Abstract
AIM: To investigate the protective effects and possible mechanisms of Veratrum nigrum L.var. ussuriense Nakai alkaloids (VnA) on hepatic ischemia/reperfusion (I/R) injury in rats.
METHODS: Forty male Wistar rats were randomly divided into four experimental groups (n = 10 in each): (A) Control group (the sham operation group); (B) I/R group (pretreated with normal saline); (C) Small-dose (10 μg/kg) VnA pretreatment group; (D) Large-dose (20 μg/kg) VnA pretreatment group. Hepatic ischemia/reperfusion (Hepatic I/R) was induced by occlusion of the portal vein and the hepatic artery for 90 min, followed by reperfusion for 240 min. The pretreatment groups were administered with VnA intraperitoneally, 30 min before surgery, while the control group and I/R group were given equal volumes of normal saline. Superoxide dismutase (SOD) activity, myeloperoxidase (MPO) activity and nitric oxide (NO) content in the liver tissue at the end of reperfusion were determined and liver function was measured. The expression of intercellular adhesion molecule-1 (ICAM-1) and E-selectin (ES) were detected by immunohistochemical examinations and Western blot analyses.
RESULTS: The results showed that hepatic I/R elicited a significant increase in the plasma levels of alanine aminotransferase (ALT: 74.53 ± 2.58 IU/L vs 1512.54 ± 200.76 IU/L, P < 0.01) and lactic dehydrogenase (LDH: 473.48 ± 52.17 IU/L vs 5821.53 ± 163.69 IU/L, P < 0.01), as well as the levels of MPO (1.97 ± 0.11 U/g vs 2.57 ± 0.13 U/g, P < 0.01) and NO (69.37 ± 1.52 μmol/g protein vs 78.39 ± 2.28 μmol/g protein, P < 0.01) in the liver tissue, all of which were reduced by pretreatment with VnA, respectively (ALT: 1512.54 ± 200.76 IU/L vs 977.93 ± 89.62 IU/L, 909.81 ± 132.76 IU/L, P < 0.01, P < 0.01; LDH: 5821.53 ± 163.69 IU/L vs 3015.44 ± 253.01 IU/L, 2448.75 ± 169.4 IU/L, P < 0.01, P < 0.01; MPO: 2.57 ± 0.13 U/g vs 2.13 ± 0.13 U/g, 2.07 ± 0.05 U/g, P < 0.01, P < 0.01; NO: 78.39 ± 2.28 μmol/g protein vs 71.11 ± 1.73 μmol/g protein, 68.58 ± 1.95 μmol/g protein, P < 0.05, P < 0.01). The activity of SOD (361.75 ± 16.22 U/mg protein vs 263.19 ± 12.10 U/mg protein, P < 0.01) in the liver tissue was decreased after I/R, which was enhanced by VnA pretreatment (263.19 ± 12.10 U/mg protein vs 299.40 ± 10.80 U/mg protein, 302.09 ± 14.80 U/mg protein, P < 0.05, P < 0.05). Simultaneously, the histological evidence of liver hemorrhage, polymorphonuclear neutrophil infiltration and the overexpression of ICAM-1 and E-selectin in the liver tissue were observed, all of which were attenuated in the VnA pretreated groups.
CONCLUSION: The results demonstrate that VnA pretreatment exerts significant protection against hepatic I/R injury in rats. The protective effects are possibly associated with enhancement of antioxidant capacity, reduction of inflammatory responses and suppressed expression of ICAM-1 and E-selectin.
Keywords: Veratrum nigrum L.var. ussuriense Nakai alkaloids, Hepatic Ischemia/Reperfusion Injury, Intracellular adhesion molecule-1, E-selectin
INTRODUCTION
Re-establishment of blood flow to the ischemic tissue is the therapeutic goal in the treatment of arterial occlusion. However, surgically restoring the blood flow often leads to continued or accelerated local tissue injury and systemic toxicity[1]. Hepatic ischemia/reperfusion (Hepatic I/R) injury is an important non-immunologic problem and a limitating factor in liver transplantation and it may result in liver dysfunction, liver failure, and even death[2]. Hepatic I/R injury is one of the major complications of hepatic resection, hemorrhagic shock with fluid resuscitation, and liver transplantation, particularly with grafts from marginal donors[3]. The mechanisms underlying ischemia/reperfusion induced organ damage are likely multifactorial and interdependent. Previous studies have identified many mediators involved in the pathogenesis of hepatic I/R injury. Among them, reactive oxygen species (ROS)[4] released by active Kupffer cells, and proinflammatory cytokines[5-7] are central to this process. Expression of the adhesion molecules is up-regulated by cytokines and mediates the recruitment of neutrophils to the liver resulting in hepatic injury[8-9]. In addition, selectins are also up-regulated during this process[10].
It has been shown that short episodes of liver ischemia confer protection against longer ischemic insults and subsequent I/R injury. This phenomenon is called ischemic or mechanical preconditioning[11-12]. Pharmacological preconditioning can be an effective alternative to ischemic preconditioning. With pharmacological preconditioning, liver protection is achieved by the administration of substances before or at the early phase of the I/R process.
Veratrum nigrum L.var. ussuriense Nakai alkaloids (VnA) is the total alkaloid extracted from the root of Veratrum nigrum L. var. ussuriense Nakai of Mount Qian in Liaoning Province, China. VnA contains at least eleven alkaloids featuring an ester-type isosteroidal structure identified by modern analytical techniques such as HPLC-MS, NMR, etc. The principal components of VnA include verussurien, verabenzoamine, verazine, germidine, jervine, germerine, 15-O-(methylbuty-royl)-germine, verussurinine, neogermbudine, zygadenine and echinuline[13-15]. Some researchers have demonstrated that VnA has powerful inhibitory effects against both arterial and venous thrombosis, and it could produce beneficial effects on brain I/R injury[16-18]. In this study, we investigated whether VnA had a protective effect against liver injury induced by hepatic I/R and explored its putative protective mechanism.
MATERIALS AND METHODS
Reagents
The VnA hydrochloride injection (100 mg/L) was prepared by the Department of Pharmaceutical Engineering, Dalian University of Science and Technology. It was diluted to the required concentrations with sterile saline solution prior to use.
Animals
Male Wistar rats (Experimental Animal Center of Dalian Medical University, Dalian, China) weighing 220 ± 20 g were used in this study. All rats were fed with standard laboratory chow and water, and housed in accordance with institutional animal care guidelines.
Experimental design
Male Wistar rats were fasted overnight but had free access to tap water. The rats were assigned randomly into four experimental groups (n = 10 in each): (A) Control group (the sham operation group); (B) I/R group (pretreated with normal saline); (C) Small-dose (10 μg/kg) VnA pretreatment group; (D) Large-dose (20 μg/kg) VnA pretreatment group. The model of hepatic I/R injury was established by the clamping and unclamping of the vessels supplying the left lateral and median hepatic lobes, which account for 70% of the rat liver mass, according to the published method[12]. We reproduced a lobar rather than a total hepatic I/R injury model to induce a severe hepatic ischemic insult without mesenteric venous congestion to avoid the development of intestinal congestion and leakage of bacteria or bacterial products into the circulation[6,19]. Briefly, 30 min before operation, the rats received intraperitoneally VnA (10 μg/kg, 20 μg/kg) or equal volumes of normal saline solution. A midline laparotomy was performed and a microvascular clip was placed to interrupt the arterial and portal venous blood flow to the left and middle lobes of the liver. Reflow was initiated after 90 min of hepatic ischemia by removing the clamp. The doses of VnA administration were determined according to previous studies, combined with our preliminary experiments[17-18]. All rats were killed at 240 min of reperfusion, and blood and liver samples were obtained for the following analyses.
Histopathological assessment
Liver samples were fixed in 10% formalin and embedded in paraffin. Five-micrometer sections of liver tissue were stained with hematoxylin and eosin according to standard procedures. Light microscopy was used to assess the degree of liver damage.
Measurement of plasma alanine aminotransferase (ALT) and lactic dehydrogenase (LDH) level
The abdominal aorta was punctured and 5 mL of blood was taken and put into heparinized tubes. The blood sample was centrifuged at 3000 r/min for 15 min at a room temperature to separate plasma for analyses. The plasma concentrations of ALT (a specific marker for hepatic parenchymal injury), and LDH were measured with an OLYMPUS AU5400 automatic analyzer. Both values were expressed as U/L.
Liver superoxide dismutase (SOD), myeloperoxidase (MPO) and nitric oxide (NO) assay
Liver samples were homogenized on ice in 5 volumes of normal saline and centrifuged at 3000 r/min for 15 min. Liver SOD, MPO and NO levels were measured using an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. The levels of SOD, MPO and NO were expressed as U/mgprot, U/g and μmol/gprot, respectively.
Immunohistochemical analyses
Formalin-fixed, paraffin-embedded liver specimens were stained by streptavidin/peroxidase immunohistochemistry technique for intercellular adhesion molecule-1 (ICAM-1) and E-selectin (ES) detection. Five-micrometer sections were treated with 0.3% H2O2 in methanol to block endogenous peroxide activity and then incubated with the polyclonal rabbit anti-rat ICAM-1 and E-selectin antibody (Wuhan Boster Biological Technology Co., Ltd, Wuhan, China, both 1:500 dilution). Biotinylated anti-rabbit immunoglobulin was added as a secondary antibody. The horseradish peroxidase labeled streptomycin-avidin complex was then used to detect the second antibody. Finally, slides were stained with 3,3’-diaminobenzidine, which was used as a chromagen, and the sections were counterstained with hematoxylin before being examined under a light microscope. The brown or dark brown stained cells were considered as positive. The results were evaluated semi-quantitatively according to the percentage of positive cells in 5 high power fields at 400 multiple signal magnification: 0, less than 5%; 1, from 6% to 25%; 2, from 26% to 50%; 3, from 51% to 75%; 4, more than 75%[20].
ICAM-1 and E-selectin Western blot analysis
Frozen liver tissue was homogenized with PBS (pH = 7.2) and centrifuged at 4°C, 10 000 g for 10 min. After precipitation the insoluble fraction was discarded, and the protein concentration in the supernatant was determined by a spectrophotometer. Aliquots (20 μg) of protein from each sample were loaded into each lane of 10% SDS-PAGE gel electrophoresis and then electroblotted onto nitrocellulose membranes (Millipore, Bedford, MA). The membranes were then probed with the rabbit antibody against rat ICAM-1(intercellular adhesion molecule-1) or E-selectin (Boster Biological Technology Co., Ltd, Wuhan, China, both 1:1000 dilution) and biotin-conjugated anti-rabbit IgG (Fuzhou Maixin Biological Technology Co., Ltd, Fuzhou, China) according to the manufacturer’s recommendations. The signals were visualized by a DAB assay kit (Fuzhou Maixin Biological Technology Co., Ltd, Fuzhou, China) and analyzed with a gel imaging system (Kodak system EDAS120, Japan).
Statistical analyses
All data were presented as mean ± SD. Statistical analyses were performed using one-way analysis of variance (ANOVA) with the SPSS 11.5 statistical software package. The differences between means were analyzed using Student-Newman-Keuls (SNK) test for multiple comparisons. P values less than 0.05 were considered statistically significant.
RESULTS
Pathological alterations of liver tissue
The histological structure of cells was normal in the control group. After 90 min of hepatic ischemia followed by 240 min of reperfusion, the occluded liver tissue appeared as dark color with an obtuse fringe. Compared with the control group, the liver tissue from the I/R group was markedly damaged characterized by edema, hemorrhage, partial exfoliation of vascular endothelium and infiltration with inflammatory cells under the microscope. Pretreatment of rats with 10 or 20 μg/kg VnA resulted in a significant amelioration of hepatic injury (Figure 1).
Figure 1.
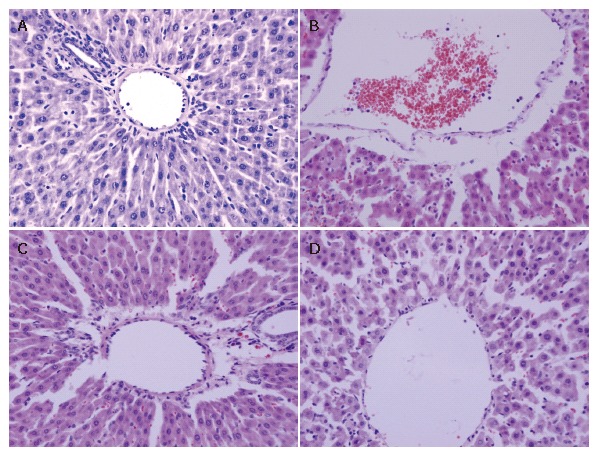
Changes of histology in the liver tissue 90 min after ischemia and 240 min after reperfusion in rats (× 400). Five μm sections of liver tissue were stained with hematoxylin and eosin according to standard procedures. For all groups, n = 10. A: Control group: Normal appearance of hepatocytes and sinusoids; B: I/R group: Histological edema, hemorrhage, partial exfoliation of blood vessel endothelium and infiltration with inflammatory cells; C: I/R + VnA (10 μg/kg) group: A significant amelioration of histological edema, hemorrhage, and exfoliation of blood vessel endothelium; D: I/R + VnA (20 μg/kg) group: Slight inflammatory cell infiltration with most hepatocytes in normal appearance.
Levels of plasma liver enzymes
Liver function was tested by measuring plasma levels of ALT and LDH. Hepatic I/R led to a marked elevation of plasma ALT and LDH activity (ALT: 74.53 ± 2.58 IU/L vs 1512.54 ± 200.76 IU/L, P < 0.01; LDH: 473.48 ± 52.17 IU/L vs 5821.53 ± 163.69 IU/L, P < 0.01, Figure 2). The increase in plasma ALT activity elicited by hepatic I/R was significantly attenuated by administration of VnA at doses of 10 and 20 μg/kg by about 35.35% and 39.85%, respectively (1512.54 ± 200.76 IU/L vs 977.93 ± 89.62 IU/L, 909.81 ± 132.76 IU/L, P < 0.01, P < 0.01); and the activity of LDH was reduced by administration of VnA at doses of 10 and 20 μg/kg by about 48.20% and 57.94%, respectively (5821.53 ± 163.69 IU/L vs 3015.44 ± 253.01 IU/L, 2448.75 ± 169.4 IU/L, P < 0.01, P < 0.01). This result demonstrated the dose-dependent protective effects of VnA on liver injury.
Figure 2.
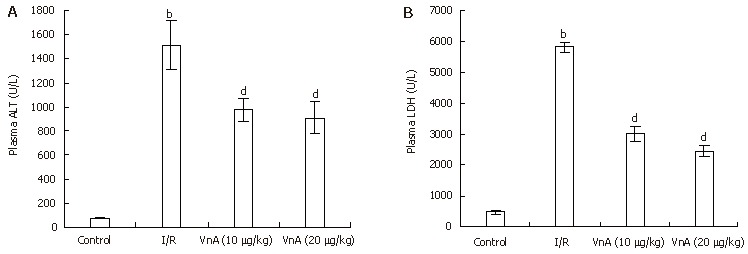
Plasma aminotransferase (ALT) (A) and acetic dehydrogenase (LDH) (B) levels in different groups (mean ± SD, n = 10). After 90 min of hepatic ischemia and 4 h of reperfusion, plasma levels of ALT and LDH were determined with an OLYMPUS AU5400 automatic analyzer. bP < 0.01 vs control group; dP < 0.01 vs I/R group.
SOD activity in liver tissue
Compared with the control group, the level of liver SOD in the I/R group reduced significantly (361.75 ± 16.22 U/mg protein vs 263.19 ± 12.10 U/mg protein, P < 0.01). After administration of VnA at doses of 10 and 20 μg/kg, respectively, the liver SOD activity was elevated significantly (263.19 ± 12.10 U/mg protein vs 299.40 ± 10.80 U/mg protein, 302.09 ± 14.80 U/mg protein, P < 0.05, P < 0.05, Figure 3).
Figure 3.
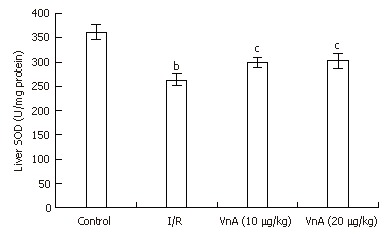
Activity of superoxide dismutase (SOD) in the liver tissue in different groups (mean ± SD, n = 10). After 90 min of ischemia and 4 h of reperfusion, the liver tissue was homogenized and assayed for SOD levels with an SOD assay kit as the index of hepatic oxidative stress. bP < 0.01 vs control group; cP < 0.05 vs I/R group.
MPO activity in liver tissue
Hepatic neutrophil recruitment was determined by liver MPO content. Hepatic I/R caused a marked increase in liver MPO content compared with the control group (1.97 ± 0.11 U/g vs 2.57 ± 0.13 U/g, P < 0.01). Administration of VnA at doses of 10 and 20 μg/kg resulted in a marked reduction of MPO in a dose-dependent manner (2.57 ± 0.13 U/g vs 2.13 ± 0.13 U/g, 2.07 ± 0.05 U/g, P < 0.01, P < 0.01), suggesting that VnA prevents leukocyte recruitment to the liver tissue (Figure 4).
Figure 4.
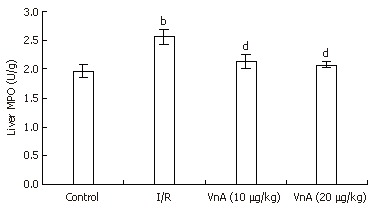
Activity of myeloperoxidase (MPO) in the liver tissue in different groups (mean ± SD, n = 10). MPO contents in liver tissue were analyzed with an MPO assay kit as the index of neutrophil recruitment. bP < 0.01 vs control group; dP < 0.01 vs I/R group.
NO content in liver tissue
The level of NO in the liver tissue was significantly higher in the I/R group compared with the control group (69.37 ± 1.52 μmol/g protein vs 78.39 ± 2.28 μmol/g protein, P < 0.01). Pretreatment with VnA at both 10 and 20 μg/kg, however, caused an obvious reduction in a dose-dependent manner when compared with the I/R group (78.39 ± 2.28 μmol/g protein vs 71.11 ± 1.73 μmol/g protein, 68.58 ± 1.95 μmol/g protein, P < 0.05, P < 0.01, Figure 5).
Figure 5.
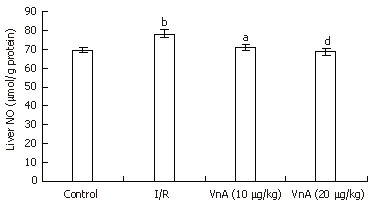
Content of nitric oxide (NO) in the liver tissue in different groups (mean ± SD, n = 10). After 90 min of ischemia and 4 h of reperfusion, the liver tissue was homogenized and assayed for NO levels with an NO assay kit as the index of hepatic oxidative stress. bP < 0.01 vs control group. aP < 0.05, dP < 0.01 vs I/R group.
Immunohistochemical analysis for liver ICAM-1 and E-selectin
The expression of ICAM-1 and E-selectin in the control group showed as light brown immunostaining. However, their expression was highly up-regulated after 90 min of ischemia and 240 min of reperfusion (P < 0.01). Compared with the I/R group, the increase of ICAM-1 and E-selectin expression was significantly suppressed by VnA in a dose-dependent manner (P < 0.05, P < 0.01; P < 0.05, P < 0.05 respectively) (Figure 6, Figure 7 and Figure 8).
Figure 6.
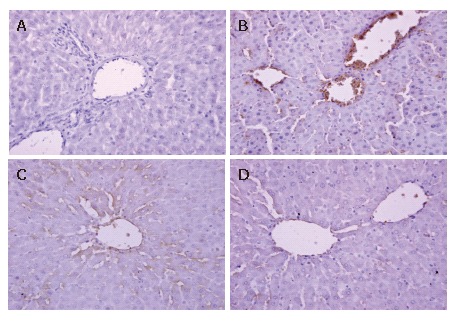
Immunohistochemical staining of adhesion molecule-1 (ICAM-1) expression in the liver tissue after 90 min of ischemia and reperfusion for 240 min in rats (× 400). Formalin-fixed, paraffin-embedded liver specimens were stained by streptavidin/peroxidase immunohistochemistry technique. For all groups, n = 10. A: Control group; B: I/R group; C: I/R + VnA (10 μg/kg) group; D: I/R + VnA (20 μg/kg) group.
Figure 7.
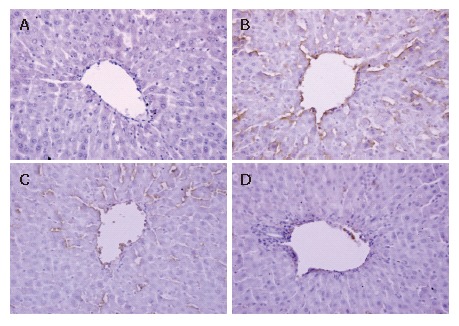
Immunohistochemical staining of E-selectin in liver tissue after ischemia for 90 min and reperfusion for 240 min in rats (× 400). Formalin-fixed, paraffin-embedded liver specimens were stained by the streptavidin/peroxidase immunohistochemistry technique. For all groups, n = 10. A: Control group; B: I/R group; C: I/R + VnA (10 μg/kg) group; D: I/R + VnA (20 μg/kg) group.
Figure 8.
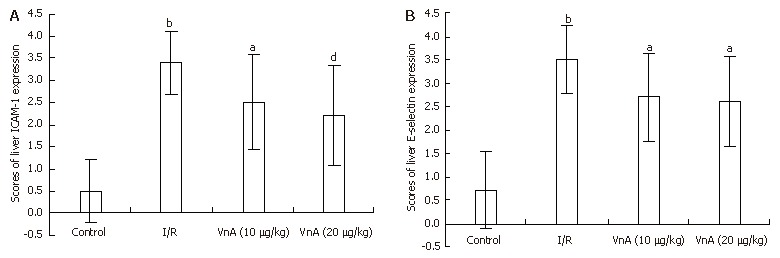
Immunohistochemical results (semi-quantitative analysis) of adhesion molecule-1 (ICAM-1) (A) and E-selectin (B) in liver tissue in different groups. Data were presented as mean ± SD. bP < 0.01 vs Control group; aP < 0.05, dP < 0.01 vs I/R group.
Western blot analysis for liver ICAM-1 and E-selectin
Western blot showed weak positive ICAM-1 and E-selectin signals in the control group. In contrast, a marked increase in ICAM-1 and E-selectin protein expression in the liver tissue was found in the I/R group. Compared with the I/R group, the signals were weakened obviously in the VnA pretreated group (Figure 9).
Figure 9.
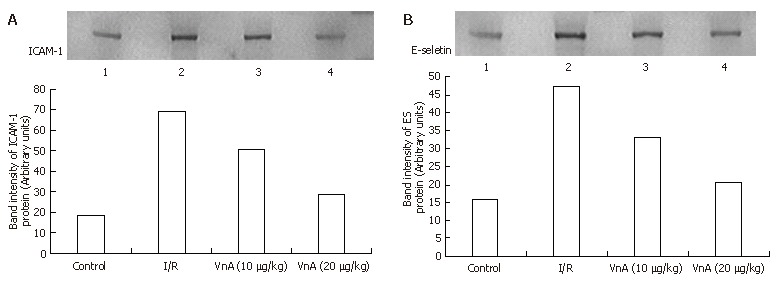
Liver adhesion molecule-1 (ICAM-1) (A) and E-selectin (ES) (B) protein signals of Western blot analyzed with a gel imaging system (Kodak system EDAS120, Japan). For all groups, n = 10. L1: Control group; L2: I/R group; L3: VnA (10 μg/kg) group; L4: VnA (20 μg/kg) group. Compared with the control group, the signals in I/R group increased and weakened in the VnA pretreated group in a dose-dependent manner.
DISCUSSION
Liver I/R injury occurs in a number of clinical settings including liver surgery, transplantation, and hemorrhagic shock. A major disadvantage of this event is an acute inflammatory response that may cause significant organ damage or dysfunction. Experimental evidence demonstrates that kupffer cells play an important part in mediating hepatic I/R[21-22]. Kuppfer cell activation leads to structural changes, formation of vascular ROS and production of proinflammatory cytokines, which in turn induce the expression of adhesion molecules in vascular endothelial cells and stimulate the production and release of neutrophil-attracting chemokines. Neutrophil recruitment in the liver tissue causes direct hepatocellular damage through exhaustion of hepatic microcirculation by blocking the capillary perfusion and releasing ROS and proteases[23-24].
VnA is a well known herbal plant widely distributed in the northeast region of China and has various pharmacological effects including antithrombotic and antihypertensive properties[15,17]. Some researchers have also demonstrated that VnA exerted beneficial effects on brain I/R injury by inhibiting oxidation and leukocyte priming and expression of inflammatory mediators[18]. In the present study, we demonstrated that VnA pretreatment at doses of 10 and 20 μg/kg could attenuate hepatic I/R injury, indicated by improved alteration in liver tissue pathology and liver function, by an enhanced antioxidant capacity with augmentation of free radical scavengers and reduced polymorphonuclear neutrophil (PMN) infiltration, as well as by suppressed overexpression of adhesion molecules and selectins.
Reperfusion of the ischemic liver in rats resulted in hepatic damage with histological evidence of liver hemorrhage, edema, accumulation of adherent leukocytes in sinusoids and terminal hepatic vein (THV), as well as partial exfoliation of blood vessel endothelia. Furthermore, hepatic I/R caused the release of liver enzymes into the blood stream, thereby eliciting a significant increase in plasma levels of ALT and LDH. VnA pretreatment abated liver pathologic injury and reduced plasma ALT and LDH activity, thus liver function was ameliorated.
SOD catalyses the dismutation of the superoxide anion (O2·) into H2O2, which can be transformed into H2O and O2 by catalase (CAT). In this study, we found that I/R impaired SOD activity, as indicated by the markedly lowered activity compared with the control group. But in the VnA pretreated group, the decrease of SOD activity was significantly counteracted. In addition, the hepatic I/R increased the levels of nitric oxide (NO), an important ROS product. This result is consistent with a previous report[25]. NO may combine with superoxide radicals to form peroxynitrite, a substance extremely toxic to cells[26]. VnA pretreatment significantly decreased liver NO content, resulting in a significant reduction in liver damage when compared with the control group. These data indicated that VnA might confer protection on the liver during I/R injury in part by improving activity of the endogenous antioxidant enzyme, which scavenges ROS and reduces their effects.
Because MPO is an enzyme restricted mainly to PMNs, the increase in MPO activity reflects neutrophil tissue infiltration. The significant increase in MPO activity in the liver tissue after hepatic I/R in the present study is consistent with another study[27]. However, VnA significantly blunted the increase of liver MPO activity compared with the I/R group. That is to say, VnA reduced infiltration of leukocytes into the inflammatory sites.
ICAM-1 is a member of the immunoglobulin superfamily that mediates firm adhesion and emigration of activated leukocytes in postcapillary venules, which process may be one of the important steps in the development of tissue injury and organ dysfunction[28]. Previous studies have shown an upregulation of ICAM-1 expression following endothelial cell activation with cytokines or LPS, accompanied by an increased binding of neutrophils and lymphocytes to endothelial cells. An anti-ICAM-1 monoclonal antibody inhibited neutrophil infiltration into pericentral sinusoids and improved and restored liver integrity in I/R injury[29]. In this study we detected overexpression of ICAM-1 after hepatic I/R, which was significantly decreased in the VnA pretreated groups. This indicated that VnA could suppress leukocyte adhesion to endothelia.
Murine E-selectin is a 110 kDa, type-1 transmembrane glycoprotein expressed only in endothelial cells after cytokine activation[30]. E-selectin participates in leukocyte rolling and firm adhesion in vitro[31] and in vivo[32]. It is down-regulated by re-internalization and by shedding from the endothelial surface into the plasma[33]. Previous studies revealed that blockade of E-selectin protected from severe acute renal failure induced by ischemia-reperfusion[34]. In our study, E-selectin was also found to be overexpressed after hepatic I/R, however, this was attenuated in the VnA pretreated groups as compared with the I/R group.
The above results suggested that ICAM-1 and E-selectin dependent neutrophil recruitment into the liver tissue was responsible partially for hepatic I/R. The dose-dependent protective effects of VnA were likely related to the suppression of ICAM-1 and E-selectin expression. This suppression reduced the neutrophil recruitment associated with the hepatic I/R, and effectively protected the liver against ischemia/reperfusion.
In conclusion, VnA has protective effects against liver injury induced by hepatic I/R. The protective effects are probably associated with enhancement of antioxidant capacities, reduction of inflammatory responses and suppressed expression of ICAM-1 and E-selectin. These findings may be useful for clinical management to reduce I/R related hepatic damage. However, the precise mechanisms of VnA protecting against hepatic I/R still need to be clarified in further studies.
ACKNOWLEDGMENTS
The authors thank Professors Sen Lü and Min Liu, Laboratory of Molecular Biology (State Administration of Traditional Chinese Medicine), Second Affiliated Hospital of Dalian Medical University for their technical assistance. The excellent technical assistance of Dr. Yu-Zhong Li and Zhen-Guo Li (Department of Docimasiology, Second Affiliated Hospital of Dalian Medical University) are acknowledged.
Footnotes
S- Editor Wang GP L- Editor Zhu LH E- Editor Bai SH
References
- 1.Haimovici H. Arterial embolism with acute massive ischemic myopathy and myoglobinuria: evaluation of a hitherto unreported syndrome with report of two cases. Surgery. 1960;47:739–747. [PubMed] [Google Scholar]
- 2.Cheng F, Li YP, Cheng JQ, Feng L, Li SF. The protective mechanism of Yisheng Injection against hepatic ischemia reperfusion injury in mice. World J Gastroenterol. 2004;10:1198–1203. doi: 10.3748/wjg.v10.i8.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozturk H, Gezici A, Ozturk H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatol Res. 2006;34:76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Gu XP, Qiu YD, Sun XM, Chen LL, Zhang LH, Ding YT. Ischemic preconditioning decreases C-X-C chemokine expression and neutrophil accumulation early after liver transplantation in rats. World J Gastroenterol. 2003;9:2025–2029. doi: 10.3748/wjg.v9.i9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368–374. [PubMed] [Google Scholar]
- 9.Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342–345. doi: 10.3748/wjg.v8.i2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 11.Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. doi: 10.1002/hep.510250424. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636–1640. doi: 10.1046/j.1365-2168.1998.00917.x. [DOI] [PubMed] [Google Scholar]
- 13.Tezuka Y, Kikuchi T, Zhao W, Chen J, Guo Y. (+)-Verussurine, a new steroidal alkaloid from the roots and rhizomes of veratrum nigrum var. ussuriense and structure revision of (+)-verabenzoamine1. J Nat Prod. 1998;61:1397–1399. doi: 10.1021/np9800811. [DOI] [PubMed] [Google Scholar]
- 14.Zhao WJ, Meng QW, Wang SS. Studies on Chemical Constituents of Veratrum nigrum L. var. ussuriense Nakai. Zhongguo Zhongyao Zazhi. 2003;28:884–885. [Google Scholar]
- 15.Li H, Gao GY, Li SY, Zhao WJ, Guo YT, Liang ZJ. Effects of Veratrum nigrum alkaloids on central catecholaminergic neurons of renal hypertensive rats. Acta Pharmacol Sin. 2000;21:23–28. [PubMed] [Google Scholar]
- 16.Li WP, Gao DY, Zhou Q, Gao GY, Han GZ, Zhao WJ. Neuroprotective effects of VnA on rats with focal cerebral ischemia injury. Zhongguo Yaoxue Zazhi. 2002;37:624. [Google Scholar]
- 17.Han GZ, Li XY, Lü L, Li WP, Zhao WJ. Antithrombotic effects of Veratrum nigrum var. ussuriense alkaloids. Zhongcaoyao. 2003;34:1107–1110. [Google Scholar]
- 18.You GX, Zhou Q, Li WP, Han GZ, Zhao WJ. Neuroprotection of Veratrum nigrum var. ussurience alkaloids on focal cerebral ischemia-reperfusion injury in rats. Zhongcaoyao. 2004;35:908–911. [Google Scholar]
- 19.Kojima Y, Suzuki S, Tsuchiya Y, Konno H, Baba S, Nakamura S. Regulation of pro-inflammatory and anti-inflammatory cytokine responses by Kupffer cells in endotoxin-enhanced reperfusion injury after total hepatic ischemia. Transpl Int. 2003;16:231–240. doi: 10.1007/s00147-002-0536-4. [DOI] [PubMed] [Google Scholar]
- 20.Song M, Xia B, Li J. Effects of topical treatment of sodium butyrate and 5-aminosalicylic acid on expression of trefoil factor 3, interleukin 1beta, and nuclear factor kappaB in trinitrobenzene sulphonic acid induced colitis in rats. Postgrad Med J. 2006;82:130–135. doi: 10.1136/pgmj.2005.037945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JX, Wu HS, Wang H, Zhang JH, Wang Y, Zheng QC. Protection against hepatic ischemia/reperfusion injury via downregulation of toll-like receptor 2 expression by inhibition of Kupffer cell function. World J Gastroenterol. 2005;11:4423–4426. doi: 10.3748/wjg.v11.i28.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schümann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, Tiegs G. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157:1671–1683. doi: 10.1016/S0002-9440(10)64804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000;15:718–724. doi: 10.1046/j.1440-1746.2000.02207.x. [DOI] [PubMed] [Google Scholar]
- 24.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 25.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karaman A, Fadillioglu E, Turkmen E, Tas E, Yilmaz Z. Protective effects of leflunomide against ischemia-reperfusion injury of the rat liver. Pediatr Surg Int. 2006;22:428–434. doi: 10.1007/s00383-006-1668-x. [DOI] [PubMed] [Google Scholar]
- 27.Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825–1828. doi: 10.3748/wjg.v11.i12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olanders K, Sun Z, Börjesson A, Dib M, Andersson E, Lasson A, Ohlsson T, Andersson R. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86–92. doi: 10.1097/00024382-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Nakano H, Nagasaki H, Yoshida K, Kigawa G, Fujiwara Y, Kitamura N, Kuzume M, Takeuchi S, Sasaki J, Shimura H, et al. N-acetylcysteine and anti-ICAM-1 monoclonal antibody reduce ischemia-reperfusion injury of the steatotic rat liver. Transplant Proc. 1998;30:3763. doi: 10.1016/s0041-1345(98)01225-1. [DOI] [PubMed] [Google Scholar]
- 30.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 32.Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- 33.Subramaniam M, Koedam JA, Wagner DD. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell. 1993;4:791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singbartl K, Ley K. Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-selectin. Crit Care Med. 2000;28:2507–2514. doi: 10.1097/00003246-200007000-00053. [DOI] [PubMed] [Google Scholar]


