Abstract
AIM: To investigate the role of glutathione S-transferase (GST) and matrix metalloproteinase-9 (MMP-9) expressions in the development and progression of reflux esophagitis-Barrett’s metaplasia-dysplasia-adenocarcinoma sequence in the esophagus.
METHODS: GST and MMP-9 expressions were analyzed in 51 paraffin-embedded tissue samples by immunohistochemistry including patients with reflux esophagitis (n = 7), Barrett’s metaplasia (n = 14), Barrett and esophagitis (n = 8), Barrett and dysplasia (n = 7), esophageal adenocarcinoma (n = 8) and a control group without any histological changes (n = 7). Immunostaining was determined semiquantitatively. Statistical analysis with one-way ANOVA, LSD test and correlation analysis were performed. P value of < 0.05 was considered significant.
RESULTS: GST expression was significantly higher while MMP-9 expression was significantly lower in control group compared to Barrett’s metaplasia and the other groups. No major changes were observed between Barrett, esophagitis, and Barrett and concomitant esophagitis. Barrett and concomitant dysplasia, and adenocarcinoma revealed a significant lower expression of GST and higher levels of MMP-9 compared to all other groups. Adenocarcinoma showed almost no expression of GST and significantly higher levels of MMP-9 than Barrett and concomitant dysplasia. Alterations of GST and MMP-9 were inversely correlated (r = - 0.82).
CONCLUSION: Decreased GST and increased expression of MMP-9 in Barrett’s metaplasia-dysplasia-adenocarcinoma sequence as compared to normal tissue suggest their association with esophageal tumorigenesis. Loss of GST and gain of MMP-9 in Barrett with dysplasia compared to non-dysplastic metaplasia indicate that these alterations may be early events in carcinogenesis. Quantification of these parameters in Barrett’s esophagus might be useful to identify patients at higher risk for progression to cancer.
Keywords: Glutathione S-transferase, Matrix metallo-proteinase-9, Barrett’s metaplasia, Esophagus, Adenocarcinoma, Dysplasia
INTRODUCTION
Esophageal cancer is still one of the most widespread diseases, and the early diagnosis of esophageal carcinoma correlates closely with improvement in prognosis. Barrett's esophagus (BE) is a precancerous condition of the lower esophagus in which the normal stratified squamous epithelium is replaced with specialized metaplastic columnar epithelium. Barrett’s mucosa represents a type of epithelium that is completely different from the normal esophageal mucosa. BE is the main precancerous condition in the development of esophageal adenocarcinoma[1,2].
BE is diagnosed in up to 20% of patients with documented chronic gastroesophageal reflux disease (GERD). Follow-up studies have shown that BE has a 30- to 125-fold increased risk of developing into an adenocarcinoma, which emerges at a rate of approximately one cancer per 100 patient-years[3]. Barrett’s adenocarcinoma displays the most rapidly increasing incidence for gastrointestinal tract cancer in the Western world. Diagnosis of Barrett’s adenocarcinoma is usually made late, and consequently, is associated with poor prognosis[4-6].
Carcinogens are one of the inducing etiological factors for esophageal adenocarcinoma. Glutathione S-transferase (GST), a family of detoxification enzymes, plays an important role in the prevention of cancer by detoxifying numerous potentially carcinogenic compounds, which can cause oxidative damage to cells[7]. Therefore, a reduction in these anti-oxidant enzymes can increase the risk of carcinogenesis[8]. Decreased GST enzyme activity has been reported in BE, and an inverse correlation was demonstrated between GST enzyme activity and tumor incidence in the gastrointestinal tract[9,10]. It has been suggested that down-regulation of GST expression could be an early event in the development of BE[11].
The degradation of the extracellular matrix (ECM), including the basement membrane, which is a specialized matrix composed of type IV collagen, laminin, entactin, proteoglycans and glycosaminoglycans, is an important feature of cancer cell invasion, and proteolytic enzymes play an important role in this event[12].
Several human solid tumors have been reported to have increased levels of proteolytic enzymes in cancer tissue, strongly suggesting that proteases may be important in tumor invasion and metastasis. With respect to the gastrointestinal tract, we have previously demonstrated that proteolytic enzymes may have a role not only in the process of gastric[13] or colorectal cancer invasion[14], but also in the progression of gastrointestinal precancerous changes into cancer[15].
Matrix metalloproteinases (MMPs) degrade components of the ECM and connective tissue surrounding the tumor cells and the basement membrane. MMPs are classified as gelatinases, collagenases, stromelysins, membrane-type matrix metalloproteinases, based mainly on the in vivo substrate specificity of the individual MMP. It was initially believed that MMPs, via breakdown of the physical barrier, were primarily involved in tumor invasion[16]. There is growing evidence, however, that the MMPs have an expanded role, as they are important for the creation and maintenance of a microenvironment that facilitates growth and angiogenesis of tumors at primary and metastatic sites[17,18].
Type IV collagen is an important protein of the basement membrane. Type IV collagenase, matrix metalloproteinase-9 (MMP-9) (gelatinase B), has been reported to be especially important in the process of tumor invasion and metastasis[19,20]. Several MMPs (gelatinase A: MMP-2; stromelysin: MMP-3; matrilysin: MMP-7; metalloelastase: MMP-12; collagenase-3: MMP-13) are expressed by tumor cells in esophageal squamous cell and adenocarcinomas, suggesting that these MMPs are responsible for tumor aggressiveness and prognosis in human esophageal carcinomas[21-24].
In the specific case of MMP-9, increased expressions have been observed in gastric cancer[25-27] and esophageal squamous cell carcinoma[28-30], but its behaviour in esophageal adenocarcinoma and in preinvasive lesions of esophageal carcinogenesis is still uncertain.
On the other hand, GST and MMP-9 as actors either in cancer prevention or in carcinogenesis have not been evaluated in the same experimental setting. Therefore, the aim of the present study was to investigate the role of GST and MMP-9 expressions using immunohistochemical analysis in the development and progression of reflux esophagitis-BE-dysplasia-adenocarcinoma sequence in the esophagus.
MATERIALS AND METHODS
Tissue specimens were obtained endoscopically from in- and outpatients with upper abdominal complaints at the 2nd Department of Medicine, Semmelweis University, Budapest.
Informed consent was obtained from all patients involved in the study, and a local ethical permission has been obtained. The patients comprised of 33 males and 18 females. The median age was 64 years with a range from 22 to 83 years. The endoscopic specimens were fixed in formalin and embedded in paraffin wax, sliced serial step sections of 4 μm thickness. GST and MMP-9 immunohistochemical expressions were analyzed in a total of 51 paraffin-embedded tissue samples by immunohistochemistry including patients with reflux esophagitis (n = 7) (4 males, 3 females, mean age 61 years, range 36-68 years); BE (n = 14) (9 males, 5 females, mean age 66 years, range 48-69 years); BE and esophagitis (n = 8) (6 males, 2 females, mean age 67 years, range 55-71 years); BE and dysplasia (n = 7) (4 males, 3 females, mean age 68 years, range 52-72 years); and esophageal adenocarcinoma (n = 8) (6 males, 2 females, mean age 71 years, range 64-83 years). Esophageal biopsies from patients with functional dyspepsia without any histological changes were used as controls (n = 7) (4 males, 3 females, mean age 49 years, range 22-56 years).
GST immunohistochemistry
The 4 micron thick tissue sections were dewaxed and rehydrated. Endogenous peroxidase activity was blocked by incubation for 30 min at room temperature in 3% hydrogen peroxide. After washing the sections 3 times in PBS for 5 min, non-specific blocking was done with 1% BSA-PBS solution for 10 min at room temperature. Next, the slides were incubated with diluted polyclonal rabbit anti-human GSTP1 antibody (1 μL GSTP1 antibody and 150 μL PBS) (Clone: A3600, DAKO) at 37°C for 60 min in a humidified chamber. After washing the specimens 3 times in PBS, signal conversion was carried out with the LSAB2 system (DAKO) as described in the manual. Finally, haematoxylin co-staining was performed.
MMP-9 immunohistochemistry
After deparaffinization in xylene and rehydration through graded ethanol, endogenous peroxidase activity was blocked by incubation for 30 min at room temperature in 3% hydrogen peroxide. After washing the sections 3 times in PBS for 5 min, non-specific blocking was carried out with 1% BSA-PBS solution for 10 min at room temperature. Next, the slides were incubated with optimally diluted monoclonal anti-human MMP-9 antibody (Clone: 36 020.111, R&D Systems) at 37°C for 60 min in a humidified chamber. After washing the samples 3 times in PBS, signal conversion was carried out with the LSAB2 system (DAKO) as described in the manual. Finally, haematoxylin co-staining was performed.
Immunohistochemical analysis of GST and MMP-9
Known immunohistochemically-positive tissue sections were used as positive controls, and negative control sections were processed immunohistochemically after having replaced the primary antibody by PBS. None of the control sections exhibited immunoreactivity. Immunostaining was determined semiquantitatively, as previously described[31]. Essentially, the intensity of staining for GST and MMP-9 under a light microscope was graded from 0 to 3, denoting no staining or light, moderate, or intense staining. An immunohistochemical staining score was calculated for each histologic area by multiplying the staining intensity level (0 to 3) by the proportion of cells in each area staining with the given intensity. The immunohistochemical staining score for an area with 100% of cells with intense staining, for example, would be 1 × 3, equalling 3, whereas an area with 50% cells with moderate staining and 40% without any staining would have a score of 0.5 × 2 plus 0.4 × 1, equalling 1.4. Two independent investigators without knowledge of the clinical outcomes evaluated the degree of immunohistochemical staining intensity. There was less than 5% variance between the results of the two counts.
Statistical analysis
Statistical analysis with one-way ANOVA, LSD test and correlation analysis were performed by the Statistica for Windows 4.3 program package. P value of < 0.05 was considered significant.
RESULTS
The immunohistochemical expression scores of GST and MMP-9 in various types of mucosal lesions of the esophagus (n = 51) are shown in Tables 1 and 2.
Table 1.
GST immunohistochemical expression according to a semiquantitative score in various types of mucosal lesions of the esophagus
| Histology | Score (mean ± SD) |
| Normal epithelium (Control group)a (n = 7) | 2.85 ± 0.24 |
| Reflux esophagitis (n = 7) | 1.14 ± 0.24 |
| Barrett’s metaplasia (n = 14) | 1.60 ± 0.34 |
| Barrett’s metaplasia and reflux esophagitis (n = 8) | 1.12 ± 0.35 |
| Barrett’s metaplasia and dysplasiab (n = 7) | 0.58 ± 0.37 |
| Adenocarcinomabc (n = 8) | 0.18 ± 0.25 |
P < 0.00001 vs the other groups;
P < 0.005 vs the other groups (normal epithelium, reflux esophagitis, barrett's metaplasia, barrett's metaplasia and Reflux esophagitis);
P < 0.05 vs barrett's metaplasia and dysplasia.
Table 2.
MMP-9 immunohistochemical expression according to a semiquantitative score in various types of mucosal lesions of the esophagus
| Histology | Score (mean ± SD) |
| Normal epithelium (Control goup)b (n = 7) | 0.28 ± 0.39 |
| Reflux esophagitis (n = 7) | 1.71 ± 0.39 |
| Barrett’s metaplasia (n = 14) | 1.46 ± 0.41 |
| Barrett’s metaplasia and reflux esophagitis (n = 8) | 1.75 ± 0.26 |
| Barrett’s metaplasia and dysplasiaa (n = 7) | 2.16 ± 0.25 |
| Adenocarcinomaac (n = 8) | 2.62 ± 0.35 |
P < 0.00001 vs the other groups;
P < 0.05 vs the other groups (normal epithelium, reflux esophagitis, barrett's metaplasia, barrett's metaplasia and Reflux esophagitis);
P < 0.05 vs barrett's metaplasia and dysplasia.
Expression of GST (Table 1) in normal esophageal epithelium (control group) was significantly higher compared to BE and the other groups (P < 0.00001), while no major changes were observed between BE, esophagitis, and BE with concomitant esophagitis.
BE with concomitant dysplasia, and adenocarcinoma revealed a significantly lower expression of GST compared to all other groups (P < 0.005). Adenocarcinoma showed almost no expression of GST and a significantly lower expression than BE and concomitant dysplasia (P < 0.05).
The semiquantitative score of MMP-9 (Table 2) in the normal esophageal epithelium (control group) was significantly lower compared to BE and the other groups (P < 0.00001); while no major changes were observed between BE, esophagitis, and BE with concomitant esophagitis.
Significantly higher expression levels of MMP-9 have been observed in BE with concomitant dysplasia and adenocarcinoma compared to all other groups (P < 0.05). Finally, MMP-9 expression was significantly higher in adenocarcinoma compared to BE and concomitant dysplasia (P < 0.05).
GST and MMP-9 were expressed mainly within the cytoplasm and cytoplasmic membranes of the esophageal epithelium in dysplastic or adenocarcinoma cells (Figures 1 and 2). Immunoexpressions of GST and MMP-9 in the esophageal tissues were inversely correlated (r = - 0.82; P = 0.001) (Figure 3).
Figure 1.
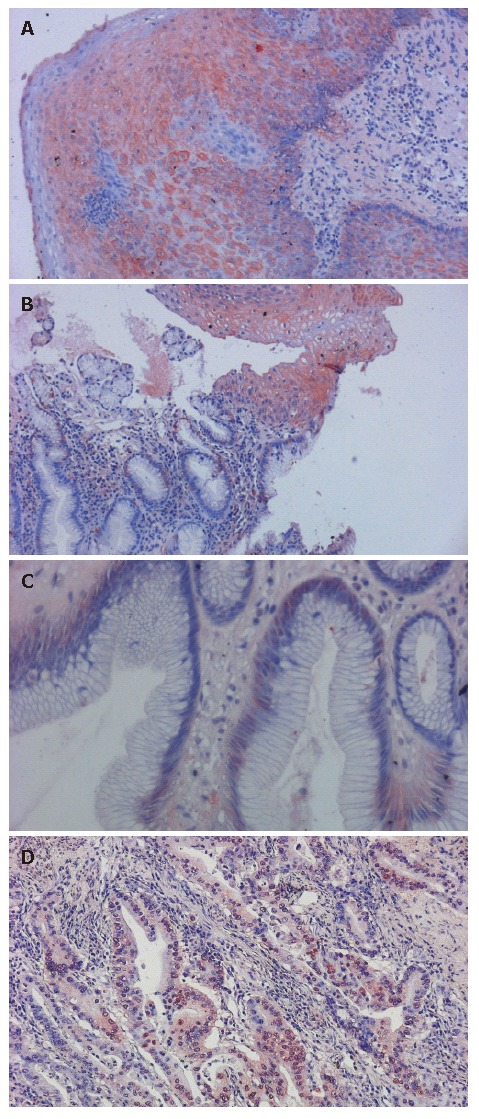
Expression of GST in different esophageal tissues. A: GST strong positive staining was observed in the normal esophagus (200 ×); B: Normal esophageal epithelium (top) with Barrett’s metaplasia (bottom) (200 ×); C: Normal esophageal epithelium shows strong positive immunostaining compared to the weaker GST expression in Barrett’s metaplasia (400 ×); D: Adenocarcinoma showing almost no expression of GST (200 ×); GST was mainly expressed within the cytoplasm.
Figure 2.
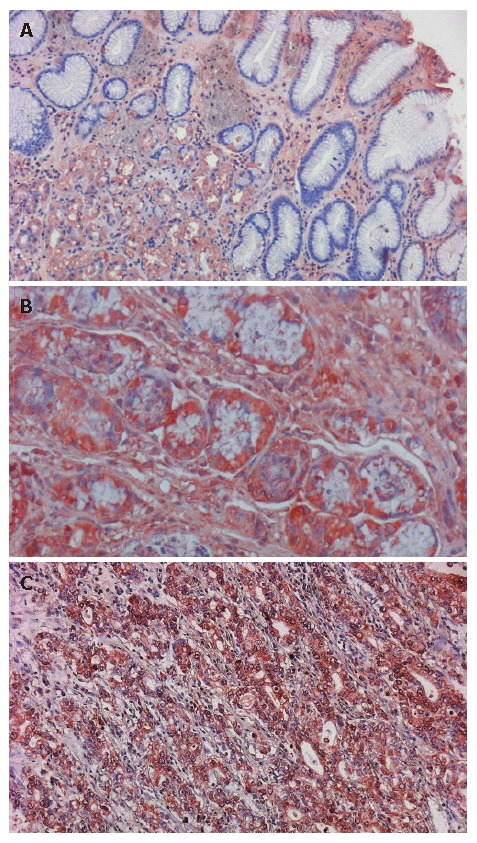
Expression of MMP-9 in different esophageal tissues. Strong positive immunostaining of MMP-9 in (A) Barrett’s metaplasia (400 ×), (B) dysplasia (400 ×) and (C) adenocarcinoma (200 ×) of the esophagus. Cytoplasm of the metaplastic and dysplastic cells and cytoplasmic membranes of the esophageal adenocarcinoma cells were stained brown. Barrett’s metaplasia with concomitant dysplasia and adenocarcinoma show the most intensive expression of MMP-9.
Figure 3.
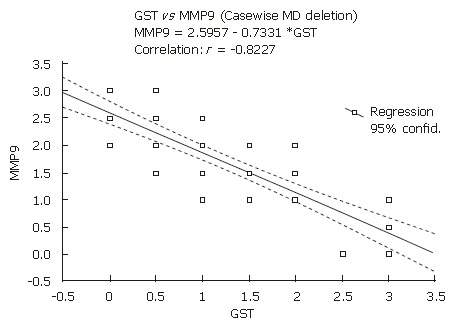
The correlation between immunohistochemical expressions of GST and MMP9 in different esophageal tissues. Immunohistochemical expressions of GST and MMP9 were inversely correlated (r = - 0.82; P = 0.001).
DISCUSSION
Despite advances in diagnosis and therapy, esophageal adenocarcinoma remains an aggressive and usually lethal tumor. BE is the main precancerous condition in the development of esophageal adenocarcinoma; however, its pathogenesis is poorly understood. BE typically progresses from metaplasia with atypia to dysplasia and adenocarcinoma. It is of great clinical importance to correctly identify changes with a high risk for malignant transformation, as high-grade dysplasias and early adenocarcinomas in patients with BE have a high chance for cure[32]. The identification of high-risk lesions in BE by histologic evaluation has drawbacks, especially regarding sampling errors and frequent intra- and inter-observer discrepancies in the histopathologic grading/staging of these lesions. Several new biomarkers are being tested to help in better determining the risk of cancer development. Although most of the biological markers need to be evaluated further, at present, aneuploidy status, p16 and p53 gene abnormalities, or allelic losses are the most extensively documented alterations[33].
Immunostaining with a variety of antibodies provides a better understanding of the process of malignant transformation and helps to identify early markers of malignant transformation in BE[34].
Given the lack in the literature of the evaluation of GST and MMP-9 expressions in the same experimental setting, we evaluated the behaviour of detoxification enzyme GST, and one member of the matrix metalloproteinases family, MMP-9, in the development and progression of normal epithelium, reflux esophagitis, BE, dysplasia and adenocarcinoma sequence in the esophagus.
A number of findings in our study confirmed that GST is involved in esophageal carcinogenesis and progression. We have demonstrated that GST expression was significantly higher in normal esophageal epithelium compared to the other groups. On the other hand, BE with dysplasia, and adenocarcinoma revealed a significantly lower expression of GST, while adenocarcinoma expressed almost no GST.
Our findings are similar to the results reported by van Lieshout et al[9] and Cobbe et al[11]. They reported that the expression of GST appeared to be reduced in BE compared to normal esophageal squamous epithelium. In contrast to the van Lieshout et al[9] and Cobbe et al[11] studies, we also demonstrated that BE with concomitant dysplasia, and adenocarcinoma revealed a significantly lower expression of GST. Brabander et al[10] also found that GST expression was highest in the basal layer of normal esophageal squamous epithelium and lowest in adenocarcinoma cells, with BE cells showing intermediate staining intensity.
These results suggest that decreased GST expression could be an early event in the development of BE and may contribute to the risk of development and progression of adenocarcinoma in BE. The observed reduction in GST expression in BE may, therefore, contribute to the increased risk in this tissue.
Degradation of the ECM and basement membrane by tumor cells is a critical step in the process of tumor invasion and metastasis. MMP-9 is one member of the matrix metalloproteinases family, which is capable of degrading several components of the ECM. Increased expression of MMP-9 has been found in various carcinomas. With respect to the gastrointestinal tract, increased MMP-9 expressions have been observed in gastric[25-27] and colorectal cancer[35-38]. In the specific case of the esophagus, increased expression of MMP-9 has been demonstrated in esophageal squamous cell carcinoma[28-30], but its role and behaviour in esophageal adenocarcinoma and BE is not well established.
The relatively small number of patients in our study can be explained by the known data about the epidemiology of BE and esophageal adenocarcinoma in Hungary; since only 4% of patients with esophageal cancers were diagnosed to have adenocarcinoma and its proportion remained stable over the observed last decade, it seems that contrary to North American and Western European countries, the prevalence of adenocarcinoma has been, until now, very low in Hungary[39].
In the present study, immunohistochemical analysis revealed a progressive increase in the expression of MMP-9 with increasing severity of esophageal lesions. MMP-9 expression was significantly lower in normal esophageal epithelium compared to other groups. BE with concomitant dysplasia revealed a significantly higher expression of MMP-9 compared to BE, reflux esophagitis or BE with concomitant esophagitis. We observed that MMP-9 expression was significantly higher in adenocarcinoma compared to BE or BE with concomitant dysplasia. These results suggest that over-expression of MMP-9 plays an important role in the progression to esophageal adenocarcinoma, and MMP-9 protein may serve as a marker for invasiveness. Our results indicate that the activation of MMP-9 may be an early event in esophageal carcinogenesis.
Our findings are relevant from both, biological and clinical points of view. Despite the advance in preoperative and postoperative medical care of esophageal carcinoma patients, their prognosis has improved only marginally. Therefore, it would be useful to have additional biomarkers to help clinicians better determine the risk of esophageal cancer development. In esophageal cancer, novel targeted treatments are still in an early phase of development. It can be speculated that the relevance of MMP-9 in esophageal carcinogenesis may also support a possible therapeutic approach[40]. Indeed, this can be obtained directly by inhibition of MMP-9. Phase II-trials with the matrix metalloproteinase inhibitor prinomastat in patients with esophageal adenocarcinoma are under evaluation[41].
The present study showed that expressions of GST and MMP-9 were reversely or negatively correlated, thus suggesting a concomitant down-regulation and up-regulation, respectively, of these systems. GST plays an important protective role in the prevention of cancer by detoxifying potentially carcinogenic compounds, while MMP-9 should be considered an aggressive factor, playing a crucial role in the progression of esophageal carcinogenesis.
In conclusion, our results demonstrate a significantly lower expression of GST and a significantly higher expression of MMP-9, respectively, in the BE-dysplasia-adenocarcinoma sequence as compared to normal esophageal tissue. The simultaneous down-regulation of GST and up-regulation of MMP-9 strongly suggest their association with esophageal tumorigenesis and particularly, their specific role in the biology of esophageal adenocarcinoma. Loss of GST and gain of MMP-9 in BE with concomitant dysplasia compared to non-dysplastic BE indicate that these alterations may be early events in esophageal carcinogenesis. Together with other biological markers, quantification of these parameters in BE might be useful to identify patients at higher risk for progression to adenocarcinoma, to prevent tumor development and to improve prognosis.
Footnotes
S- Editor Liu Y L- Editor Lakatos PL E- Editor Liu WF
References
- 1.Shaheen NJ. Advances in Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 2005;128:1554–1566. doi: 10.1053/j.gastro.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett's metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim R, Weissfeld JL, Reynolds JC, Kuller LH. Etiology of Barrett's metaplasia and esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 1997;6:369–377. [PubMed] [Google Scholar]
- 4.Olliver JR, Hardie LJ, Gong Y, Dexter S, Chalmers D, Harris KM, Wild CP. Risk factors, DNA damage, and disease progression in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:620–625. doi: 10.1158/1055-9965.EPI-04-0509. [DOI] [PubMed] [Google Scholar]
- 5.Wong A, Fitzgerald RC. Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005;3:1–10. doi: 10.1016/s1542-3565(04)00602-0. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski JA, Anderson M. Review article: management of oesophageal adenocarcinoma -- control of acid, bile and inflammation in intervention strategies for Barrett's oesophagus. Aliment Pharmacol Ther. 2004;20 Suppl 5:71–80; discussion 95-96. doi: 10.1111/j.1365-2036.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Zhang SH, Zhang KH, Li BM, Chen J. Value of endoscopic methylene blue and Lugol's iodine double staining and detection of GST-Pi and telomerase in the early diagnosis of esophageal carcinoma. World J Gastroenterol. 2005;11:6090–6095. doi: 10.3748/wjg.v11.i39.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coles B, Ketterer B. The role of glutathione and glutathione transferases in chemical carcinogenesis. Crit Rev Biochem Mol Biol. 1990;25:47–70. doi: 10.3109/10409239009090605. [DOI] [PubMed] [Google Scholar]
- 9.van Lieshout EM, Tiemessen DM, Witteman BJ, Jansen JB, Peters WH. Low glutathione and glutathione S-transferase levels in Barrett's esophagus as compared to normal esophageal epithelium. Jpn J Cancer Res. 1999;90:81–85. doi: 10.1111/j.1349-7006.1999.tb00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brabender J, Lord RV, Wickramasinghe K, Metzger R, Schneider PM, Park JM, Hölscher AH, DeMeester TR, Danenberg KD, Danenberg PV. Glutathione S-transferase-pi expression is downregulated in patients with Barrett's esophagus and esophageal adenocarcinoma. J Gastrointest Surg. 2002;6:359–367. doi: 10.1016/s1091-255x(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Cobbe SC, Scobie GC, Pohler E, Hayes JD, Kernohan NM, Dillon JF. Alteration of glutathione S-transferase levels in Barrett's metaplasia compared to normal oesophageal epithelium. Eur J Gastroenterol Hepatol. 2003;15:41–47. doi: 10.1097/00042737-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–5059s. [PubMed] [Google Scholar]
- 13.Plebani M, Herszènyi L, Cardin R, Roveroni G, Carraro P, Paoli MD, Rugge M, Grigioni WF, Nitti D, Naccarato R. Cysteine and serine proteases in gastric cancer. Cancer. 1995;76:367–375. doi: 10.1002/1097-0142(19950801)76:3<367::aid-cncr2820760304>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Herszènyi L, Plebani M, Carraro P, De Paoli M, Roveroni G, Cardin R, Tulassay Z, Naccarato R, Farinati F. The role of cysteine and serine proteases in colorectal carcinoma. Cancer. 1999;86:1135–1142. doi: 10.1002/(sici)1097-0142(19991001)86:7<1135::aid-cncr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Farinati F, Herszényi L, Plebani M, Carraro P, De Paoli M, Cardin R, Roveroni G, Rugge M, Nitti D, Grigioni WF, et al. Increased levels of cathepsin B and L, urokinase-type plasminogen activator and its inhibitor type-1 as an early event in gastric carcinogenesis. Carcinogenesis. 1996;17:2581–2587. doi: 10.1093/carcin/17.12.2581. [DOI] [PubMed] [Google Scholar]
- 16.Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996;119:209–215. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 18.Auvinen MI, Sihvo EI, Ruohtula T, Salminen JT, Koivistoinen A, Siivola P, Rönnholm R, Rämö JO, Bergman M, Salo JA. Incipient angiogenesis in Barrett's epithelium and lymphangiogenesis in Barrett's adenocarcinoma. J Clin Oncol. 2002;20:2971–2979. doi: 10.1200/JCO.2002.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 20.Roeb E, Schleinkofer K, Kernebeck T, Pötsch S, Jansen B, Behrmann I, Matern S, Grötzinger J. The matrix metalloproteinase 9 (mmp-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J Biol Chem. 2002;277:50326–50332. doi: 10.1074/jbc.M207446200. [DOI] [PubMed] [Google Scholar]
- 21.Shima I, Sasaguri Y, Kusukawa J, Yamana H, Fujita H, Kakegawa T, Morimatsu M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer. 1992;70:2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita K, Mori M, Shiraishi T, Shibuta K, Sugimachi K. Clinical significance of matrix metalloproteinase-7 expression in esophageal carcinoma. Clin Cancer Res. 2000;6:1169–1174. [PubMed] [Google Scholar]
- 23.Salmela MT, Karjalainen-Lindsberg ML, Puolakkainen P, Saarialho-Kere U. Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett's oesophageal adenocarcinoma. Br J Cancer. 2001;85:383–392. doi: 10.1054/bjoc.2001.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etoh T, Inoue H, Yoshikawa Y, Barnard GF, Kitano S, Mori M. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut. 2000;47:50–56. doi: 10.1136/gut.47.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Li L, Lin JY, Lin H. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. 2003;9:899–904. doi: 10.3748/wjg.v9.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun WH, Sun YL, Fang RN, Shao Y, Xu HC, Xue QP, Ding GX, Cheng YL. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in gastric carcinoma and its correlation with angiogenesis. Jpn J Clin Oncol. 2005;35:707–713. doi: 10.1093/jjco/hyi196. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JF, Zhang YP, Hao FY, Zhang CX, Li YJ, Ji XR. DNA ploidy analysis and expression of MMP-9, TIMP-2, and E-cadherin in gastric carcinoma. World J Gastroenterol. 2005;11:5592–5600. doi: 10.3748/wjg.v11.i36.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama H, Iwata H, Kuwabara Y, Iwase H, Kobayashi S, Fujii Y. Gelatinolytic activity of matrix metalloproteinase-2 and -9 in oesophageal carcinoma; a study using in situ zymography. Eur J Cancer. 2000;36:2164–2170. doi: 10.1016/s0959-8049(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 29.Samantaray S, Sharma R, Chattopadhyaya TK, Gupta SD, Ralhan R. Increased expression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:37–44. doi: 10.1007/s00432-003-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto H, Vinitketkumnuen A, Adachi Y, Taniguchi H, Hirata T, Miyamoto N, Nosho K, Imsumran A, Fujita M, Hosokawa M, et al. Association of matrilysin-2 (MMP-26) expression with tumor progression and activation of MMP-9 in esophageal squamous cell carcinoma. Carcinogenesis. 2004;25:2353–2360. doi: 10.1093/carcin/bgh270. [DOI] [PubMed] [Google Scholar]
- 31.Hritz I, Kuester D, Vieth M, Herszenyi L, Stolte M, Roessner A, Tulassay Z, Wex T, Malfertheiner P. Secretory leukocyte protease inhibitor expression in various types of gastritis: a specific role of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2006;18:277–282. doi: 10.1097/00042737-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Theisen J, Nigro JJ, DeMeester TR, Peters JH, Gastal OL, Hagen JA, Hashemi M, Bremner CG. Chronology of the Barrett's metaplasia-dysplasia-carcinoma sequence. Dis Esophagus. 2004;17:67–70. doi: 10.1111/j.1442-2050.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 33.Krishnadath KK, Reid BJ, Wang KK. Biomarkers in Barrett esophagus. Mayo Clin Proc. 2001;76:438–446. doi: 10.4065/76.4.438. [DOI] [PubMed] [Google Scholar]
- 34.Kleeff J, Friess H, Liao Q, Büchler MW. Immunohistochemical presentation in non-malignant and malignant Barrett's epithelium. Dis Esophagus. 2002;15:10–15. doi: 10.1046/j.1442-2050.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- 35.Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res. 2004;10:8229–8234. doi: 10.1158/1078-0432.CCR-04-0424. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi T, Hisanaga M, Nagao M, Ikeda N, Fujii H, Koyama F, Mukogawa T, Matsumoto H, Kondo S, Takahashi C, et al. The membrane-anchored matrix metalloproteinase (MMP) regulator RECK in combination with MMP-9 serves as an informative prognostic indicator for colorectal cancer. Clin Cancer Res. 2004;10:5572–5579. doi: 10.1158/1078-0432.CCR-03-0656. [DOI] [PubMed] [Google Scholar]
- 37.Ishida H, Murata N, Tada M, Okada N, Hashimoto D, Kubota S, Shirakawa K, Wakasugi H. Determining the levels of matrix metalloproteinase-9 in portal and peripheral blood is useful for predicting liver metastasis of colorectal cancer. Jpn J Clin Oncol. 2003;33:186–191. doi: 10.1093/jjco/hyg035. [DOI] [PubMed] [Google Scholar]
- 38.Guzińska-Ustymowicz K. MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC) Anticancer Res. 2006;26:1589–1594. [PubMed] [Google Scholar]
- 39.Lakatos PL, Lakatos L, Fuszek P, Lukovich P, Kupcsulik P, Halbász J, Schaff Z, Papp J. Incidence and pathologic distribution of esophageal cancers at the gastro-esophageal junction between 1993-2003. Orv Hetil. 2005;146:411–416. [PubMed] [Google Scholar]
- 40.Tew WP, Kelsen DP, Ilson DH. Targeted therapies for esophageal cancer. Oncologist. 2005;10:590–601. doi: 10.1634/theoncologist.10-8-590. [DOI] [PubMed] [Google Scholar]
- 41.Heath EI, Burtness BA, Kleinberg L, Salem RR, Yang SC, Heitmiller RF, Canto MI, Knisely JP, Topazian M, Montgomery E, et al. Phase II, parallel-design study of preoperative combined modality therapy and the matrix metalloprotease (mmp) inhibitor prinomastat in patients with esophageal adenocarcinoma. Invest New Drugs. 2006;24:135–140. doi: 10.1007/s10637-006-5934-5. [DOI] [PubMed] [Google Scholar]


