Abstract
AIM: To observe the therapeutic effects of dexamethasone on rats with severe acute pancreatitis (SAP) and investigate the influences of dexamethasone on the inflammatory mediators and NF-κB expression in multiple organs of SAP rats as well as the mechanisms involved.
METHODS: Ninety Sprague-Dawley (SD) rats with SAP were randomly divided into the model group (n = 45) and dexamethasone treatment group (n = 45), and another 45 rats were selected for the sham operation group. All groups were randomly subdivided into the 3 h, 6 h and 12 h groups, each group containing 15 rats. The survival of all groups and pathological changes of multiple organs (liver, kidney and lung) were observed at different time points after the operation. The pathological score of multiple organs was carried out, followed by the determination of amylase, endotoxin and TNF-α contents in blood. The tissue microarray was used to detect the expression levels of NF-κB p65 protein in multiple organs.
RESULTS: There was no marked difference between the model group and treatment group in the survival rate. The amylase content of the treatment group was significantly lower compared to the model group at 12 h (P < 0.01, 7791.00 vs 9195.00). Moreover, the endotoxin and TNF-α levels of the treatment group were significantly lower than that of the model group at 6 h and 12 h (P < 0.01, 0.040 vs 0.055, 0.042 vs 0.059 and P < 0.05, 58.30 vs 77.54, 38.70 vs 67.30, respectively). Regarding the changes in liver NF-κB expression, the model group significantly exceeded the sham operation group at 3 h (P < 0.01, 1.00 vs 0.00), and the treatment group significantly exceeded the sham operation group at 12 h (P < 0.01, 1.00 vs 0.00), whereas no marked difference was observed between the model group and treatment group at all time points. The kidney NF-κB expression level in the treatment group significantly exceeded the model group (P < 0.05, 2.00 vs 0.00) and the sham operation group (P < 0.01, 2.00 vs 0.00) at 12 h. No NF-κB expression in the lung was found in any group.
CONCLUSION: Dexamethasone can lower the amylase, endotoxin and TNF-α levels as well as mortality of SAP rats. NF-κB plays an important role in multiple organ injury. Further studies should be conducted to determine whether dexamethasone can ameliorate the pathological changes of multiple organs by reducing the NF-κB expression in the liver and kidney. The advantages of tissue microarrays in pancreatitis pathological examination include time- and energy- saving, and are highly efficient and representative. The restriction of tissue microarrays on the representation of tissues to various extents due to small diameter may lead to the deviation of analysis.
Keywords: Severe acute pancreatitis, Dexamethasone;NF-κB, Tissue microarrays, Mutiple organs
INTRODUCTION
Severe acute pancreatitis (SAP), as one of the common presentations of clinically acute abdomen is a systemic disease in which the local inflammatory pathological changes of pancreas involve multiple organs[1]. It is recently believed the systemic inflammatory response syndrome (SIRS) due to the excessive inflammatory reactions plays an extremely important role in SAP pathogenesis, which is relatively complicated[2,3]. Although the exact pathogenesis remains unclear[4]. Studies in recent years have found that NF-κB (nuclear factor kappa-B) which is a main factor in the genetic transcription of inflammation, presents high expression state in acute pancreatitis and plays an important role in the onset and turnover of acute pancreatitis together with other inflammatory cytokines[5-7]. Dexamethasone is the antagonist of a non-specific inflammatory mediator[8]. Studies have shown that dexamethasone can lower the expression level of NF-κB by inducing the release of NF-κB profilin (IκB)[9]. In this study, we prepared rat SAP models by using the improved Aho’s method[10], and investigated the therapeutic effects of dexamethasone on the SAP rats and examined the influences of dexamethasone on the inflammatory mediators and NF-κB expression in multiple organs of the rats.
MATERIALS AND METHODS
Materials
Clean grade healthy male Sprague-Dawley (SD) rats weighing 250-300 g were purchased from the Experimental Animal Center of Medical School, Zhejiang University, China. Sodium taurocholate and sodium pentobarbital were purchased from Sigma Company, USA. Dexamethasone injection was purchased from Zhejiang Xinchang Pharmaceutical Company. NF-κB p65 antibody was purchased from Santa Cruz Company, USA. The full automatic biochemical analyzer was used to determine the plasma amylase level (U/L). Plasma endotoxin Tachypleus Amebocyte Lysate Kit was purchased from Shanghai Yihua Medical Science and Technology Corporation (Institute of Medical Analysis in Shanghai, China), the calculation unit for content is EU/mL. TNF-α ELISA kit was purchased from Jingmei Bioengineering Corporation, China, the calculation unit for content is pg/mL (ng/L).
Animal grouping
We adopted the improved Aho’s method[10] to prepare 90 SAP rat models and randomly divided into the model group (45 rats) and treatment group (45 rats). Another 45 rats were selected as the sham operation group. All groups were randomly subdivided into the 3, 6 and 12 h groups, each group containing 15 rats. The dexamethasone treatment group was injected once with dexamethasone (1 mL = 5 mg) via the vena caudalis, 0.5 mg/100 g body weight 15 min after successful preparation of SAP model. During the laparotomy in the sham operation group, we performed pancreas and duodenum manipulation, observed pathological changes of multiple organs and finally closed the abdomen. The sham operation group and model group were injected with same amount of normal saline (0.1 mL/100 g body weight) via the vena caudalis 15 min after the operation.
Animal model preparation
Fasting and water restriction was imposed on all rat groups 12 h prior to the operation. The rats were anesthetized by intraperitoneal injection of 20 g/L sodium pentobarbital (0.25 mL/100 g body weight) and the operation was performed under aseptic conditions. Model group: After entering the abdomen via median epigastric incision, the bile-pancreatic duct, hepatic hilus and common hepatic duct were identified; the duodenal papilla inside the duodenum duct wall was identified, and then a No. 5 needle was used to drill a hole in the mesenteric avascular area. A segmental epidural catheter was inserted into the duodenum cavity via the hole, and then inserted into the bile-pancreatic duct toward the direction of papilla in a retrograde way, a microvascular clamp was used to nip the catheter head temporarily. Meanwhile, another microvascular clamp was used to temporarily occlude the common hepatic duct at the confluence of the hepatic duct. After connecting the epidural catheter end with the transfusion converter, 3.5% sodium taurocholate (0.1 mL/100 g) was transfused via the microinjection pump at a speed of 0.2 mL/min, stayed for 4 min after injection, and then the microvascular clamp and epidural catheter were removed. After checking for bile leakage, the hole in the lateral duodenal wall was sutured. A sterile cotton ball was used to absorb up the anaesthetic in the abdominal cavity and then the abdomen was closed.
Preparation of tissue microarrays of mutiple organs
We fixed the tissue sample with neutral formalin, prepared the routine paraffin block (named donor block), cut the donor block into 5-μm thick tissue section and carried out routine hematoxylin-eosin (HE) staining as well as microscopic morphological observation under microscope, and then selected the required representative area, marked the locations on the HE sections and also on the corresponding part of the donor block. We prepared the blank block as the recipient block in the size of 45 mm × 20 mm × 15 mm and drilled the recipient block with the tissue microarrays section (Beecher Instruments, USA), the diameter of the drilling needle is 2.0 mm. In obtaining of the donor block tissue microarrays, we used another drilling needle (its inner diameter is equal to the outer diameter of the former drilling needle) to drill the marked location of paraffin block and collected the tissue microarray. Its length was about 0.1 mm shorter than the depth of hole. The tissue microarrays collecting method was just the same as that of drilling the recipient block. After pushing out the tissue microarrays, we directly inserted it or inserted it with forceps into the hole of recipient block. After pressing the tissue microarrays downwards with common glass slide, we used the distance adjuster to correctly move the drilling needle to a proper distance forward and back or right and left. This process was repeated and could insert tens of tissue microarrays into the recipient block in an orderly fashion. Finally, we piled up three glass slides to press all the tissue microarrays and thus the prepared tissue microarrays section block had flat and smooth surface. We put the prepared tissue microarrays section block into the paraffin block again to make the mold, and put it into a 60°C oven for 1 h so that the paraffin of the tissue microarrays and recipient block could be melted together. We took the mold out of the oven gently, cooled the half melted paraffin at the room temperature (about 30 min) and then cooled it in a -20°C refrigerator for 6 min. Later, we took the tissue microarrays section block out of the mold and stored it in a 4°C refrigerator for later use. We took out the standby paraffin block and rapidly nipped it on the sectioning machine for correction till all the tissue microarrays were on the same plane, then stuck the ice block on the paraffin block for about 5 min and cut into 10-30 successive sections of 5-μm thickness and used the ice block to freeze the paraffin block. We repeated the above process until finishing sectioning of the tissue, floated the successive sections on the cool water and let it spread naturally. Then we used the ophthalmic elbowed forceps and glass slide to separate the sections during which the first section on the head part of the successive sections could be stuck to the glass slide, fixed and separated it with forceps to avoid loss of tissue microarrays sample due to the leakage of tissue section during separation. The sections were transferred into 45°C warm water to spread for 1 min to ensure their full spreading without scattering. The sections were backed by the glass slide processed by 10% APES acetone solution for staining. We incubated the prepared tissue microarrays sections into a 60°C oven for 1 h, took them out, cooled it at room temperature and put it into a -20°C refrigerator for later use.
NF-κB p65 immunohistochemical staining (supersensitive S-P method)
We baked the section at 60°C for 16 h and dewaxed in a routine fashion. We carried out antigen retrieval at high temperature and high pressure for 2 min, dropped reagent A to block the endogenous peroxidase, incubated at room temperature for 10 min, followed by washing with distilled water thrice, with biotin blocking reagent A at room temperature for 10 min, twice with PBS for 5 min each, with biotin blocking reagent B, at room temperature for 10 min, and twice with PBS for 5 min each. We added the normal goat serum-blocking liquid, incubated at room temperature for 20 min and removed the extra liquid, then added primary antibody (1:100 dilution), incubated over night at 4°C, washed thrice with PBS for 5 min each, and again incubated with secondary antibody at room temperature for 10 min, washed thrice with PBS for 5 min each. We added streptomycete antibiotin-peroxidase solution, put at room temperature for 10 min, washed four times with PBS for 5 min each, and then added freshly prepared DAB solution for coloration. The sections were observed under microscope and washed with distilled water.
Observational index
Survival rate: The rat mortality observed at 3 h, 6 h, and 12 h after operation and the survival rate was calculated.
Pathological changes of mutiple organs: After mercy killing the rats anesthetized by sodium pentobarbital in batches, the gross samples of mutiple organs (liver, kidney, lung) were collected and observed for the pathological changes.
NF-κB p65 protein expression in the mutiple organs: We applied tissue microarrays to prepare microarray sections of the mutiple organs, and, using immunohistochemical S-P method, observed the NF-κB p65 protein expression and carried out the comprehensive assessment according to the positive cell percentage: < 10% (-); 10%-20% (+); 20%-50% (++); > 50% (+++).
Statistical analysis
The statistical analysis was conducted using the SPSS11.5 software. The Kruskal-Wallis test was applied for comparison of the three groups. The Bonferroni test was applied to the two-group comparison. The likelihood ratio Chi-square test was applied to compare the survival rate. P < 0.05 was considered statistically significant.
RESULTS
Survival rate
The 3 h, 6 h and 12 h mortality of the model group were 0% (0/15), 0% (0/15), 13.33% (2/15), respectively. The entire survival rate was 86.67%, while the survival rate of sham operation group and treatment group at all time points were 100%. But the survival rate at different time points was not significantly different between the model group and treatment group.
Comparison of plasma amylase content of all groups
Plasma amylase content was significantly increased in the model group and dexamethasone treatment group compared to the sham operation group at all time points (P < 0.001). No marked difference was observed in plasma amylase content between the dexamethasone treatment group and model group at 3 h and 6 h. However, plasma amylase content was found to be significantly less in the dexamethasone treatment group than the model group at 12 h (P < 0.01) (Table 1).
Table 1.
Comparison of plasma amylase [M (QR)]
| Group (time/h) | 3 h | 6 h | 12 h |
| Sham operation | 2038.00 (346.00) | 2117.00 (324.00) | 1725.00 (434.00) |
| Model | 7423.00 (2275.00) | 8149.00 (1540.00) | 9195.00 (1298.00) |
| Treatment | 6739.00 (2310.00) | 7839.00 (2258.00) | 7791.00 (1863.00) |
Comparison of plasma endotoxin content of all groups
Plasma endotoxin content was significantly increased in the model group and dexamethasone treatment group than the sham operation group at all time points (P < 0.001). No marked difference was observed in plasma endotoxin content between the dexamethasone treatment group and model group at 3 h. However, plasma endotoxin content was found to be significantly less in the dexamethasone treatment group compared to the model group at 6 h and 12 h (P < 0.01) (Table 2).
Table 2.
Comparison of plasma endotoxin [M (QR)]
| Group (time/h) | 3 h | 6 h | 12 h |
| Sham operation | 0.015 (0.007) | 0.015 (0.007) | 0.016 (0.005) |
| Model | 0.035 (0.0170) | 0.055 (0.025) | 0.059 (0.020) |
| Treatment | 0.030 (0.0140) | 0.040 (0.012) | 0.042 (0.018) |
Comparison of serum TNF-α content of all groups
Serum TNF-α content was obviously increased in the model group and dexamethasone treatment group compared to the sham operation group at all time points (P < 0.001). No obvious difference was observed in serum TNF-α content between the dexamethasone treatment group and model group at 3 h. Serum TNF-α content was found to be significantly less in the dexamethasone treatment group compared to the model group at 6 h and 12 h (P < 0.05) (Table 3).
Table 3.
Comparison of serum TNF-α [M (QR)]
| Group (time/h) | 3 h | 6 h | 12 h |
| Sham operation | 3.30 (3.60) | 4.90 (2.60) | 3.70 (2.30) |
| Model | 46.13 (37.95) | 77.54 (42.16) | 67.30 (32.13) |
| Treatment | 38.40 (26.60) | 58.30 (26.40) | 38.70 (28.50) |
Macroscopic and microscopic changes of the liver
Sham operation group: Macroscopically, we observed normal color without obvious swelling of the liver in all groups. Microscopically, roughly normal hepatic tissue, slight inflammatory cell infiltration in the portal area, normal morphous of most liver cells, some with acidophilia apomorphosis or slight expansion and congestion of sinus hepaticus were observed.
Model group: Macroscopically, in the 3 h group, slight swelling of the liver was observed, and some rats had local grey plaques with unclear boundary, while in the 6 h and 12 h groups, pale, turbid color or congestion on the liver, and some with scattered grey plague in irregular shape or necrosis were observed. Microscopically, we observed swelling or acidophilia apomorphosis of the liver cells, inflammatory cell infiltration in the portal area, expansion and congestion of sinus hepaticus, and scattered spotty necrosis in the hepatic lobule in the 3 h group, obvious swelling of the liver cells, increased range and area of the liver cell necrosis, visible focal or massive hemorrhagic necrosis, inflammatory cell infiltration in necrosis focus, obvious congestion of partial sinus hepaticus, bile duct proliferation and scattered necrosis of single cell in the portal area (concentration and fragmentation of nucelus) in the 6 h group, obviously damaged structure of the hepatic lobule, further increased necrosis range and area of the liver cells, more inflammatory cell infiltration in the lobule and/or portal area, and obvious congestion of sinus hepaticus in the 12 h group.
Dexamethasone treatment group: Macroscopically, the gross liver pathological changes of the dexamethasone treatment group at 6 h and 12 h were milder than those of the model group, most significantly at 12 h. Microscopically, we observed slight swelling of the liver cells, slight expansion and congestion of the sinus hepaticus, scattered inflammatory cell infiltration but with significantly less scale in the portal area at all time points in the dexamethasone treatment group, while more limited necrosis range of the liver cells, no obvious lamellar necrosis at 6 h and 12 h groups. The gross pathological changes of the dexamethasone treatment group were milder than those of the model group at 6 h and 12 h, most significantly at 12 h.
Macroscopic and microscopic changes of the kidney
Sham operation group: Macroscopically, no swelling of the kidney with normal morphous, and no bleeding point on the renal cortex surface were observed. Microscopically, normal structures of renal glomerulus, renal tubule and renal interstitium without any obvious pathological changes were observed in most of the rats, while unclear boundary of the renal tubular epithelial cells (especially proximal tubule), stenosis and atresia of lumens, congestion of renal glomerulus and interstitial edema were observed in a small number of rats.
Model group: Macroscopically, in the 3 h group, no obvious gross changes in the kidney, while in the 6 h and 12 h groups, renal swelling, tension of the kidney envelope, scattered bleeding points on surface of the kidney envelope in some rats and slight hemorrhagic urine within the pelvis in severe cases were observed. Microscopically, in the 3 h group, congestion of glomerular capillary, swelling of the renal tubular epithelial cells, scattered necrosis, unclear cell boundary, stenosis or atresia of lumens, visible protein cast, interstitial edema and inflammatory cell infiltration, while in the 6 h and 12 h groups, obvious congestion of glomerular capillary, swelling of the renal tubular epithelial cells, scattered necrosis, interstitial edema, inflammatory cell infiltration were observed. Moreover, eosinophilic staining floss, red cells and eosinophilic staining homogen cast or red cell cast in the glomerular capsule were observed. There were expansion of renal tubule lumens in medulla, atrophia of endothelial cells and the pathological changes grew worse with time; lamellar necrosis of the renal tubular epithelial cells in a small number of rats.
Dexamethasone treatment group: Macroscopically, in the 6 h and 12 h groups, the gross pathological changes of the dexamethasone treatment group were milder than those of the model group. Microscopically, we observed milder congestion of glomerular capillary, swelling of the renal tubular epithelial cells as well as less eosinophilic staining floss and red cells in the renal capsule and less inflammatory cell infiltration than those of the model group; edema of renal interstitium and scattered necrosis in a small part of the renal tubular epithelial cells.
Macroscopic and microscopic changes of the lung
Sham operation group: Macroscopically, normal color and structure of the lung on both sides, no bleeding point on the surface and no effusion in the thoracic cavity were observed. Microscopically, normal function of the most lung tissues, but quite few with slight edema and inflammatory cell infiltration of interstitium were observed.
Model group: Macroscopically, in the 3 h group, obvious hyperemia and edema of the pulmonary lobes on both sides, dark red bleeding points on the local pulmonary lobe surface, small amount of amber and dilute effusion in the thoracic cavity, while in the 6 h and 12 h groups, aggravated pathological changes of the lung on both sides with prolonged time after modeling, lump-like pruinosus plaque on the lung surface, increased effusion in the thoracic cavity, and some hemorrhagic changes were observed. Microscopically, in the 3 h group, edema of the lung interstitium and alveolar space, widened interstitium of alveolar wall, visible inflammatory cell infiltration, telangiectasis and congestion of alveolar wall and widened alveolar septum, while in the 6 h and 12 h groups, further increased range of pathological changes of pulmonary lobes, obviously increased effusion in alveolar space, edema and bleeding of interstitium and alveolar space, obviously widened alveolar septum, more inflammatory cell infiltration and lucent kytoplasm of local tunica mucosa bronchiorum epithelium were observed.
Dexamethasone treatment group: Macroscopically, no obvious bleeding point on the pulmonary lobe surface, sound elasticity of pulmonary lobes, no obvious effusion in the thoracic cavity were observed; the gross lung pathological changes were milder than those of the model group at all time points, indicating obvious therapeutic effects. Microscopically, most lung tissue restored normal structures, and few with slight edema of interstitium and alveolar space were observed, indicating obvious therapeutic effects.
Changes of NF-κB expression levels in the liver of all groups
The positive NF-κB staining was located in the cytoplasm of the liver cells. The NF-κB expressions were all negative in the sham operation group at different time points, partly negative and partly + or ++ at 3 h in the model group. Most expressions of the model group were negative at 6 h and 12 h and a small part + or ++, while the negative rate of the model group at 12 h surpassed that at 6 h. There was no marked difference among all groups at 6 h. There was no marked difference between the model group and treatment group at all time points. However, the model group significantly exceeded the sham operation group at 3 h (P < 0.01) and the treatment group significantly exceeded the sham operation group at 12 h (P < 0.01) (Tables 4 and 5, Figure 1A and B).
Table 4.
The changes of expression level of NF-κB in the liver
| Group | (Time/h) | Cases |
Pathologic grade |
|||
| - | + | ++ | +++ | |||
| Sham operation | 3 | 15 | 15 | 0 | 0 | 0 |
| 6 | 15 | 15 | 0 | 0 | 0 | |
| 12 | 15 | 15 | 0 | 0 | 0 | |
| Model | 3 | 15 | 7 | 4 | 4 | 0 |
| 6 | 15 | 10 | 3 | 2 | 0 | |
| 12 | 13 | 11 | 1 | 1 | 0 | |
| Treatment | 3 | 15 | 12 | 1 | 2 | 0 |
| 6 | 15 | 11 | 1 | 3 | 0 | |
| 12 | 15 | 9 | 3 | 3 | 0 | |
Table 5.
Comparison of the expression level of NF-κB in the liver [M (QR)]
| Group | 3 h | 6 h | 12 h |
| Sham operation | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Model | 1.00 (2.00) | 0.00 (1.00) | 0.00 (0.00) |
| Treatment | 0.00 (0.00) | 0.00 (1.00) | 0.00 (1.00) |
Figure 1.
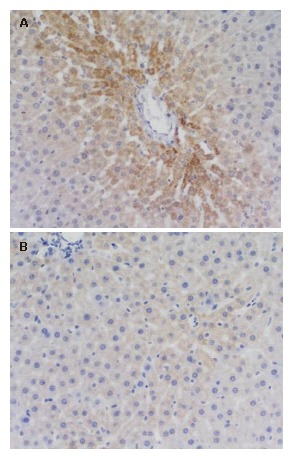
NF-κB expressions in the liver in the model group-3 h, (++) × 200 (A) and the treatment group-3 h, (-) ×200 (B).
Changes of NF-κB expression levels in the kidney of all groups
The positive NF-κB staining was located in the cytoplasm of renal tubular epithelial cells (Table 3). The expressions of the sham operation group were all negative at different time points. Most expressions of the model group were negative at 3 h and 6 h and a small part + or ++. The expressions of the model group were all negative at 12 h. Most expressions of the treatment group were negative at 3 h and a quite small part + or ++. Most expressions of the treatment group were negative at 6 h and a quite small part ++. The expressions of the treatment group were partly negative and partly + or ++ at 12 h. There were no marked differences between the model group and treatment group at all time points, moreover, no obvious differences among all groups at 3 h and 6 h. However, the treatment group significantly exceeded the model group (P < 0.05) and sham operation group (P < 0.01) at 12 h (Tables 6 and 7, Figure 2A, B and C).
Table 6.
The changes of expression level of NF-κB in the kidney
| Group | (Time/h) | Cases |
Pathologic grade |
|||
| - | + | ++ | +++ | |||
| Sham operation | 3 | 15 | 15 | 0 | 0 | 0 |
| 6 | 15 | 15 | 0 | 0 | 0 | |
| 12 | 15 | 15 | 0 | 0 | 0 | |
| Model | 3 | 15 | 13 | 1 | 1 | 0 |
| 6 | 15 | 12 | 1 | 2 | 0 | |
| 12 | 13 | 13 | 0 | 0 | 0 | |
| Treatment | 3 | 15 | 12 | 2 | 1 | 0 |
| 6 | 15 | 13 | 0 | 2 | 0 | |
| 12 | 15 | 9 | 2 | 3 | 1 | |
Table 7.
Comparison of the expression level of NF-κB in the kidney [M (QR)]
| Group | 3 h | 6 h | 12 h |
| Sham operation | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Model | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Treatment | 0.00 (0.00) | 0.00 (0.00) | 0.00 (2.00) |
Figure 2.
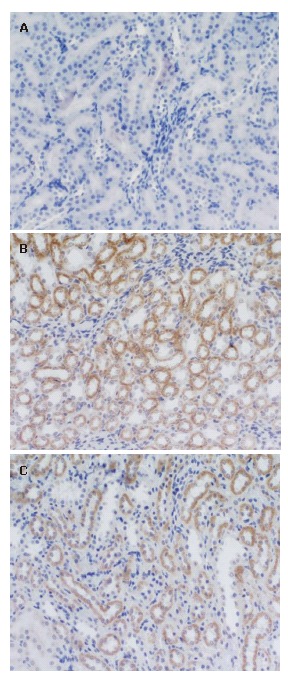
NF-κB expressions in the kidney in the sham operation group-12 h, (-) ×200 (A), model group-6 h, (++) × 200 (B) and model group-12 h, (++) × 200 (C).
Changes of NF-κB expression levels in the lung of all groups
NF-κB expressions in the lung of all groups were negative (Figure 3).
Figure 3.
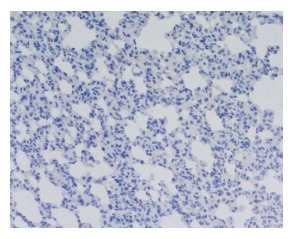
NF-κB expressions in the lung in the sham operation group-3 h, (-) ×200.
DISCUSSION
As one of the common clinical acute abdomen, severe acute pancreatitis (SAP) usually accompanied by the obvious inflammatory reactions besides local pathological injuries can lead to systemic inflammatory response syndrome (SIRS) or even the complication of multiple organ injury, further multiple organ dysfunction syndrome (MODS)[11], resulting in quite high mortality. Although people have conducted enormous studies on AP pathogenesis and brought forward many valuable theories such as the theory of oxygen-free radicals[12], the exact mechanism remains unclear. Studies in recent years have proven that the activation of NF-κB plays an extremely important role in the onset process of SAP[7,13,14].
NF-κB, a protein with multi-attribute transcription-regulating effect, consists of NF-κB/Rel protein family members. NF-κB is usually combined with NF-κB profilin (IκB) to form an inactive trimer that cannot enter the cell nucleus but exists in the cytoplasm[15,16]. A series of enzymes can be activated through signal transduction pathway after the stimulation of TNF-α, IL-1, LPS[17] to activate NF-κB and next IκB kinase to realize the phosphorylation of IκB. When IκB falls off the NF-κB complex, the activated NF-κB will move into the cell nucleus, bind to the κB structural domain of the promoter or enhancer of target gene and cause the transcription of many factors[18-20], including cytokines, chemotatic factor, macrophage chemotactic peptide, cellular adhesion molecule, growth factor, immune receptor and acute phase reactive protein.
The NF-κB p65 involved in this experiment is one of the important composing members of NF-κB/Rel family with 65 000 of relative molecular weight. Its most common form in cell is the heterotic dimer of NF-κB consisting of p65 and p50. When resting, it is combined with its inhibitor IκB to exist in the cytoplasm. When stimulated, the NF-κB will be transferred into nucleus through IκB degradation to combine the κB sequence of the promoter and enhancer area of the regulated gene, promote the transcription of these genes[5] and participate in the tissue injury caused by manifold factors[21]. Tietz et al[22] respectively determined the serum concentration of TNF-α and IL-6 as well as their mRNA expression levels in mice with acute pancreatitis through ELISA and RT-PCR methods, and found that the genetic expression of inflammation promoting cytokines like TNF-α and IL-6 played an extremely important role in the progression of acute pancreatitis.
Dexamethasone, a kind of glucocorticoid, can inhibit the gene synthesis of manifold inflammatory mediators and inhibit inflammatory mediators by increasing the synthesis of anti-inflammatory protein[8]. The possible mechanism for dexamethasone inhibition of NF-κB activation could be: (1) As a hormone where the ligand binds to its corresponding receptor and activates it. The activated glucocorticoid receptor directly couples the RelA subunit of NF-κB in the cell nucleus to inhibit the functions of NF-κB. (2) The activated glucocorticoid receptor blocks the nucleus shifting of NF-κB and its combination with DNA by enhancing the genetic transcription of IκB and raising its level.
Acute liver injury is also a common complication of SAP. Studies found that NF-κB plays an important role in the liver injury process of SAP rats[23,24]. NF-κB after activation can promote liver injury by promoting the genetic transcription of TNF-α and IL-6[25]. NF-κB inhibitor can protect the liver by inhibiting the activation of NF-κB. Some studies believe liver cells are not only target cells of SAP liver injury but also could be the effector cells of inflammatory reactions secreting cytokines. They can act on the liver cells through autocrine or paracrine fashion to aggravate the liver injury[26]. In this experiment, we observed the rise of NF-κB p65- positive cell percentage and expression of manifold inflammation-inducing factors which participated in the liver injury complicated with SAP. The positive cell percentage of the dexamethasone treatment group dropped at 3 h and 6 h possibly because dexamethasone had inhibited the activation of NF-κB, reduced the expression of relevant cytokines and thereby alleviated the liver injury. It should be mentioned that the positive cell rate of the treatment group had risen compared with the model group at 12 h, while the statistical results showed no marked difference between the model group and treatment group at all time points. Theoretically, the positive cell rate should decline possibly due to the following factors: (1) With features like time- and energy- saving and high efficiency, the tissue microarray sections of 2.0 mm in diameter may not reflect the whole tissue picture and, to a certain extent, its representation is limited. (2) With different case numbers, the two groups cannot be compared directly. Statistical results showed no marked difference between the model group and treatment group at all time points; (3) There were also possible faults in reagent selection and staining.
Acute kidney injury is also one of the common complications of SAP with few relevant reports currently. In this experiment, the NF-κB expression level of the treatment group significantly exceeded that of the model group at 12 h after dexamethasone treatment, which is contradictory to the theory to certain extent and we can hardly understand. The reason could resemble that of the aforementioned liver. The main reason is still that the representation of tissue microarrays sections of 2.0 mm in diameter is doubtful and could lead to the deviation of the experimental results. We have planned to select three 2.0-mm points for each tissue in subsequent studies to ensure the representation of tissue. In common studies, the determination and analysis of a single index usually could pose many limitations. Therefore, we add the determination of multiple indexes into the experiment including the plasma amylase content, plasma endotoxin content and serum TNF-α content in order to completely evaluate the therapeutic effects and mechanism of dexamethasone.
Acute lung injury is one of the most common severe complications of SAP[27]. The mechanism responsible for acute lung injury caused by SAP is quite complicated and remains unclear till now. It is now believed that pancreatin, adhension molecule, neutrophil, various inflammatory mediators, etc play extremely important roles in the onset process[28-30]. It has been shown an increase in expression of manifold inflammation-inducing cytokines in the lung tissue at early injury stage, while the activation of transcription factor NF-κB can stimulate the expression of manifold cytokines. Studies show the selective use of NF-κB inhibitor can markedly lower the injury degree of the pancreas and lung, indicating the important role of NF-κB in SAP complicated with the lung injury[31-33]. This experiment has observed the NF-κB p65 expression in the lung tissue but the NF-κB expression levels in the lungs of all groups were negative, indicating that the non-expression of NF-κB p65 in the lung could be related to the reagents. The reagents used in this experiment did not stain in the lung.
In conclusion, NF-κB plays an important role in multiple organ injury. Further studies should be conducted to determine whether dexamethasone could alleviate the pathological changes of multiple organs by reducing the NF-κB expression of the liver and kidney. The advantages of tissue microarrays in pancreatitis pathological examination include saving time and energy, they are highly efficient and representative. The restriction on the representation of tissues to various extents due to small diameter may lead to the deviation of analysis.
Produced mainly by the pancreas, amylase (AMS) has an important effect on digestion of polysaccharide in food and acute pancreatitis is the most common cause of its rise. The rising degree of AMS activity is not certainly related to the injury degree of pancreatic tissue. But the more obviously AMS rises, the more severe the injury becomes. Our study showed that the amylase content of the model group increased with time and the amylase content in plasma dropped after treatment.
Endotoxin is a kind of compound of lipopolysaccharide (LPS) and small protein (Protein) on the cell wall of Gram-negative bacteria. It is specific not because it is a bacteria or metabolite of bacteria but a substance with endotoxin bioactivity only released after death or disintegration of bacteria. Its chemical composition mainly consists of O-specific chain, core polysaccharide and lipoid A. Endotoxemia results when the endotoxin can be detected in the circulatory blood, which could cause a series of pathophysiological changes including sepsis, shock, and diffuse intravascular coagulation and multiple organ dysfunction syndrome (MODS). The role of endotoxin in onset of acute pancreatitis covers the following aspects: (1) Interfering the normal function of cell membrane by non-specific combination with it; (2) Directly destroying the lysosomal membrane within cells of mononuclear phagocytic system to cause cell damage; (3) Damaging mitochondria structure, affecting the coupling process of ATP enzyme and oxidative phosphorylation and causing disturbance in energy metabolism; (4) Changing the immune function of body; (5) Causing a series of pathological or pathophysiological changes of body, affecting the vasomotor function, activating vasoactive substance, reducing platelet and leukocyte, lowering blood pressure or even causing DIC, MOSF, etc.
Mainly generated from the activated mononuclear macrophage, TNF-α is a kind of polypeptide cytokine with extensive biologic activities. The secretion of appropriate amounts of TNF-α had protecting effects and can promote the chemotaxis and antimicrobial effects of PMN, macrophage and eosinophile granulocyte and is one of the defense mechanisms of the body. The excessive secretion of TNF-α could cause inflammatory reactions. High concentration of TNF-α entering the blood flow could also cause fever, drop of blood pressure and reduction of tissue perfusion through lowering myocardial contraction force and tension of vascular smooth muscle as well as metabolic disorder, organ injury and even multi-system damage. It has potentially lethal effects.
Our experiment found no marked difference in the plasma amylase content at 3 h and 6 h between the treatment group and model group, while the plasma amylase content of the treatment group was significantly lower than that of the model group at 12 h (P < 0.01). The plasma endotoxin content of the treatment group was significantly lower than that of the model group at 6 h and 12 h (P < 0.01). The serum TNF-α level of the treatment group was significantly lower than that of the model group at 6 h and 12 h (P < 0.05). Thus, it is concluded that dexamethasone can lower the plasma amylase, plasma endotoxin and serum TNF-α content of rats with SAP as well as their mortality.
The tissue microarrays (TMA) technology adopted by us in this study is exactly the new biochip technology currently extensively applied to basic and clinical applications as well as in other fields. The tissue microarray technology has exceeded the traditional histopathological section technology which is single in sample and low in efficiency[34]. The advantages of TMA are high-throughput, economic, time-saving, reliable result, convenient for experimental control, etc. Since the most distinguished feature of tissue microarrays is to combine the study of gene and its expression products with histomorphology, it possesses great potential in oncopathology studies[35,36], and current studies also mainly focus on this area[37-44]. The chip preparation, staining, examination, etc have restrained the application of this technology in non-tumor diseases. To the best of our knowledge, there was no study report in literature on applying tissue microarrays to pancreatitis pathological examination before our experiment was conducted. Thus this article has taken the lead.
We used the tissue microarray section maker (Beecher Instruments, USA) to drill a hole of 2.0 mm in diameter on recipient block and combined the immunohistochemical method to examine the NF-κB expression levels of the multiple organs. The experimental results are unsatisfactory mainly because the tissue representation has been restricted to different extents due to the small diameter. However, there are advantages of tissue microarrays shown in its application in the pathological examination of pancreatitis, including time- and energy-saving and high efficiency.
Footnotes
Supported by Technological Foundation Project of Traditional Chinese Medicine Science of Zhejiang Province, NO. 2003C130 and NO. 2004C142; Foundation Project for Medical Science and Technology of Zhejiang province, No. 2003B134; Grave Foundation Project for Technological and Development of Hangzhou, No. 2003123B19; Intensive Foundation Project for Technology of Hangzhou, NO. 2004Z006; Foundation Project for Medical Science and Technology of Hangzhou, No. 2003A004; and Foundation Project for Technology of Hangzhou, No. 2005224
S- Editor Liu Y L- Editor Kumar M E- Editor Lu W
References
- 1.Renzulli P, Jakob SM, Täuber M, Candinas D, Gloor B. Severe acute pancreatitis: case-oriented discussion of interdisciplinary management. Pancreatology. 2005;5:145–156. doi: 10.1159/000085266. [DOI] [PubMed] [Google Scholar]
- 2.Kim CD. Pancreatitis--etiology and pathogenesis. Korean J Gastroenterol. 2005;46:321–332. [PubMed] [Google Scholar]
- 3.Hirota M, Sugita H, Maeda K, Ichibara A, Ogawa M. Concept of SIRS and severe acute pancreatitis. Nihon Rinsho. 2004;62:2128–2136. [PubMed] [Google Scholar]
- 4.Weber CK, Adler G. From acinar cell damage to systemic inflammatory response: current concepts in pancreatitis. Pancreatology. 2001;1:356–362. doi: 10.1159/000055834. [DOI] [PubMed] [Google Scholar]
- 5.Algül H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-kappaB/Rel activation. Pancreatology. 2002;2:503–509. doi: 10.1159/000066090. [DOI] [PubMed] [Google Scholar]
- 6.Satoh A, Masamune A, Kimura K, Kaneko K, Sakai Y, Yamagiwa T, Satoh M, Kikuta K, Asakura T, Shimosegawa T. Nuclear factor kappa B expression in peripheral blood mononuclear cells of patients with acute pancreatitis. Pancreas. 2003;26:350–356. doi: 10.1097/00006676-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 7.O'Reilly DA, Roberts JR, Cartmell MT, Demaine AG, Kingsnorth AN. Heat shock factor-1 and nuclear factor-kappaB are systemically activated in human acute pancreatitis. JOP. 2006;7:174–184. [PubMed] [Google Scholar]
- 8.Dong R, Wang ZF, LV Y. Treatment of severe acute pancreatitis with large dosage of dexamethasone in rats. Zhonghua Putong Waike Zazhi. 2001;10:314–317. [Google Scholar]
- 9.Brummer E, Choi JH, Stevens DA. Interaction between conidia, lung macrophages, immunosuppressants, proinflammatory cytokines and transcriptional regulation. Med Mycol. 2005;43 Suppl 1:S177–S179. doi: 10.1080/13693780500051497. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XP, Zhou YF. An ideal rat model of acute necrosis pancreatitis. Weichangbingxue yu Ganzangbingxue Zazhi. 2003;12:530–531. [Google Scholar]
- 11.Shi C, Zhao X, Lagergren A, Sigvardsson M, Wang X, Andersson R. Immune status and inflammatory response differ locally and systemically in severe acute pancreatitis. Scand J Gastroenterol. 2006;41:472–480. doi: 10.1080/00365520500318965. [DOI] [PubMed] [Google Scholar]
- 12.Shi C, Andersson R, Zhao X, Wang X. Potential role of reactive oxygen species in pancreatitis-associated multiple organ dysfunction. Pancreatology. 2005;5:492–500. doi: 10.1159/000087063. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 14.Weber CK, Adler G. Acute pancreatitis. Curr Opin Gastroenterol. 2003;19:447–450. doi: 10.1097/00001574-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Pande V, Ramos MJ. NF-kappaB in human disease: current inhibitors and prospects for de novo structure based design of inhibitors. Curr Med Chem. 2005;12:357–374. doi: 10.2174/0929867053363180. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto Y, Gaynor RB. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 18.Choi EK, Jang HC, Kim JH, Kim HJ, Kang HC, Paek YW, Lee HC, Lee SH, Oh WM, Kang IC. Enhancement of cytokine-mediated NF-kappaB activation by phosphatidylinositol 3-kinase inhibitors in monocytic cells. Int Immunopharmacol. 2006;6:908–915. doi: 10.1016/j.intimp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Su JR, Zhao ZC, Chen WL, Wang X. The effect of activated nuclear factor-kappaB in pathogenesis of acute pancreatitis. Zhonghua YiXue ZaZhi. 2003;83:1497–1500. [PubMed] [Google Scholar]
- 20.Meng Y, Ma QY, Kou XP, Xu J. Effect of resveratrol on activation of nuclear factor kappa-B and inflammatory factors in rat model of acute pancreatitis. World J Gastroenterol. 2005;11:525–528. doi: 10.3748/wjg.v11.i4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Sun SQ, Zhang ZY. Experimental Study on Intrapulmonary Expression of Nuclear Factor-κB, Inflammatory Mediators and Lung Injury in Rats With Severe Acute Pancreatitis. Zhongguo Linchuang Yixue. 2003;20:84–87. [Google Scholar]
- 22.Tietz AB, Malo A, Diebold J, Kotlyarov A, Herbst A, Kolligs FT, Brandt-Nedelev B, Halangk W, Gaestel M, Göke B, et al. Gene deletion of MK2 inhibits TNF-alpha and IL-6 and protects against cerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1298–G1306. doi: 10.1152/ajpgi.00530.2005. [DOI] [PubMed] [Google Scholar]
- 23.Zhao YF, Zhai WL, Zhang SJ, Chen XP. Protection effect of triptolide to liver injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2005;4:604–608. [PubMed] [Google Scholar]
- 24.Jaffray C, Yang J, Norman J. Elastase mimics pancreatitis-induced hepatic injury via inflammatory mediators. J Surg Res. 2000;90:95–101. doi: 10.1006/jsre.2000.5832. [DOI] [PubMed] [Google Scholar]
- 25.Murr MM, Yang J, Fier A, Kaylor P, Mastorides S, Norman JG. Pancreatic elastase induces liver injury by activating cytokine production within Kupffer cells via nuclear factor-Kappa B. J Gastrointest Surg. 2002;6:474–480. doi: 10.1016/s1091-255x(01)00071-3. [DOI] [PubMed] [Google Scholar]
- 26.Yuan YZ, Ji L, Zhu Y. The role of nuclear factor-κB in the pathogenesis of severe acute pancreatitis-associated hepatic injury. Shanghai Yixue Zazhi. 2002;25:172–175. [Google Scholar]
- 27.Liu XM, Xu J, Wang ZF. Pathogenesis of acute lung injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2005;4:614–617. [PubMed] [Google Scholar]
- 28.Surbatović M, Jovanović K, Radaković S, Filipović N. Pathophysiological aspects of severe acute pancreatitis-associated lung injury. Srp Arh Celok Lek. 2005;133:76–81. doi: 10.2298/sarh0502076s. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Dib M, Andersson E, Shi C, Widegren B, Wang X, Andersson R. Alterations of adhesion molecule expression and inflammatory mediators in acute lung injury induced by septic and non-septic challenges. Lung. 2005;183:87–100. doi: 10.1007/s00408-004-2522-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhao QL, Huang CY, Huang Y, Wang JF, Liu J. Study on acute pancreatitis-associated lung injury induced by L-arginine in mice. Sichuan DaXue XueBao YiXueBan. 2004;35:839–842. [PubMed] [Google Scholar]
- 31.Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497–505. doi: 10.1016/s1072-7515(02)01222-x. [DOI] [PubMed] [Google Scholar]
- 32.Virlos I, Mazzon E, Serraino I, Genovese T, Di Paola R, Thiemerman C, Siriwardena A, Cuzzocrea S. Calpain I inhibitor ameliorates the indices of disease severity in a murine model of cerulein-induced acute pancreatitis. Intensive Care Med. 2004;30:1645–1651. doi: 10.1007/s00134-004-2328-z. [DOI] [PubMed] [Google Scholar]
- 33.Virlos I, Mazzon E, Serraino I, Di Paola R, Genovese T, Britti D, Thiemerman C, Siriwardena A, Cuzzocrea S. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock. 2003;20:544–550. doi: 10.1097/01.shk.0000093543.78705.aa. [DOI] [PubMed] [Google Scholar]
- 34.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Shi QL. Application of tissue microarrays technique in lymphoma studies. Zhenduan Binglixue Zazhi. 2003;110:182–184. [Google Scholar]
- 36.Yan XC, Duan GJ. Study on tumors by tissue microarrays (tissue chip) Zhongguo Zhongliu Fangzhi Zazhi. 2003;30:519–521. [Google Scholar]
- 37.Gaiser T, Thorns C, Merz H, Noack F, Feller AC, Lange K. Gene profiling in anaplastic large-cell lymphoma-derived cell lines with cDNA expression arrays. J Hematother Stem Cell Res. 2002;11:423–428. doi: 10.1089/152581602753658619. [DOI] [PubMed] [Google Scholar]
- 38.Husson H, Carideo EG, Neuberg D, Schultze J, Munoz O, Marks PW, Donovan JW, Chillemi AC, O'Connell P, Freedman AS. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002;99:282–289. doi: 10.1182/blood.v99.1.282. [DOI] [PubMed] [Google Scholar]
- 39.Oka T, Yoshino T, Hayashi K, Ohara N, Nakanishi T, Yamaai Y, Hiraki A, Sogawa CA, Kondo E, Teramoto N, et al. Reduction of hematopoietic cell-specific tyrosine phosphatase SHP-1 gene expression in natural killer cell lymphoma and various types of lymphomas/leukemias : combination analysis with cDNA expression array and tissue microarray. Am J Pathol. 2001;159:1495–1505. doi: 10.1016/S0002-9440(10)62535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natkunam Y, Warnke RA, Montgomery K, Falini B, van De Rijn M. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Mod Pathol. 2001;14:686–694. doi: 10.1038/modpathol.3880373. [DOI] [PubMed] [Google Scholar]
- 41.Florell SR, Coffin CM, Holden JA, Zimmermann JW, Gerwels JW, Summers BK, Jones DA, Leachman SA. Preservation of RNA for functional genomic studies: a multidisciplinary tumor bank protocol. Mod Pathol. 2001;14:116–128. doi: 10.1038/modpathol.3880267. [DOI] [PubMed] [Google Scholar]
- 42.Kipps TJ. Advances in classification and therapy of indolent B-cell malignancies. Semin Oncol. 2002;29:98–104. doi: 10.1053/sonc.2002.30146. [DOI] [PubMed] [Google Scholar]
- 43.Manley S, Mucci NR, De Marzo AM, Rubin MA. Relational database structure to manage high-density tissue microarray data and images for pathology studies focusing on clinical outcome: the prostate specialized program of research excellence model. Am J Pathol. 2001;159:837–843. doi: 10.1016/S0002-9440(10)61759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB. Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol. 2002;117:723–728. doi: 10.1309/PEF8-GL6F-YWMC-AG56. [DOI] [PubMed] [Google Scholar]


