PPC with adenosine is primarily mediated via A1ARs.
The findings of the present study revealed that non-receptor mediated (e.g. KCl-induced) and receptor mediated (e.g. carbacol- and substance P-induced) ileal contractions that were reduced significantly due to I/R, were improved remarkably and returned to sham-control levels by systemic administration of adenosine or CPA. During the process of preconditioning, adenosine is generated in the ischemic tissue. The endogenous adenosine or selective pharmacological agonists activate A1ARs. Preischemic activation of A1ARs has been demonstrated to prevent from I/R damage in various organs including heart[15-18,25], kidney[26,27] and brain[28,29]. In these studies, the activation of A1ARs has been strongly implicated in the mediation of IPC. Adenosine therapy before induction of ischemia has been reported to attenuate ischemic injury in heart[23,24] and intestine[37]. Furthermore, pharmacological blockade of A1AR during preconditioning eliminates the achivement of protection[15-18]. The protective effect of A1AR activation is accomplished through the activation of PKC, leading to translocation of PKC to sarcolemmal and to mitochondrial membranes. Activated PKC then induces an increase in opening of ATP-sensitive K+ channels in heart[11,12]. Stimulation of A1ARs also precedes the early activation of some other kinases such as tyrosine kinases, p38, MAPK[11,19,25,31,33], ERK[32], and Akt[20]. Additionally, in protection obtained by agonist-induced stimulation of A1AR, elevated content or activity of many proteins have been demonstrated such as HSP 27[11] and Mn-SOD[15]. Despite the existence and involvement of relatively large number of effector molecules, it appears that they vary among tissues. In small intestine, for instance, Davis et al[38]report that pharmacological modulation of A1ARs is involved in reduced expression of P-selectin, which is a downstream effector target of the adenosine-initiated, PKC dependent, anti-inflammatory signaling pathway in preconditioning. In our study, I/R of small intestine elevated the tissue TBARS content, indicating enhanced generation of OFRs; therefore, inducing lipid peroxidation. Systemic administration of adenosine or CPA appeared to be protective against I/R-induced reduction of contractility via, at least, inhibiting lipid peroxidation and neutrophil infiltration as confirmed by reduction of TBARS and MPO levels, respectively. Another significant observation reported recently is that activation of A1ARs in vitro prevents cellular functions from H2O2-induced injury through signaling pathways related to PKC in renal proximal tubular cells[53]. The same observation has been demonstrated in other studies on heart[54,55] and kidney[53]. In these studies, activation of A1ARs in vivo and in vitro is reported to be associated with protection against H2O2-induced oxidative injury by modulation of the detrimental increases in intracellular calcium concentration and by means of activation of cardiomyocyte K+ channels after H2O2 exposure.
GSH is an endogenous antioxidant and present in all animal cells. Reacting with free radicals, it can provide protection from singlet oxygen, hydroxyl radical and superoxide anion[31]. Many published studies indicate that tissue injury, induced by various stimuli (e.g. I/R), is coupled with glutathione depletion[9]. In the present study, we showed that depleted GSH content in ischemic ileal tissue was recovered by adenosine or CPA therapies. In other words, inducing PPC with these drugs maintained GSH content during reperfusion. This effect may be related to activation of PKC since adenosine has been reported to induce the activation of antioxidant enzymes in vitro and since it is suggested that the stimulatory action of adenosine is likely involved in PKC-mediated phosphorylation. Such a mechanism could serve to decrease the levels of OFRs, which would otherwise be harmful to the cell. This very effect of adenosine is also evident in vivo, and may account for adenosine-induced reduction of lipid peroxidation in cochlea[34]. Although the present study has not examined antioxidant enzymes or PKC, the elevated level of GSH implicates the potential involvement of a cytoprotective mechanism related to adenosine and A1 receptor activation.
Modulation of the inflammatory response following I/R injury is an important component of tissue defense, mostly because inflammation is the major component of cell death and motor alteration in intestine subjected to intestinal injury. In the initial period of I/R, generation of OFRs occurs, which is the most likely the initial factor responsible for the induction of neutrophil chemotactic activity. Afterwards an influx of leukocytes during reperfusion triggers an intricate cascade of proinflammatory events associated with cytokine/chemokine release and free radical-mediated intestinal injury[1,3]. Upon attachment to endothelium, neutrophils cause the secretion of additional OFRs, contributing to the damage. At this point, the enzyme MPO, found largely in leukocytes particularly in neutrophils, provides an opportunity to check the tissue level of the cells since it is a marker of neutrophil infiltration and accumulation into tissues[27]. In the present study, we have demonstrated that the therapy with A1AR agonist CPA prevented neutrophil infiltration into the reperfused-intestine as shown by the decrese in MPO content. This finding is in agreement with those of previous studies which reports that A1AR stimulation is associated with decreased inflammation and MPO levels[22,27,38].
In the present study, we have demonstrated that administration of adenosine and the A1AR agonist CPA ameliorated intestinal contractile dysfunction induced by I/R. The outcome of the study suggests that preischemic administration of adenosine or CPA may protect intestine, as indicated by recovery of contractile response, possibly through decreasing oxidative strees and reducing neutrophil infiltration. In conclusion, our findings suggest the cellular mechanism by which adenosine and pharmacological stimulation of A1ARs attenuate intestinal injury, which may indicate the possible therapeutic usage of adenosine as an adjunct for ischemia and ischemia related small bowel diseases.
ACKNOWLEDGMENTS
We are greatful to Hasan Tahsin Yilmaz and Ramazan Temel for their unfailing assistance in animal care and maintaining standard conditions of animal research laboratory.
COMMENTS
Background
A number of experimental studies have shown that reperfusion injury in the small intestine is prevented by ischemic preconditioning (IPC). Moreover, various studies using rat small intestine have demonstrated that establishing pharmacological preconditioning (PPC) by administration of either adenosine or adenosine A1 receptor agonist mimic the protective effects of IPC. On the other hand, relatively little data is available on the role of the different adenosine receptors in mediating cytoprotection during intestinal I/R injury. There is no direct evidence which confirms the possible effects of adenosine and adenosine A1 receptor activation on I/R injury- related decreased in contractility of intestinal smooth muscle.
Research frontiers
That adenosine exerts anti-ischaemic actions is indicated by a number of studies using adenosine receptor agonists and antagonists as well as animals overexpressing or lacking the adenosine A1 receptor. Administration of adenosine either prior to ischemia or during reperfusion has been shown to attenuate myocardial injury. Treatment with adenosine A1 receptor agonist initiates preconditioning not only in heart but also in such tissues as kidney and brain, resulting in attenuation of ischemic injury.
Innovations and breakthroughs
IPC of the small intestine reduces postischemic leukocyte adhesion by maintaining the bioavailability of nitric oxide. Moreover, it lowers the expression of P-selectin, which is a downstream effector target of the adenosine-initiated, PKC dependent, signalling pathway in intestine. Although activation of PKC triggered by adenosine is a crucial factor for initiating the beneficial actions of IPC in most tissues, the effector of the preconditioning phenomenon appears to vary among tissues. Activated-K+ channels, nitric oxide, and endogenous opioid peptides have reported to be the other downstream effectors of IPC in intestine. Furthermore, currently published studies suggest important anti-ischemic roles of the A1, A3 or A2a adenosine receptors in heart.
Applications
The therapeutic efficacy of adenosine and the adenosine A1 agonist, 2-chloro-N6-cyclopentyladenosine (CPA) should be examined for potential clinical application in the treatment of conditions related to intestinal ischemia-reperfusion injury, such as small bowel transplantation, strangulated hernias, and abdominal aortic aneurysm. In addition, it would be worthwhile to focus onthe possible effector molecules (e.g. involvement of PKC, opening of mitochondrial ATP-sensitive K+ channels, or activation of Akt) which underlie the mechanism (s) responsible for the beneficial effects of adenosine and CPA observed in the present study.
Terminology
Ischemia: deficient supply of blood to a body part (e.g. any organ) that is due to obstruction of the inflow of arterial blood (for example, by narrowing of arteries as a result of spasm or disease); Ischemia-reperfusion: interruption of the blood flow to a tissue for a period of time followed by restoration of blood flow. During the ischaemic period , a sequence of events is initiated that may ultimately lead to cellular dysfunction or even cell death; Reperfusion injury: When ischemia is ended by restoration of blood flow, a second series of injurious events ensue producing additional damage. The injury produced by reperfusion is more severe that that induced by ischemia and is called reperfusion injury. The primary harmful events are the formation of cytotoxic oxidants (also commonly called oxygen free radicals) derived from molecular oxygen, oxygen free radical-mediated damage to cellular membranes via lipid peroxidation, loss of cellular calcium balance, and generation of inflammatory reaction at the site of damage; Oxidative stress: stress on the body or organism that results from the cumulative damage done by oxygen free radicals which are inadequately neutralized by antioxidants; Agonist: a chemical substance capable of combining with a receptor on a cell and initiating the same reaction or activity typically produced by the binding of an endogenous substance; Antagonist: a chemical substance that acts through receptor to reduce the physiological activity of another chemical or endogenous substance.
Peer review
The present study is interesting, well designed, and contained novel findings. The study is set up throughly and the paper is well written. The conclusions are well based and are of clinical value.
Footnotes
Supported by Zonguldak Karaelmas University Research Projects Fund, No. 2003-01-09
S- Editor Liu Y L- Editor Lalor PF E- Editor Bi L
References
- 1.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 2.Lodato RF, Khan AR, Zembowicz MJ, Weisbrodt NW, Pressley TA, Li YF, Lodato JA, Zembowicz A, Moody FG. Roles of IL-1 and TNF in the decreased ileal muscle contractility induced by lipopolysaccharide. Am J Physiol. 1999;276:G1356–G1362. doi: 10.1152/ajpgi.1999.276.6.G1356. [DOI] [PubMed] [Google Scholar]
- 3.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam TV, Shiels IA, Woodruff TM, Reid RC, Fairlie DP, Taylor SM. Protective effect of a new C5a receptor antagonist against ischemia-reperfusion injury in the rat small intestine. J Surg Res. 2002;103:260–267. doi: 10.1006/jsre.2002.6369. [DOI] [PubMed] [Google Scholar]
- 5.Bielefeldt K, Conklin JL. Intestinal motility during hypoxia and reoxygenation in vitro. Dig Dis Sci. 1997;42:878–884. doi: 10.1023/a:1018899927786. [DOI] [PubMed] [Google Scholar]
- 6.Khanna A, Rossman JE, Fung HL, Caty MG. Attenuated nitric oxide synthase activity and protein expression accompany intestinal ischemia/reperfusion injury in rats. Biochem Biophys Res Commun. 2000;269:160–164. doi: 10.1006/bbrc.2000.2266. [DOI] [PubMed] [Google Scholar]
- 7.Poussios D, Andreadou I, Papalois A, Rekka E, Gavalakis N, Aroni K, Kourounakis PN, Fotiadis C, Sechas MN. Protective effect of a novel antioxidant non-steroidal anti-inflammatory agent (compound IA) on intestinal viability after acute mesenteric ischemia and reperfusion. Eur J Pharmacol. 2003;465:275–280. doi: 10.1016/s0014-2999(03)01488-2. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Tomomasa T, Kaneko H, Watanabe T, Tabata M, Morikawa H, Tsuchida Y, Kuwano H. Intestinal motility in an in vivo rat model of intestinal ischemia-reperfusion with special reference to the effects of nitric oxide on the motility changes. J Pediatr Gastroenterol Nutr. 2001;33:283–288. doi: 10.1097/00005176-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ozacmak VH, Sayan H, Arslan SO, Altaner S, Aktas RG. Protective effect of melatonin on contractile activity and oxidative injury induced by ischemia and reperfusion of rat ileum. Life Sci. 2005;76:1575–1588. doi: 10.1016/j.lfs.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Hassoun HT, Weisbrodt NW, Mercer DW, Kozar RA, Moody FG, Moore FA. Inducible nitric oxide synthase mediates gut ischemia/reperfusion-induced ileus only after severe insults. J Surg Res. 2001;97:150–154. doi: 10.1006/jsre.2001.6140. [DOI] [PubMed] [Google Scholar]
- 11.Baxter GF. Role of adenosine in delayed preconditioning of myocardium. Cardiovasc Res. 2002;55:483–494. doi: 10.1016/s0008-6363(02)00280-8. [DOI] [PubMed] [Google Scholar]
- 12.Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki M, Tokita K, Nagakura Y, Takeda M, Hanaoka K, Tomoi M. Adenosine A1 receptor blockade reverses dysmotility induced by ischemia-reperfusion in rat colon. Eur J Pharmacol. 2000;409:319–323. doi: 10.1016/s0014-2999(00)00867-0. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls J, Hourani SM. Characterization of adenosine receptors on rat ileum, ileal longitudinal muscle and muscularis mucosae. Eur J Pharmacol. 1997;338:143–150. doi: 10.1016/s0014-2999(97)81942-5. [DOI] [PubMed] [Google Scholar]
- 15.Dana A, Jonassen AK, Yamashita N, Yellon DM. Adenosine A(1) receptor activation induces delayed preconditioning in rats mediated by manganese superoxide dismutase. Circulation. 2000;101:2841–2848. doi: 10.1161/01.cir.101.24.2841. [DOI] [PubMed] [Google Scholar]
- 16.Mozzicato S, Joshi BV, Jacobson KA, Liang BT. Role of direct RhoA-phospholipase D1 interaction in mediating adenosine-induced protection from cardiac ischemia. FASEB J. 2004;18:406–408. doi: 10.1096/fj.03-0592fje. [DOI] [PubMed] [Google Scholar]
- 17.De Jonge R, Out M, Maas WJ, De Jong JW. Preconditioning of rat hearts by adenosine A1 or A3 receptor activation. Eur J Pharmacol. 2002;441:165–172. doi: 10.1016/s0014-2999(01)01611-9. [DOI] [PubMed] [Google Scholar]
- 18.Kristo G, Yoshimura Y, Keith BJ, Stevens RM, Jahania SA, Mentzer RM, Lasley RD. Adenosine A1/A2a receptor agonist AMP-579 induces acute and delayed preconditioning against in vivo myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287:H2746–H2753. doi: 10.1152/ajpheart.00493.2004. [DOI] [PubMed] [Google Scholar]
- 19.Headrick JP, Hack B, Ashton KJ. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol. 2003;285:H1797–H1818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- 20.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 21.Maddock HL, Mocanu MM, Yellon DM. Adenosine A(3) receptor activation protects the myocardium from reperfusion/reoxygenation injury. Am J Physiol Heart Circ Physiol. 2002;283:H1307–H1313. doi: 10.1152/ajpheart.00851.2001. [DOI] [PubMed] [Google Scholar]
- 22.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 23.Randhawa MP, Lasley RD, Mentzer RM. Salutary effects of exogenous adenosine administration on in vivo myocardial stunning. J Thorac Cardiovasc Surg. 1995;110:63–74. doi: 10.1016/s0022-5223(05)80010-8. [DOI] [PubMed] [Google Scholar]
- 24.Sekili S, Jeroudi MO, Tang XL, Zughaib M, Sun JZ, Bolli R. Effect of adenosine on myocardial 'stunning' in the dog. Circ Res. 1995;76:82–94. doi: 10.1161/01.res.76.1.82. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura Y, Kristo G, Keith BJ, Jahania SA, Mentzer RM, Lasley RD. The p38 MAPK inhibitor SB203580 blocks adenosine A(1) receptor-induced attenuation of in vivo myocardial stunning. Cardiovasc Drugs Ther. 2004;18:433–440. doi: 10.1007/s10557-004-6220-4. [DOI] [PubMed] [Google Scholar]
- 26.Sugino H, Shimada H, Tsuchimoto K. Role of adenosine in renal protection induced by a brief episode of ischemic preconditioning in rats. Jpn J Pharmacol. 2001;87:134–142. doi: 10.1254/jjp.87.134. [DOI] [PubMed] [Google Scholar]
- 27.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Nakakimura K, Matsumoto M, Sakabe T. Rapid tolerance to focal cerebral ischemia in rats is attenuated by adenosine A1 receptor antagonist. J Cereb Blood Flow Metab. 2002;22:161–170. doi: 10.1097/00004647-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Hiraide T, Katsura K, Muramatsu H, Asano G, Katayama Y. Adenosine receptor antagonists cancelled the ischemic tolerance phenomenon in gerbil. Brain Res. 2001;910:94–98. doi: 10.1016/s0006-8993(01)02647-6. [DOI] [PubMed] [Google Scholar]
- 30.Kudo M, Wang Y, Xu M, Ayub A, Ashraf M. Adenosine A(1) receptor mediates late preconditioning via activation of PKC-delta signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H296–H301. doi: 10.1152/ajpheart.01087.2001. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Alexander JS, Zhang S, Zhu Y, Sieber NJ, Aw TY, Carden DL. Redox regulation of endothelial barrier integrity. Am J Physiol Lung Cell Mol Physiol. 2001;281:L879–L886. doi: 10.1152/ajplung.2001.281.4.L879. [DOI] [PubMed] [Google Scholar]
- 32.Reid EA, Kristo G, Yoshimura Y, Ballard-Croft C, Keith BJ, Mentzer RM, Lasley RD. In vivo adenosine receptor preconditioning reduces myocardial infarct size via subcellular ERK signaling. Am J Physiol Heart Circ Physiol. 2005;288:H2253–H2259. doi: 10.1152/ajpheart.01009.2004. [DOI] [PubMed] [Google Scholar]
- 33.Ballard-Croft C, Kristo G, Yoshimura Y, Reid E, Keith BJ, Mentzer RM, Lasley RD. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. Am J Physiol Heart Circ Physiol. 2005;288:H1359–H1366. doi: 10.1152/ajpheart.01006.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ramkumar V, Hallam DM, Nie Z. Adenosine, oxidative stress and cytoprotection. Jpn J Pharmacol. 2001;86:265–274. doi: 10.1254/jjp.86.265. [DOI] [PubMed] [Google Scholar]
- 35.Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8:86–94. doi: 10.1097/00024382-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischaemic preconditioning improves microvascular perfusion and oxygenation following reperfusion injury of the intestine. Br J Surg. 2005;92:1169–1176. doi: 10.1002/bjs.4988. [DOI] [PubMed] [Google Scholar]
- 37.Unal S, Demirkan F, Arslan E, Cin I, Cinel L, Eskandari G, Cinel I. Comparison of ischemic and chemical preconditioning in jejunal flaps in the rat. Plast Reconstr Surg. 2003;112:1024–1031. doi: 10.1097/01.PRS.0000076224.23190.52. [DOI] [PubMed] [Google Scholar]
- 38.Davis JM, Gute DC, Jones S, Krsmanovic A, Korthuis RJ. Ischemic preconditioning prevents postischemic P-selectin expression in the rat small intestine. Am J Physiol. 1999;277:H2476–H2481. doi: 10.1152/ajpheart.1999.277.6.H2476. [DOI] [PubMed] [Google Scholar]
- 39.Vlasov TD, Smirnov DA, Nutfullina GM. Preconditioning of the small intestine to ischemia in rats. Neurosci Behav Physiol. 2002;32:449–453. doi: 10.1023/a:1015896614819. [DOI] [PubMed] [Google Scholar]
- 40.Sola A, De Oca J, González R, Prats N, Roselló-Catafau J, Gelpí E, Jaurrieta E, Hotter G. Protective effect of ischemic preconditioning on cold preservation and reperfusion injury associated with rat intestinal transplantation. Ann Surg. 2001;234:98–106. doi: 10.1097/00000658-200107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222:27–32. doi: 10.1006/bbrc.1996.0692. [DOI] [PubMed] [Google Scholar]
- 42.Yang SP, Hao YB, Wu YX, Dun W, Shen LH, Zhang Y. Ischemic preconditioning mediated by activation of KATP channels in rat small intestine. Zhongguo Yao Li Xue Bao. 1999;20:341–344. [PubMed] [Google Scholar]
- 43.Zhang Y, Wu YX, Hao YB, Dun Y, Yang SP. Role of endogenous opioid peptides in protection of ischemic preconditioning in rat small intestine. Life Sci. 2001;68:1013–1019. doi: 10.1016/s0024-3205(00)01004-3. [DOI] [PubMed] [Google Scholar]
- 44.Boucher M, Pesant S, Falcao S, de Montigny C, Schampaert E, Cardinal R, Rousseau G. Post-ischemic cardioprotection by A2A adenosine receptors: dependent of phosphatidylinositol 3-kinase pathway. J Cardiovasc Pharmacol. 2004;43:416–422. doi: 10.1097/00005344-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Boucher M, Wann BP, Kaloustian S, Massé R, Schampaert E, Cardinal R, Rousseau G. Sustained cardioprotection afforded by A2A adenosine receptor stimulation after 72 hours of myocardial reperfusion. J Cardiovasc Pharmacol. 2005;45:439–446. doi: 10.1097/01.fjc.0000159047.73359.08. [DOI] [PubMed] [Google Scholar]
- 46.Kaya TT, Koyluoglu G, Soydan AS, Arpacik M, Karadas B. Effects of nimesulide and pentoxifylline on decreased contractile responses in rat ileum with peritonitis. Eur J Pharmacol. 2002;442:147–153. doi: 10.1016/s0014-2999(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 47.Casini AF, Ferrali M, Pompella A, Maellaro E, Comporti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene-intoxicated mice. Am J Pathol. 1986;123:520–531. [PMC free article] [PubMed] [Google Scholar]
- 48.Aykaç G, Uysal M, Yalçin AS, Koçak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36:71–76. doi: 10.1016/0300-483x(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 49.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 50.Ferrer JV, Ariceta J, Guerrero D, Gomis T, Larrea MM, Balén E, Lera JM. Allopurinol and N-acetylcysteine avoid 60% of intestinal necrosis in an ischemia-reperfusion experimental model. Transplant Proc. 1998;30:2672. doi: 10.1016/s0041-1345(98)00784-2. [DOI] [PubMed] [Google Scholar]
- 51.Jacob T, Ascher E, Hingorani A, Kallakuri S. Glycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat model. Surgery. 2003;134:457–466. doi: 10.1067/s0039-6060(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 52.Aban N, Cinel L, Tamer L, Aktas A, Aban M. Ischemic preconditioning reduces caspase-related intestinal apoptosis. Surg Today. 2005;35:228–234. doi: 10.1007/s00595-004-2918-y. [DOI] [PubMed] [Google Scholar]
- 53.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human proximal tubular cells via A(1) and A(2a) adenosine receptors. Am J Physiol Renal Physiol. 2002;282:F844–F852. doi: 10.1152/ajprenal.00195.2001. [DOI] [PubMed] [Google Scholar]
- 54.Narayan P, Mentzer RM, Lasley RD. Adenosine A1 receptor activation reduces reactive oxygen species and attenuates stunning in ventricular myocytes. J Mol Cell Cardiol. 2001;33:121–129. doi: 10.1006/jmcc.2000.1282. [DOI] [PubMed] [Google Scholar]
- 55.Thomas GP, Sims SM, Cook MA, Karmazyn M. Hydrogen peroxide-induced stimulation of L-type calcium current in guinea pig ventricular myocytes and its inhibition by adenosine A1 receptor activation. J Pharmacol Exp Ther. 1998;286:1208–1214. [PubMed] [Google Scholar]


