Abstract
AIM: To investigate the suppressive effect of saikosaponin-d (SSd) on hepatic fibrosis in rats induced by CCl4 injections in combination with alcohol and high fat, low protein feeding and its relationship with the expression of nuclear factor-κB (NF-κB), tumor necrosis factor-alpha (TNF-α) and interleukins-6 (IL-6).
METHODS: Hepatic fibrosis models were induced by subcutaneous injection of CCl4 at a dosage of 3 mL/kg in rats. At the same time, rats in treatment groups were injected intraperitoneally with SSd at different doses (1.0, 1.5 and 2.0 mg/kg) once daily for 6 wk in combination with CCl4, while the control group received olive oil instead of CCl4. At the end of the experiment, rats were anesthetized and killed (except for 8 rats which died during the experiment; 2 from the model group, 3 in high-dose group, 1 in medium-dose group and 2 in low-dose group). Hematoxylin and eosin (HE) staining and Van Gieson staining were used to examine the changes in liver pathology. The levels of alanine aminotransferase (ALT), triglyeride (TG), albumin (ALB), globulin (GLB), hyaluronic acid (HA) and laminin (LN) in serum and the content of hydroxyproline (HYP) in liver were measured by biochemical examinations and radioimmuneoassay, respectively. In addition, the expression of TNF-α and IL-6 in liver homogenate was evaluated by enzyme-linked immunosorbent assay (ELISA) and the levels of NF-κBp65 and I-κBα in liver tissue were analyzed by Western blotting.
RESULTS: Both histological examination and Van Gieson staining demonstrated that SSd could attenuate the area and extent of necrosis and reduce the scores of liver fibrosis. Similarly, the levels of ALT, TG, GLB, HA, and LN in serum, and the contents of HYP, TNF-α and IL-6 in liver were all significantly increased in model group in comparison with those in control group. Whereas, the treatment with SSd markedly reduced all the above parameters compared with the model group, especially in the medium group (ALT: 412 ± 94.5 IU/L vs 113.76 ± 14.91 IU/L, TG: 0.95 ± 0.16 mmol/L vs 0.51 ± 0.06 mmol/L, GLB: 35.62 ± 3.28 g/L vs 24.82 ± 2.73 g/L, HA: 42.15 ± 8.25 ng/mL vs 19.83 ± 3.12 ng/mL, LN: 27.56 ± 4.21 ng/mL vs 13.78 ± 2.57 ng/mL, HYP: 27.32 ± 4.32 μg/mg vs 16.20 ± 3.12 μg/mg, TNF-α: 4.38 ± 0.76 ng/L vs 1.94 ± 0.27 ng/L, IL-6: 28.24 ± 6.37 pg/g vs 12.72 ± 5.26 pg/g, respectively, P < 0.01). SSd also decreased ALB in serum (28.49 ± 4.93 g/L vs 37.51 ± 3.17 g/L, P < 0.05). Moreover, the expression of NF-κB p65 in the liver of treated groups was lower than that in model groups while the expression of I-κBα was higher in treated group than in model group (P < 0.01). The expression of NF-κBp65 and TNF-α had a positive correlation with the level of HA in serum of rats after treatment with CCl4 (r = 0.862, P < 0.01; r = 0.928, P < 0.01, respectively).
CONCLUSION: SSd attenuates CCl4-induced hepatic fibrosis in rats, which may be related to its effects of hepato-protective and anti-inflammation properties, the down-regulation of liver TNF-α, IL-6 and NF-κBp65 expression and the increased I-κBα activity in liver.
Keywords: Saikosaponin-d, Hepatic fibrosis, Tumor necrosis factor, Interleukins-6, Nuclear factor-κB, Inhibitory κB alpha
INTRODUCTION
Hepatic fibrosis represents the wound healing response of the liver to repeated liver injuries, and is associated with increased inflammatory cell infiltration and may involve the interplay of different inflammatory mediators, which is a common stage in most chronic liver diseases[1-5]. If treated properly in this stage, hepatic fibrosis can be reversed and its progression to irreversible cirrhosis often leading to lethal complications and high mortality may be prevented[6-9]. Nuclear factor-κB (NF-κB) as a critical component in inflammatory conditions can produce proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which are involved in the process of fibrogenesis[10-13]. Therefore, suppressing the inflammatory response and reducing the release of proinflammatory cytokines such as NF-κB, TNF-α and IL-6, may prevent and reverse hepatic fibrosis. Saikosaponin-d (SSd) is a major active component extracted from the root of Bupleurum falcatum. It has been demonstrated that SSd has a wide variety of pharmacological activities, such as liver-protective activity, and anti-hepatic fibrosis or anti-microbial or anti-tumor and anti-inflammatory activities[14-17]. However, its molecular mechanism involved in therapeutic effects of SSd on hepatic fibrosis has not been completely elucidated. Our present study was designed to further evaluate the effect of SSd on hepatic fibrosis in rats induced by CCl4 and its relationship with the expression of NF-κB, TNF-α and IL-6.
MATERIALS AND METHODS
Reagents
SSd was purchased from Jiangxi Herbfine Hi-tech Co. Ltd. CCl4 (Xi’an Chemical Factory) was diluted into 400 g/L in olive oil before it was used. Enzyme-linked immunosorbent assay (ELISA) kit for mouse TNF-α and IL-6 was purchased from R&D Systems Co. Ltd (USA). Hydroxyproline (HYP) assay kit was a product of Nanjing Jiancheng Bioengineering Institute. Kits for HA and LN were bought from Senxiong Company, Shanghai, China. Polyclonal rabbit anti-rat P65 and I-κBα were purchased from Santa Cruz Biotechnology (USA). HRP-labeled goat-anti-rabbit IgG was obtained from HuaMei Company, Shanghai, China.
Animals
Seventy-five adult male SD rats weighing 160-200 g were provided by the Laboratory Animal Center of Medical College, Xi’an Jiaotong University. The rats were randomly divided into 5 groups (n = 15): control group, model group, and three treatment groups. Except for the control rats, all rats were subcutaneously injected with 400 g/L CCl4 (CCl4: olive oil = 2:3), 3 mL/kg, b.w, at every 3 d for 6 wk, and fed with high fat, low protein diet (75% pure maize plus 20% lard and 0.5% cholesterol) and 300 mL/L alcohol in the drinking water. In the 3 treatment groups, SSd was administered daily, via intraperitoneal injection at a dosage of 2.0, 1.5 and 1.0 mg/kg for 6 wk, respectively. After 6 wk, all rats were anesthetized with 200 g/L urethane (5 mL/kg, abdominal injection). Blood was taken from the abdominal aorta. Serum was separated by centrifugation at 4°C and kept at -20°C for assay. Liver tissue was homogenized in cold saline for pathological diagnosis.
Light microscopic examination
Liver tissue was fixed in a 40 g/L solution of formaldehyde in 0.1 mol/L phosphate-buffed saline (pH 7.4), and embedded in paraffin. Five-micrometer thick sections were prepared. All the sections stained with HE and standard Van Gieson (VG) were coded and scored by blind reading. Van Gieson’s method was used to detect collagen fibers[18]. Liver condition was classified according to the standard formulated by China Medical Association in 1995[19], and fibrosis was graded from 0 to 4 (0: no fibrosis; 1: portal area fibrosis; 2: fibrotic septa between portal tracts; 3: fibrosis septa and structure disturbance of hepatic lobule and 4: cirrhosis).
Biochemical determination
Serum levels of alanine transaminase (ALT), albumin (ALB), triglyeride (TG) and globulin (GLB) were measured by routine laboratory methods using a 7170-automatic biochemistry analyzer (Tokyo, Japan). Serum hyaluronic acid (HA) and laminin (LN) were detected by radioimmunoassay, and the content of hydroxyproline (HYP) in liver was determined according to the method described by Jamall et al[20]. The contents of TNF-α and IL-6 protein in liver homogenate were determined by ELISA according to the corresponding protocols of the kits.
Western blotting detection
Nuclear and cytosolic protein extracts were prepared according to manufacturer’s instructions provided with the kits (Active Motif Corp, USA). Nuclear or cytosolic proteins (100 μg each) were run on a 10% SDS-PAGE gel and transferred electrophoretically onto a nitro-cellulose membrane respectively (Shanghai Huashun Corp, China). The membrane was blocked overnight with 10% nonfat milk prior to incubation with polyclonal rabbit anti-rat I-κBα antibody (1:800) or anti-NF-κBp65 antibody (1:1000) at room temperature for 2 h. After washed with PBS, the blots were incubated with HRP-labeled goat-anti-rabbit serum for 1 h and colored on X-ray film by ECL.
Statistical analysis
Quantitative data were analyzed using ANOVA by SPSS 13.0 statistic package and RIDIT test was used for statistical analysis of the qualitative data. All data were expressed as mean ± SD. The correlation was analyzed by Spearman’s correlation analysis. All P values were two-tailed. P < 0.05 was considered statistically significant.
RESULTS
Pathological assay
At the end of the experiment, liver tissue samples from control rats showed normal lobular architecture with central veins and radiating hepatic cords (Figure 1A). Liver tissue samples from model group showed that more fibrous tissues were formed extending into the hepatic lobules to separate them completely. A large number of inflammatory cells infiltrated in the intralobular and interlobular regions. The liver structure was disordered and there were more necrotic and fatty degenerated liver cells compared with the controls (Figures 1B and 1D). In the 3 treatment groups, however, hepatocyte degeneration, necrosis and infiltration of inflammatory cells were all apparently ameliorated and collagen deposition was also markedly reduced (Figures 1C and 1E). Compared with model group, the liver condition of rats in SSd treatment groups was significantly improved (Table 1).
Figure 1.
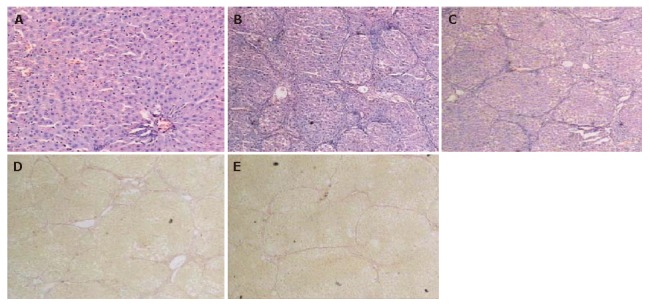
Light microscopy showing normal liver tissue in control group (A) (HE × 100), degenerated and necrotic liver cells associated with inflammatory cells in model group (B) (HE × 40), attenuated necrosis and infiltration of inflammatory cells after SSd treatment (C) (HE × 40), collagen fibers deposited in spaces of Disse and formation of pseudoloculi in model group (D) (Van Gieson × 40), and liver fibrosis tissue in SSd group (E). The pathological change of liver was much milder in SSd group than in model group (Van Gieson × 40).
Table 1.
Pathological observation of liver condition
| Group | n |
Liver condition |
U | ||||
| 0 | I | II | III | IV | |||
| Model | 13 | 0 | 0 | 0 | 4 | 9 | |
| High-dose | 12 | 0 | 5 | 3 | 2 | 2 | 3.26a |
| Medium-dose | 14 | 0 | 6 | 4 | 3 | 1 | 4.17b |
| Low-dose | 13 | 0 | 3 | 2 | 6 | 2 | 2.96a |
U represents the RIDIT value of the two groups, P < 0.05 indicates U > 1.96, P < 0.01 indicates U > 2.58.
P < 0.05,
P < 0.01 vs model group.
Detection of serum HA, LN and liver function
As is shown in Figure 2A, HA and LN levels in serum were significantly higher in model group than in the controls, but they were markedly decreased in 3 treatment groups compared with the model group. Compared with the controls, the serum ALT, TG and GLB levels in model group were all significantly increased while the level of ALB was decreased (P < 0.001, P < 0.05), respectively. However, the levels of ALT, TG and GLB were all in the 3 treatment groups, especially in the group receiving the middle dose of SSd, and the level of ALB was increased compared with the model group (P < 0.05) (Table 2).
Figure 2.
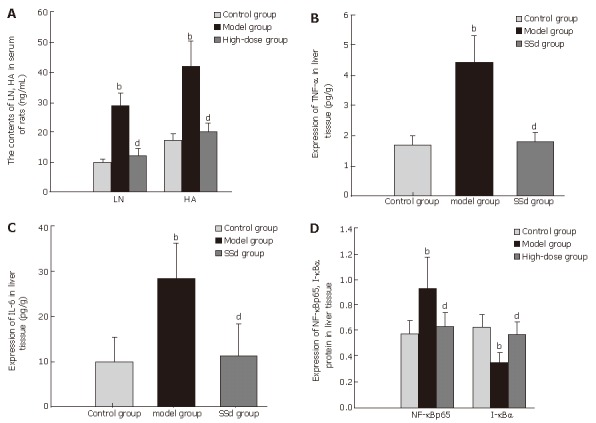
Analysis of serum LN and HA levels (A), expressions of TNF-α (B), IL-6 (C), and NF-κBp65 and I-κBα (D) in liver tissue after treatment with SSd. bP < 0.01 vs control group; dP < 0.01 vs model group.
Table 2.
Serum level of ALT, ALB, GLB, TG and liver HYP (mean ± SD)
| Group | n | ALT (IU/L) | ALB (g/L) | GLB (g/L) | TG (mmol/L) | Liver HYP (μg/mg protein) |
| Control | 15 | 67.58 ± 11.21 | 41.12 ± 2.54 | 21.48 ± 3.24 | 0.39 ± 0.08 | 9.80 ± 1.07 |
| Model | 13 | 412 ± 94.50 | 28.49 ± 4.93 | 35.62 ± 3.28 | 0.95 ± 0.16 | 27.54 ± 4.32 |
| High-dose | 12 | 173.09 ± 24.62bc | 35.73 ± 2.73a | 25.59 ± 3.61a | 0.61 ± 0.10b | 12.83 ± 2.54ad |
| Medium-dose | 14 | 113.76 ± 14.91ad | 37.51 ± 3.17a | 24.82 ± 2.73a | 0.51 ± 0.06a | 16.20 ± 3.12bd |
| Low-dose | 13 | 152.86 ± 19.19bc | 34.31 ± 4.52b | 27.51 ± 2.41b | 0.58 ± 0.07b | 14.38 ± 2.18bd |
ALT: Alanine aminotransferase; ALB: Albumin; GLB: Globulin; TG: Triglyeride; HYP: Hydroxyproline.
P < 0.05,
P < 0.01 vs control group;
P < 0.05,
P < 0.01 vs model group.
TNF-α, IL-6 and HYP contents in liver tissue
The contents of TNF-α, IL-6 and HYP were all significantly lower in SSd treatment groups than in the model group (Figures 2B and 2C). Furthermore, among the 3 treatment groups the high-dose group showed the best effect. The liver HYP level in three SSd treatment groups and TNF-α, IL-6 content in high-dose group were higher than those in the controls, with no significant difference between them (P > 0.05). However, there was a significant difference in the contents of TNF-α, IL-6 between low- and medium-SSd treatment groups and control group (P < 0.05).
Western blot analysis
NF-κBp65 expression was increased significantly in model group compared with the control group, whereas it was markedly decreased in all SSd treatment groups (P < 0.01), especial in the high-dose group. There was no significant difference in the expression of NF-κBp65 between SSd treatment groups and control group (P > 0.05) (Figure 2D). On the contrary, its inhibitory IκBα was significantly decreased in model group while increased in SSd treatment group compared with the model group (P < 0.01) (Figures 3A and 3B). Therefore, SSd could significantly inhibit the activation of NF-κB, which might be associated with increased I-κBα degradation.
Figure 3.
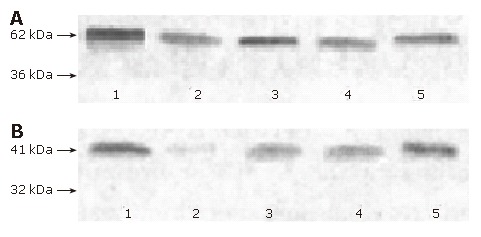
Images of Western blotting of NF-κBp65 (A) and I-κBα (B) in liver tissue of rats. Lane 1: NF-κBp65 and I-κBα protein in control group; lane 2: NF-κBp65 and I-κBα in model group; lanes 3-5: NF-κBp65 and I-κBα in SSd group (from low to high-dose group).
Correlation analysis
Correlation analysis revealed that NF-κBp65 had a highly positive correlation with the expression of TNF-α protein (r = 0.823, P < 0.01). Both NF-κBp65 and TNF-α had a strong positive correlation with the levels of HA in serum of rats induced by CCl4 (r = 0.862, P < 0.01; r = 0.928, P < 0.01, respectively).
DISCUSSION
Hepatic fibrosis is a chronic inflammation-associated disease, which is involved in the infiltration of inflammatory cells and releasing of proinflammatory cytokines, such as TNF-α and IL-6. As a result, hepatic stellate cells (HSCs) are transformed into myofibroblast cells to synthesize more collagen and proteoglycans, increasing deposition and altered composition of extra-cellular matrix (ECM) in liver[21-24]. Based on the current knowledge, a “three-step cascade theory of inflammation involving in liver fibrogenesis” including preinflammatory phases 1-3, has been proposed by Gressner[25], which implies that multiple inflammatory cell interactions with Kupffer cells, platelets, endothelial cells and hepatocytes mediated by various cytokines and growth factors (TNF-α, IL-6 and TGF-β) are involved in the mechanism of fibrogenesis. Therefore, suppressing the inflammatory response can prevent and reverse hepatic fibrosis. Our study showed that, 6 wk treatment with SSd, especially at the middle dose used, could decrease serum levels of ALT, TG, GLB and ALB in rats with hepatic injury caused by CCl4. Histological examination also demonstrated that a large number of inflammatory cells infiltrated the intralobular and interlobular regions, more fibrous tissue was formed and the margin of liver was uneven in model group compared with the control group. In contrast, SSd especially its medium-dose could obviously attenuate the extent of necrosis and reduce the immigration of inflammatory cells compared with the model group, and no pseudoloculus could be observed. Moreover, SSd could decrease the scores of hepatic fibrosis grading (Table 1), indicating that SSd can significantly protect liver against fibrosis, which may be related to its inhibitory effects on inflammation. These findings are consistent with previously reported results[26]. It has been demonstrated that SSd has marked inhibitory actions on the processes of inflammation, including capillary permeability, releasing of inflammation mediators, leukoplasia and desmoplasia[15,27,28]. In addition, SSd can increase serum concentrations of adrenocorticotropic hormone and corticosterone[29] as well as corticotropin-releasing factor (mRNA) level in the hypothalamus[16].
HA and LN levels in serum and HYP in liver are the important indices reflecting the degree of hepatic fibrosis[30-32]. In this study, the contents of HA and LN in serum and HYP in liver were much higher than those in the controls, but markedly lower in treatment groups (P < 0.01), indicating that SSd can prevent hepatic fibrosis due to chronic liver injury, thus delaying the development of cirrhosis.
Recent studies have identified NF-κB as a critical component to bridge inflammation by producing proinflammatory cytokines (such as TNF-α and IL-6) and more ECM in liver, thus further boosting inflammatory processes and activating HSCs[21-23,33-36]. It was also reported that TNF-α released from activating macrophages can turn up NF-κB activity both in target tissue cells and in macrophages themselves[37-40].
NF-κB is a transcription factor consisting of p65 and p50 subunits of the Rel protein family[41]. In most cells, it binds to its inhibitory counterpart I-κBα and other IκB proteins to form P65-P50-IκB trimer which is located in the cytoplasm as an inactive complex. Following I-κBα degradation by a complex signaling cascade initiated on the cell surface, the activated NF-κBp65 disassociates from I-κBα and shifts into nuclei where it binds to specific DNA motifs to regulate transcriptional activity of its target genes involved in HSC activation[42], releasing of proinflammatory cytokines including IL-6 and TNF-α. Thus, inhabiting IκBα phosphorylation is an indispensable step to activate the NF-κB signaling pathway[43-46]. In the present study, NF-κBp65 expression increased significantly in model group compared with normal control group, whereas it was markedly decreased in all SSd treatment groups, especial in the high-dose group. There was no difference in the expression of NF-κBp65 between SSd treatment groups and control group. On the contrary, its inhibitory I-κBα was significantly lower in model group but higher in SSd treatment group, suggesting that SSd can significantly inhibit the activation of NF-κBp65, which may be associated with a reduction in I-κBα degradation.
In addition, Spearman’s correlation analysis showed that NF-κBp65 was highly correlated with the expression of TNF-α protein (r = 0.823, P < 0.01) and both of them had a strong positive correlation with the serum levels of HA induced by CCl4 (r = 0.862, P < 0.01; r = 0.928, P < 0.01, respectively).
In conclusion, SSd has beneficial effects on hepatic fibrosis. Down-regulation of TNF-α, IL-6 and NF-κBp65 expression and increased I-κBα activity of SSd in rat liver may play an important role in the improvement of hepatic fibrosis induced by CCl4. Since hepatic fibrogenesis is a very complicated process, the underlying mechanisms of SSd remain to be further explored.
COMMENTS
Background
Hepatic fibrosis is a chronic inflammation-associated disease, which is involved in the infiltration of inflammatory cells and releasing of proinflammatory cytokines, such as NF-κB, TNF-α and IL-6. Recently, more and more clinical and experimental observations have demonstrated that SSd, a traditional Chinese medicine, is of some preventive and therapeutic values against liver fibrosis, whereas, the molecular mechanism involved in therapeutic effects of SSd on hepatic fibrosis has not been completely elucidated. Therefore, the aim of our study was to further evaluate the anti-hepatic fibrosis effect of SSd in rats and to study its relationship with the expression of NF-κBp65, TNF -α and IL-6.
Research frontiers
NF-κB as a critical component to bridge inflammation, can produce proinflammatory cytokines such as TNF-α and IL-6, which are involved in the process of fibrogenesis. Our study aimed at investigating the suppressive effect of SSd on hepatic fibrosis in rats induced by CCl4 from the level of cytokine and its relationship with the expression of NF-κBp65, TNF-α and IL-6.
Innovations and breakthroughs
SSd has beneficial effects on hepatic fibrosis, and the down-regulation of TNF-α, IL-6 and NF-κBp65 expression and increased I-κBα activity of SSd in rat liver may play an important role in the improvement of hepatic fibrosis induced by CCl4.
Applications
SSd may play a role in antifibrotic therapy. It protects liver cells against fibrosis and inhibits collagen fiber deposition in liver, and therefore can be used in the treatment of cirrhosis in clinic practice.
Terminology
Nuclear factor-κB (NF-κB) is a transcription factor consisting of p65 and p50 subunits of the Rel protein family. In most cells, it binds to its inhibitory counterpart I-κBα and other I-κB proteins to form P65-P50-IκB trimer that is located in the cytoplasm as an inactive complex. Following I-κBα degradation by a complex signaling cascade initiated at the cell surface, the activated NF-κBp65 disassociates from I-κBα and shifts into nuclei where it binds to specific DNA motifs to regulate transcriptional activity of its target genes involved in HSC activation, releasing of proinflammatory cytokines.
Peer review
In this study, rats with liver fibrosis were treated with CCl4 in combination with ethanol, high fat and low protein diet. Rats receiving SSd in combination with CCl4 injection developed less liver fibrosis. The effect was associated with less liver damage indicated by lower transaminase and higher albumin levels. Additionally, less activation of NF-κB was observed in the liver of treated rats, suggesting that SSd could attenuate CCl4-induced liver fibrosis by down-regulating the inflammatory response in the liver. The study addresses an interesting issue, but the value of this study in its current form is limited. The data provided are preliminary but do not sufficiently support the conclusions drawn by the authors.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30471982
S- Editor Wang GP L- Editor Wang XL E- Editor Bi L
References
- 1.Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28–36. [PubMed] [Google Scholar]
- 2.Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, Groothuis GM, Meijer DK, Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719–728. doi: 10.1053/jhep.2001.27805. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 4.Lamireau T, Desmoulière A, Bioulac-Sage P, Rosenbaum J. Mechanisms of hepatic fibrogenesis. Arch Pediatr. 2002;9:392–405. doi: 10.1016/s0929-693x(01)00800-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang LJ, Yu JP, Li D, Huang YH, Chen ZX, Wang XZ. Effects of cytokines on carbon tetrachloride-induced hepatic fibrogenesis in rats. World J Gastroenterol. 2004;10:77–81. doi: 10.3748/wjg.v10.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazaki I, Watanabe T, Hozawa S, Niioka M, Arai M, Maruyama K. Reversibility of hepatic fibrosis: from the first report of collagenase in the liver to the possibility of gene therapy for recovery. Keio J Med. 2001;50:58–65. doi: 10.2302/kjm.50.58. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Wang GJ, Diao Y, Xu RA, Xie HT, Li XY, Sun JG. Adeno-associated virus mediated interferon-gamma inhibits the progression of hepatic fibrosis in vitro and in vivo. World J Gastroenterol. 2005;11:4045–4051. doi: 10.3748/wjg.v11.i26.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki I, Watanabe T, Hozawa S, Arai M, Maruyama K. Molecular mechanism of the reversibility of hepatic fibrosis: with special reference to the role of matrix metalloproteinases. J Gastroenterol Hepatol. 2000;15 Suppl:D26–D32. doi: 10.1046/j.1440-1746.2000.02185.x. [DOI] [PubMed] [Google Scholar]
- 9.Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D, et al. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol. 2002;37:773–780. doi: 10.1016/s0168-8278(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 10.Schwabe RF, Schnabl B, Kweon YO, Brenner DA. CD40 activates NF-kappa B and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol. 2001;166:6812–6819. doi: 10.4049/jimmunol.166.11.6812. [DOI] [PubMed] [Google Scholar]
- 11.Sudo K, Yamada Y, Moriwaki H, Saito K, Seishima M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine. 2005;29:236–244. doi: 10.1016/j.cyto.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Chong LW, Hsu YC, Chiu YT, Yang KC, Huang YT. Anti-fibrotic effects of thalidomide on hepatic stellate cells and dimethylnitrosamine-intoxicated rats. J Biomed Sci. 2006;13:403–418. doi: 10.1007/s11373-006-9079-5. [DOI] [PubMed] [Google Scholar]
- 13.Luedde T, Beraza N, Trautwein C. Evaluation of the role of nuclear factor-kappaB signaling in liver injury using genetic animal models. J Gastroenterol Hepatol. 2006;21 Suppl 3:S43–S46. doi: 10.1111/j.1440-1746.2006.04588.x. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Cui R. Advances in the study of anti-inflammatory and immunoregulatory effects of saikosaponins and their similar substances. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:446–448. [PubMed] [Google Scholar]
- 15.Bermejo Benito P, Abad Martínez MJ, Silván Sen AM, Sanz Gómez A, Fernández Matellano L, Sánchez Contreras S, Díaz Lanza AM. In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci. 1998;63:1147–1156. doi: 10.1016/s0024-3205(98)00376-2. [DOI] [PubMed] [Google Scholar]
- 16.Dobashi I, Tozawa F, Horiba N, Sakai Y, Sakai K, Suda T. Central administration of saikosaponin-d increases corticotropin-releasing factor mRNA levels in the rat hypothalamus. Neurosci Lett. 1995;197:235–238. doi: 10.1016/0304-3940(95)11933-n. [DOI] [PubMed] [Google Scholar]
- 17.Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69:705–709. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- 18.Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, Cheng JL, Xu QF. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis: effects of RAS inhibitors on hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:824–828. doi: 10.3748/wjg.v6.i6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.China Medical Association infectious branch. The standard of grading and staging of viral hepatitis. Zhonghua Chuanranbing Zazhi. 1995;13:241–247. [Google Scholar]
- 20.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 21.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luckey SW, Taylor M, Sampey BP, Scheinman RI, Petersen DR. 4-hydroxynonenal decreases interleukin-6 expression and protein production in primary rat Kupffer cells by inhibiting nuclear factor-kappaB activation. J Pharmacol Exp Ther. 2002;302:296–303. doi: 10.1124/jpet.102.033522. [DOI] [PubMed] [Google Scholar]
- 23.Sung CK, She H, Xiong S, Tsukamoto H. Tumor necrosis factor-alpha inhibits peroxisome proliferator-activated receptor gamma activity at a posttranslational level in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G722–G729. doi: 10.1152/ajpgi.00411.2003. [DOI] [PubMed] [Google Scholar]
- 24.Lee KS, Lee SJ, Park HJ, Chung JP, Han KH, Chon CY, Lee SI, Moon YM. Oxidative stress effect on the activation of hepatic stellate cells. Yonsei Med J. 2001;42:1–8. doi: 10.3349/ymj.2001.42.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Gressner AM. Mediators of hepatic fibrogenesis. Hepatogastroenterology. 1996;43:92–103. [PubMed] [Google Scholar]
- 26.Yamamoto M, Kumagai A, Yamamura Y. Structure and action of saikosaponins isolated from Bupleurum falcatum L. II. Metabolic actions of saikosaponins, especially a plasma cholesterol-lowering action. Arzneimittelforschung. 1975;25:1240–1243. [PubMed] [Google Scholar]
- 27.Navarro P, Giner RM, Recio MC, Máñez S, Cerdá-Nicolás M, Ríos JL. In vivo anti-inflammatory activity of saponins from Bupleurum rotundifolium. Life Sci. 2001;68:1199–1206. doi: 10.1016/s0024-3205(00)01019-5. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, Kumagai A, Yamamura Y. Structure and actions of saikosaponins isolated from Bupleurum falcatum L. I. Anti-inflammatory action of saikosaponins. Arzneimittelforschung. 1975;25:1021–1023. [PubMed] [Google Scholar]
- 29.Hiai S, Yokoyama H, Nagasawa T, Oura H. Stimulation of the pituitary-adrenocortical axis by saikosaponin of Bupleuri radix. Chem Pharm Bull (Tokyo) 1981;29:495–499. doi: 10.1248/cpb.29.495. [DOI] [PubMed] [Google Scholar]
- 30.Hayasaka A, Saisho H. Serum markers as tools to monitor liver fibrosis. Digestion. 1998;59:381–384. doi: 10.1159/000007493. [DOI] [PubMed] [Google Scholar]
- 31.Körner T, Kropf J, Gressner AM. Serum laminin and hyaluronan in liver cirrhosis: markers of progression with high prognostic value. J Hepatol. 1996;25:684–688. doi: 10.1016/s0168-8278(96)80239-x. [DOI] [PubMed] [Google Scholar]
- 32.Murawaki Y, Ikuta Y, Koda M, Nishimura Y, Kawasaki H. Clinical significance of serum hyaluronan in patients with chronic viral liver disease. J Gastroenterol Hepatol. 1996;11:459–465. doi: 10.1111/j.1440-1746.1996.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 33.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen K, Long YM, Wang H, Lan L, Lin ZH. Activation of nuclear factor-kappa B and effects of pyrrolidine dithiocarbamate on TNBS-induced rat colitis. World J Gastroenterol. 2005;11:1508–1514. doi: 10.3748/wjg.v11.i10.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 36.Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71:226–240. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- 37.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura A, Mori H, Ohishi M, Aki D, Hanada T. Negative regulation of cytokine signaling influences inflammation. Curr Opin Immunol. 2003;15:704–708. doi: 10.1016/j.coi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Baldwin AS. Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 41.Cowburn AS, Deighton J, Walmsley SR, Chilvers ER. The survival effect of TNF-alpha in human neutrophils is mediated via NF-kappa B-dependent IL-8 release. Eur J Immunol. 2004;34:1733–1743. doi: 10.1002/eji.200425091. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 43.Hellerbrand C, Jobin C, Iimuro Y, Licato L, Sartor RB, Brenner DA. Inhibition of NFkappaB in activated rat hepatic stellate cells by proteasome inhibitors and an IkappaB super-repressor. Hepatology. 1998;27:1285–1295. doi: 10.1002/hep.510270514. [DOI] [PubMed] [Google Scholar]
- 44.Elsharkawy AM, Wright MC, Hay RT, Arthur MJ, Hughes T, Bahr MJ, Degitz K, Mann DA. Persistent activation of nuclear factor-kappaB in cultured rat hepatic stellate cells involves the induction of potentially novel Rel-like factors and prolonged changes in the expression of IkappaB family proteins. Hepatology. 1999;30:761–769. doi: 10.1002/hep.510300327. [DOI] [PubMed] [Google Scholar]
- 45.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]


