Abstract
AIM: To assess the outcome of patients, who under-went transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) and subsequently liver transplantation (OLT) irrespective of tumor size when no tumor progression was observed.
METHODS: Records, imaging studies and pathology of 84 patients with HCC were reviewed. Ten patients were not treated at all, 67 patients had TACE and 35 of them were listed for OLT. Tumor progression was monitored by ultrasound and AFP level every 6 wk. Fifteen patients showed signs of tumor progression without transplantation. The remaining 20 patients underwent OLT. Further records of 7 patients with HCC seen in histological examination after OLT were included.
RESULTS: The patients after TACE without tumor progression underwent transplantation and had a median survival of 92.3 mo. Patients, who did not qualify for liver transplantation or had signs of tumor progression had a median survival of 8.4 mo. The patients without treatment had a median survival of 3.8 mo. Independent of International Union Against Cancer (UICC) stages, the patients without tumor progression and subsequent OLT had longer median survival. No significant difference was seen in the OLT treated patients if they did not fulfill the Milan criteria.
CONCLUSION: Selection of patients for OLT based on tumor progression results in good survival. The evaluation of HCC patients should not only be based on tumor size and number of foci but also on tumor progression and growth behavior under therapy.
Keywords: Liver transplantation, Hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related mortality and the fifth most common cancer[1] worldwide. Nearly 500 000 cases are diagnosed each year in the United States and the incidence increased from 1.4 cases per 100 000 between 1976 and 1980 to 2.4 cases per 100 000 from 1990 to 1995. Furthermore, the associated mortality rate and hospitalization showed an increase of 41% and 46%, which demands a significant challenge in the management of HCC patients[2].
The stage of disease divides HCC therapy into curative versus palliative approaches. Curative treatments are reserved for patients without portal vein invasion or distant metastases and include percutaneous ablation, surgical resection and liver transplantation[3-5]. Palliative attempts include liver-directed therapies, rarely systemic chemotherapy, and are offered when local extra-hepatic spread or distant metastases are present. Palliative therapy via transarterial chemoembolization (TACE) may be offered for unresectable HCC. TACE involves the injection of chemotherapeutic agents into the hepatic artery[6,7]. Multiple large randomized and controlled trials have failed to demonstrate a beneficial effect in survival of patients treated with TACE, but meta-analysis of these trials show a slight beneficial effect in survival in comparison with conservative treatment[8].
For localized HCC in curative situations, which is defined by the absence of macro-vascular invasion and metastatic disease with well-preserved hepatic function, the initial treatment of choice is hepatic resection. In case of cirrhosis, optimal candidates for surgical resection show a single lesion less than 5 cm in size, with no complications of end-stage liver disease and no significant portal hypertension (portal pressure gradient less than 10 mmHg). Nevertheless, after 5 years there are significant recurrence rates (70%) in HCC patients after surgical resection, and the 5-year survival rate is 30%. Taken together only about 5% of patients are ideal candidates for hepatic resection[9-11]. Therefore liver transplantation (OLT) is the only curative approach that addresses the HCC lesion as well as the underlying liver cirrhosis[12-15].
Initial reports of liver transplantation in patients with HCC showed poor outcomes with a recurrence rate up to 50% and a 5-year survival of less than 40%. In these reports many patients underwent OLT in the setting of advanced HCC. As a consequence the Milan criteria have been put forward to provide guidelines that help select HCC patients for curative OLT. The goal of this effort was to achieve comparable survival rates in liver transplanted patients with HCC and patients without concomitant neoplasias[12].
Patients fulfilling the criteria (single nodule < 5 cm or up to three nodules each < 3 cm) have a favorable prognosis with 3-year survival rates of 75% up to 85% and a recurrence rate of less than 15%. However, a retrospective cohort analysis showed comparable survival rates in patients who had solitary nodules less than 6.5 cm or 3 nodules with a combined diameter of less than 8 cm[13], demonstrating that OLT is a potentially curative approach for patients with HCC extending the Milan criteria.
However, the general scarcity of donor livers hampers timely liver transplantation. In the interim specific therapy such as TACE can be initiated to stabilize the patient’s health condition. Because the Milan criteria do not take into account tumor progression following non-surgical intervention strategies, patients treated with TACE cannot be necessarily evaluated on the basis of these criteria. We therefore selected patients for liver transplantation based on the lack of tumor progression during the waiting time and determined the clinical outcome in patients who were treated with TACE and subsequently underwent liver transplantation.
MATERIALS AND METHODS
Study design and characteristics of patients
From January 1995 to March 2002, 77 patients with HCC were seen at the Department of Surgery, University of Goettingen. The diagnosis of HCC was confirmed in all patients either by biopsy of the tumor or by a serum alpha-fetoprotein (AFP) measurement. In addition, in 7 patients who underwent OLT, HCC was diagnosed in the histological examination of the explanted organ.
Patient demographics showed a male: female ratio of 70:14 and a mean age of 59 ± 11.4 years (range 31-84). The main underlying disease of HCC was liver cirrhosis (n = 63, 75%), which could be assigned to the diagnoses of alcohol (n = 23), hepatits C (n = 22) and hepatitis B (n = 18) (Table 1).
Table 1.
Diagnostic chart of patients with HCC
| Diagnoses | n | Age (yr, mean ± SD) |
| No liver disease | 10 | 61 ± 12.2 |
| Fibrosis | 3 | 66.7 ± 0.6 |
| Alcohol induced cirrhosis | 23 | 60 ± 8.3 |
| Hepatitis (C) cirrhosis | 22 | 55.9 ± 9.9 |
| Hepatitis (B) cirrhosis | 18 | 55 ± 14.1 |
| Others1 | 8 | 56 ± 16.9 |
| Total | 84 | 59 ± 11.4 |
Haemochromatosis 2, Caroli-syndrome 1, primary biliary cirrhosis 1, primary sclerosing cholangitis 1, hepatitis C 1, acute liver faliure 1, cryptogene cirrhosis 1. The majority of patients had liver cirrhosis secondary to viral hepatitis and alcohol-related liver disease.
Age, gender, Child-Pugh score and tumor stage of all patients are shown in Tables 2 and 3. At the time of HCC diagnosis, 10 (12%) patients had no evidence of impaired liver function, 28 (33%) patients presented with class A, 29 (35%) with class B, and 17 (20%) with class C impaired liver function according to the Child-Pugh classification. The tumor staging according to the UICC criteria of patients used in this study is shown in Table 3.
Table 2.
Cirrhosis scoring of study patients according to Child-Pugh Score
| Child-Pugh Score | n | Age |
Gender |
|
| (yr, mean ± SD) | Male | Female | ||
| A | 28 | 56 ± 13.2 | 26 | 2 |
| B | 29 | 57 ± 9.7 | 25 | 4 |
| C | 17 | 56 ± 9.1 | 15 | 2 |
| Total | 74 | 56.3 ± 11.3 | 66 | 8 |
HCC was classified at hospital admission
Table 3.
Staging of the patients according to UICC
| UICC stage | n | No cirrhosis | Cirrhosis | Age (yr, mean ± SD) |
| I | 5 | 1 | 4 | 52 ± 11.7 |
| II | 13 | 1 | 12 | 60 ± 13.5 |
| III | 11 | 3 | 8 | 60.8 ± 8.0 |
| IV | 55 | 5 | 50 | 58 ± 11.6 |
| Total | 84 | 10 | 74 | 59 ± 11.4 |
Ten (12%) out of 84 patients were not treated because they died before the treatment was started (n = 4), they refused treatment (n = 1) or TACE could not be done due to their cardio-pulmonary risk (n = 5). The remaining 74 patients were treated as seen in Table 4. In 7 transplanted patients, the HCC was diagnosed after liver transplantation and therefore they were not treated before OLT. In the other 67 patients TACE was done and 35 of them were listed for OLT. The reasons for not listing a patient for liver transplantation were no additional liver cirrhosis (n = 10), age older than 65 (n = 11) and persistent alcohol disease (n = 11). From the initial 35 patients who were listed for OLT, 15 showed tumor progression after TACE and were therefore subsequently removed from the transplantation list. These patients showed a median time of tumor progression of 3.1 mo. The remaining 20 patients underwent OLT with a median time on waiting list of 7.6 mo.
Table 4.
Treatment modalities of patients with HCC
| Treatment | n | No cirrhosis | Cirrhosis |
| No treatment | 10 | 3 | 7 |
| TACE, not listed for OLT | 32 | 6 | 41 |
| TACE, died waiting for OLT | 15 | ||
| TACE and liver transplantation | 20 | 0 | 20 |
| Liver transplantation | 7 | 1 | 6 |
| Total | 84 | 10 | 74 |
Chest X-ray, computed tomography (CT) and staging by the TNM scoring system of the UICC was performed in all the patients. Tumors that were first identified by histopathology of the explanted liver were classified as incidental tumors.
Selection criteria for OLT
Patients were selected for OLT based on the guidelines of Transplantions Gesellschaft (DTG). In addition, patients with extrahepatic tumor manifestation did not qualify for OLT. Tumor size or number of tumors were not taken into account for listing the patient. The patients received TACE and were restaged every 6 wk during waiting time. Evidence of tumor progression resulted in removement of the patients from the waiting list.
Transaterial chemoembolization protocol
Patients were listed for OLT and immediately obtain TACE. TACE was performed in cases of advanced HCC stage or when tumors progressed during the staging work-up every 6 wk. Patients in advanced tumor stage (downstaging group) were listed when they responded to the first TACE treatment cycle. Sixty-seven patients were subjected to selective TACE before transplantation. The chemoembolization solution contained 50 mg epirubicin, 10 mL lipiodol and 3 mL water-soluble contrast material. Embolization was performed until blood flow to the tumor stopped.
The following day CT scanning was performed to determine the lipiodol uptake by the tumor tissue. Each TACE cycle was repeated every 6 wk and ultrasound, CT scan and AFP levels were assessed. Response to TACE is defined as constant size of the tumor and/stable AFP levels. Patients showing a positive response to TACE remained on the waiting list and were monitored by a CT scan (every 3 mo) and determination of AFP level (monthly). Patients with tumor progression under TACE treatment were discharged from the waiting list (non-responder).
Post-transplantation management and follow-up
Immunosuppressive therapy following OLT consisted of a drug regimen of Prograf in combination with corticosteroids. Corticosteroids were gradually tapered and discontinued within 3 mo. Prograf was continued for one year after OLT unless side-effects were seen. The frequency of the outpatient visits thereafter varied according to the patient conditions and types of compli-cations. No anti-cancer treatment was given after transplantation. All patients were followed up weekly in the outpatient clinic for the first month after discharged from the hospital. Screening for tumor recurrence was assessed by determination of a-fetoprotein (AFP) and ultrasonography every 3 mo. A routine CT scan of the abdomen and chest was performed every year, and additional imaging techniques (bone scan, magnetic resonance imaging) were done if HCC recurrence was suspected. The medical records and pathologic reports were analyzed retrospectively.
Statistical analysis
We analyzed the statistical significance on recurrence and survival of tumor-related risk factors, tumor size, number of nodules, and Milan criteria. We used the Kaplan-Meier method to measure survival and the log-rank test to analyze statistical differences. Results are expressed as mean ± SD. One-way ANOVA was used for comparisons between the groups when one measurement per experiment was available. Non-normally distributed data was calculated after rank transformation. Data were calculated by univariate ANOVA. Tukey’s-HSD test was used for post hoc comparisons. P < 0.05 was considered significant in difference. Survival was estimated using Kaplan-Meier/log-rank analysis. All calculations were performed using SPSS 10.0®, standard version.
RESULTS
Tumor progression after TACE
Chemoembolization was well tolerated in the majority of patients. The most common complaints after TACE were pain, transient fever, and nausea. No patient developed major complications, which required surgical intervention. After TACE, 20 (29.2%) showed response and 47 (70.1%) showed tumor progression.
Tumor progression defines a group with good survival after OLT
As expected, the patients without treatment had the worst outcome with a median survival of 3.8 mo as seen in Figure 1. The Patients who were treated with TACE but did not qualify for liver transplantation due missing sighs of liver cirrhosis (n = 10), age older than 65 (n = 11), persistent alcohol disease (n = 11) or tumor progression (n = 15) had a median survival of 8.4 mo (Figure 1). The patients who showed no signs of tumor progression during waiting time had an average of 3 sessions of TACE (range, 1 to 10) before OLT. The TACE treated and liver transplanted patients (n = 20) had a significant better outcome compared with TACE treated patients with a median survival of 92.3 mo (Figure 1). The time of median survival in the transplanted group is comparable to OLT patients with non-malignant diseases (median survival of 101.6 mo) and confirms the selection criteria for OLT are suitable to select HCC patients for liver transplantation.
Figure 1.
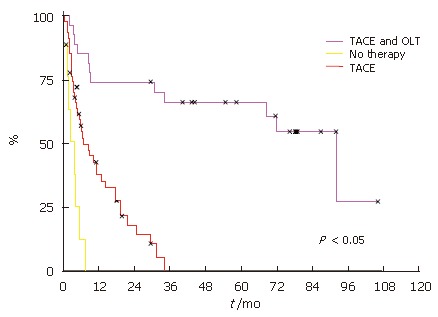
Survival probabilities for the first 5 yr after treatment. Overall HCC patient survival who had received TACE and OLT, only TACE and patients who received no treatment.
Survival dependent on UICC stage
Survival analysis revealed that the patient overall survival was dependent on the UICC stage. Patients with UICC stageI(n = 5) had an overall survival of 100%, stage II (n = 13) 64%, stage III (n = 11) 45% and stage IV only 8% (n = 55) (Figure 2).
Figure 2.
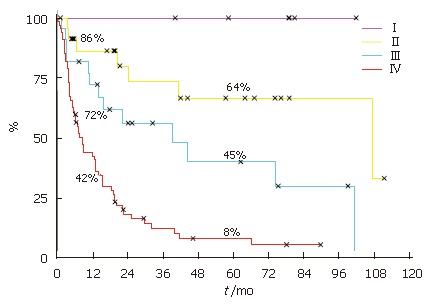
Kaplan-Meier patient survival curves showing survival dependent on UICC stage.
Lack of tumor progression and subsequent OLT improves survival irrespective of UICC stage
Patients, who fulfilled the selection criteria for liver transplantation and had no signs of tumor progression, underwent subsequently OLT. Independent of UICC stage, the patients with TACE and OLT had a better survival (Figure 3, Figure 4 and Figure 5). None of the patients with UICC stageIon the waiting list showed signs of tumor progression. In UICC stage II and III, 4 patients had signs of tumor progression during waiting time. Seven of the UICC stage IV patients displayed signs of tumor progression. Independent of the UICC stage the patients without tumor progression and subsequent OLT had a significant better median survival. Patients who did not fulfill the criteria for liver transplantation or showed signs of tumor progression in UICC stage III had a median survival of 14.2 mo compared to 39.5 mo in the liver transplanted group (Figure 4). In UICC stage IV patients the median survival was 8.6 versus 15.6 mo, respectively. The patients who were not treated at all had the worst survival with a median survival time of 3.8 mo (Figure 5). Taken together, independent of the UICC stage, the survival was significantly better in patients who qualified for liver transplantation and without any evidence of tumor progression while waiting for OLT (P > 0.05).
Figure 3.
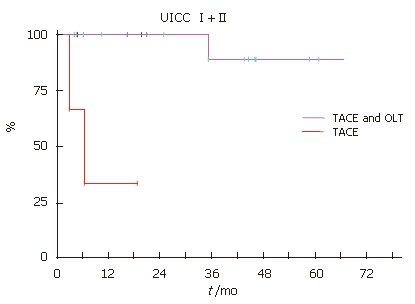
Kaplan-Meier patient survival curves demonstrating that OLT improved survival UICC stage I and II.
Figure 4.
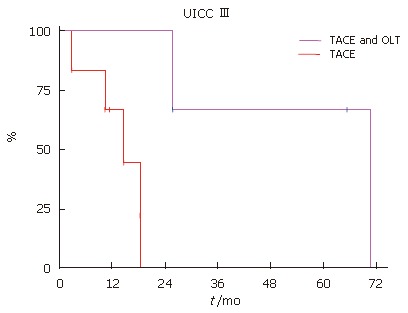
Kaplan-Meier patient survival curves. OLT improved survival UICC stage III.
Figure 5.
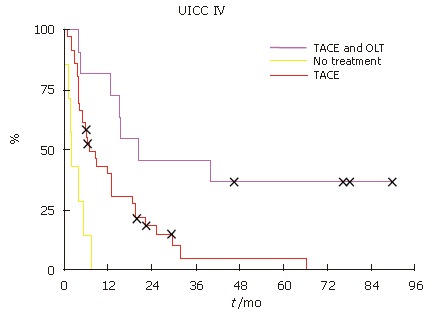
Kaplan-Meier patient survival curves. OLT improved survival UICC stage IV.
Patients without tumor progression have a comparable outcome irrespective of the Milan criteria
Patients, who fullfilled the Milan criteria, are considered to have an excellent outcome. Interestingly, in our study there was no significant difference (P = 0.19) in survival of patients, who fullfilled the Milan criteria compared to the more advanced patients (Figure 6A). However, the patients with tumors less than 5cm had a significantly better median survival (P = 0.03) compared to patients with tumors larger than 5 cm (Figure 6B).
Figure 6.
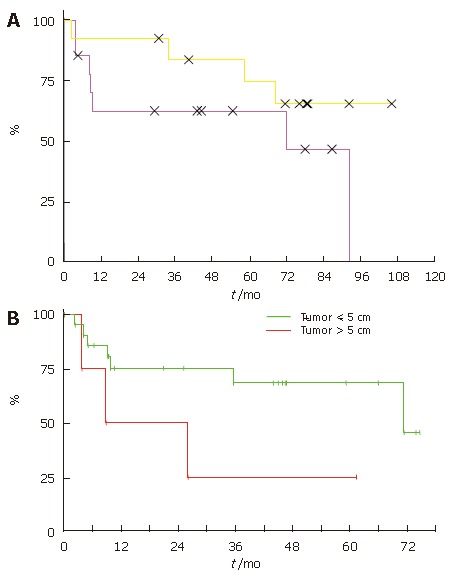
Survival probabilities for the first 5 years after liver transplantation according to the Milan criteria (A) and tumor size (B).
DISCUSSION
Liver transplantation is the only approach that addresses the multifocal hepatocellular carcinoma and also treats the underlying cirrhosis but the limited availability of donor organs and the subsequent immunosuppression urge criteria of patient selection. Our data suggest that patients, who do not show signs of tumor progression after TACE treatment benefit from OLT irrespective of UICC stage. Without tumor progression, even patients outside of the Milan criteria do have a good outcome. Therefore we suggest that patient selection for OLT should be based on tumor size and additionally on assessment of tumor progression.
The current UNOS policy for organ allocation among patients with HCC favors those with tumors confined within the limits of diameter and number of nodules defined by the Milan criteria[12]. These were derived from a prospective study showing significantly better recurrence-free survival for 35 patients meeting the proposed criteria than for 13 patients with HCC exceeding criteria because of preoperative tumor stage underestimation based on pathologic analysis of the liver explants (92% vs 59% at 4 years). Other study groups proposed to “expand” the limits concerning OLT for HCC[16], because studies using the extended UCSF criteria (solitary tumor < 6.5 cm, three or fewer nodules with the largest lesion < 4.5 cm, total tumor diameter < 8 cm or without gross vascular invasion) do not worse the outcome of patients after OLT[13]. Therefore, concerns arise whether the Milan criteria may be too restrictive, thus excluding patients who would otherwise benefit from OLT. The decision for OLT is based on tumor size and number of tumor within the liver but not on growth behavior of the tumor or response of the tumor after bridging therapy. In our present study, 12 patients were found to have tumor characteristics exceeding the Milan criteria and underwent OLT. These patients were selected on the tumor progression after TACE and the difference in survival among those patients compared to patients meeting the Milan criteria did not reach statistical significance (P = 0.19). Thus patients without tumor progression not fulfilling the Milan criteria might benefit from OLT.
Vitale et al[17] described a very low occurrence of drop-outs from waiting list, though they had a quite long median waiting time of 11 mo[18]. In their study they used the following criteria to exclude patients from OLT: short-listing, general contraindication to transplant, extrahepatic spread, vascular invasion or poorly differentiated HCC (G3) at pre-OLT percutaneous biopsy. Size and number were not considered absolute selection criteria. They suggest that adoption of different selection criteria accounting more for tumor biology (grading) rather than tumor size and number and a use of a pre-OLT multimodal strategy probably may guarantee a lower number of drop-outs. Our results demonstrate that patients who show no further tumor progression under TACE treatment significantly (P < 0.05) benefit from OLT. It seems to be an easier biological grading method than the routine percutaneous biopsy for liver lesions because of the given potential risk of tumor seeding along the biopsy tract.
Furthermore, current imaging techniques have a high incidence of false-negative and false-positive results when evaluating HCC in cirrhosis. Only in 14.3% of OLT patients, tumor diameter was correctly identified by pretransplant radiological examinations shown by Sotiropoulos et al[19,20]. Sensitivity of radiological imaging was especially poor for tumors between 1 and 2 cm and less than 1 cm (21% and 0%, respectively), indicating that detection of small HCCs, especially in end-stage cirrhotic livers, remains problematic. A critical appraisal of patient characteristics together with great caution when interpreting imaging studies is recommended to determine candidacy for transplantation. Otherwise many patients are not given the opportunity to undergo OLT.
TACE involves the injection into the hepatic artery of chemotherapeutic agents such as doxorubicin, mitomycin, or cisplatin with lipiodol, to promote intra-tumor retention of the medications. TACE has the advantage of treating larger tumor-areas, being repeatable and perhaps downstaging patients. Disadvantages of TACE include significant toxicity and acute liver failure, especially when treating large areas[17]. Even if downstaging by TACE has been reported, TACE has not been shown to improve survival or recurrence of HCC after transplantion. Even in patients who were not transplanted, multiple large randomized controlled trials have failed to demonstrate a survival benefit. Only a small survival benefit in comparison with conservative treatment was seen in meta-analysis of these trials. In addition, none of the current studies have examined the ability of TACE to sustain patients on the transplantation waiting list. Our data demonstrate that the patients, who have no tumor progression after TACE benefit substantially from OLT. Whether these patients would progress without TACE remains speculative[19,21-26].
In contrast to other malignant diseases, assessment of HCC patients on the waiting list should not only account for the current stage, it although should evaluate the tumor progression. Multiple genetic lesions within the HCC cells, which modulate growth, cell cycle, apoptosis and invasion, define tumor progression[27-31]. These genetic lesions are not defined in number and location and therefore prospective evaluation based on molecular pattern is not possible. On the other hand it is practicable to assess tumor growth by size and AFP levels.
Therefore, we conclude that the growth behavior of the tumor (defined as progression under anti-cancer therapy) could provide simple but helpful information about the recurrence rate and the outcome of HCC patients after OLT. We propose that growth behavior (P0 for no progression and P1 for progression) should be added to staging systems for classification of HCC to select patients for OLT[32-34].
Footnotes
S- Editor Wang J L- Editor Ma JY E- Editor Liu WF
.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 4.Sakon M, Umeshita K, Nagano H, Eguchi H, Kishimoto S, Miyamoto A, Ohshima S, Dono K, Nakamori S, Gotoh M, et al. Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg. 2000;135:1456–1459. doi: 10.1001/archsurg.135.12.1456. [DOI] [PubMed] [Google Scholar]
- 5.Orlando A, Cottone M, Virdone R, Parisi P, Sciarrino E, Maringhini A, Caltagirone M, Simonetti RG, Pagliaro L. Treatment of small hepatocellular carcinoma associated with cirrhosis by percutaneous ethanol injection. A trial with a comparison group. Scand J Gastroenterol. 1997;32:598–603. doi: 10.3109/00365529709025106. [DOI] [PubMed] [Google Scholar]
- 6.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 7.Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, Rudich S, McClure LA, Arenas J. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. doi: 10.1053/jlts.2002.36394. [DOI] [PubMed] [Google Scholar]
- 8.Oldhafer KJ, Chavan A, Frühauf NR, Flemming P, Schlitt HJ, Kubicka S, Nashan B, Weimann A, Raab R, Manns MP, et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol. 1998;29:953–959. doi: 10.1016/s0168-8278(98)80123-2. [DOI] [PubMed] [Google Scholar]
- 9.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–285. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 10.Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–228; discussion 228-229;. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bismuth H, Chiche L, Adam R, Castaing D. Surgical treatment of hepatocellular carcinoma in cirrhosis: liver resection or transplantation? Transplant Proc. 1993;25:1066–1067. [PubMed] [Google Scholar]
- 12.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 13.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 14.Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631–636. doi: 10.1053/jlts.2001.25458. [DOI] [PubMed] [Google Scholar]
- 15.McPeake JR, O'Grady JG, Zaman S, Portmann B, Wight DG, Tan KC, Calne RY, Williams R. Liver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcome. J Hepatol. 1993;18:226–234. doi: 10.1016/s0168-8278(05)80250-8. [DOI] [PubMed] [Google Scholar]
- 16.Broelsch CE, Frilling A, Malago M. Should we expand the criteria for liver transplantation for hepatocellular carcinoma--yes, of course! J Hepatol. 2005;43:569–573. doi: 10.1016/j.jhep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Vitale A, Brolese A, Zanus G, Bassanello M, Montin U, Gringeri E, D'Amico F, Ciarleglio FA, Carraro A, Cappuzzo G, et al. Multimodal therapy before liver transplantation for hepatocellular carcinoma. Hepatol Res. 2005;31:112–115. doi: 10.1016/j.hepres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Fisher RA, Maluf D, Cotterell AH, Stravitz T, Wolfe L, Luketic V, Sterling R, Shiffman M, Posner M. Non-resective ablation therapy for hepatocellular carcinoma: effectiveness measured by intention-to-treat and dropout from liver transplant waiting list. Clin Transplant. 2004;18:502–512. doi: 10.1111/j.1399-0012.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 19.Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki EI, Verhagen R, Dirsch O, Gerken G, Lang H, et al. Efficacy of transarterial chemoembolization prior to liver transplantation for hepatocellular carcinoma as found in pathology. Hepatogastroenterology. 2005;52:329–332. [PubMed] [Google Scholar]
- 20.Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki E, Kühl H, Dirsch O, Lang H, Broelsch CE. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumor classification before transplantation realistic? Transplantation. 2005;79:483–487. doi: 10.1097/01.tp.0000152801.82734.74. [DOI] [PubMed] [Google Scholar]
- 21.Biselli M, Andreone P, Gramenzi A, Trevisani F, Cursaro C, Rossi C, Ricca Rosellini S, Cammà C, Lorenzini S, Stefanini GF, et al. Transcatheter arterial chemoembolization therapy for patients with hepatocellular carcinoma: a case-controlled study. Clin Gastroenterol Hepatol. 2005;3:918–925. doi: 10.1016/s1542-3565(05)00425-8. [DOI] [PubMed] [Google Scholar]
- 22.Arimura E, Kotoh K, Nakamuta M, Morizono S, Enjoji M, Nawata H. Local recurrence is an important prognostic factor of hepatocellular carcinoma. World J Gastroenterol. 2005;11:5601–5606. doi: 10.3748/wjg.v11.i36.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC) Eur Radiol. 2006;16:661–669. doi: 10.1007/s00330-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 24.Liem MS, Poon RT, Lo CM, Tso WK, Fan ST. Outcome of transarterial chemoembolization in patients with inoperable hepatocellular carcinoma eligible for radiofrequency ablation. World J Gastroenterol. 2005;11:4465–4471. doi: 10.3748/wjg.v11.i29.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 26.Guan YS, Sun L, Zhou XP, Li X, Zheng XH. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol. 2004;10:3543–3548. doi: 10.3748/wjg.v10.i24.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez Saborido B, Meneu JC, Moreno E, García I, Moreno A, Fundora Y. Is transarterial chemoembolization necessary before liver transplantation for hepatocellular carcinoma? Am J Surg. 2005;190:383–387. doi: 10.1016/j.amjsurg.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Marui Y, McCall J, Gane E, Holden A, Duncan D, Yeong ML, Chow K, Munn S. Liver transplantation for hepatocellular carcinoma in New Zealand: a prospective intent-to-treat analysis. N Z Med J. 2005;118:U1532. [PubMed] [Google Scholar]
- 29.Lambert B, Praet M, Vanlangenhove P, Troisi R, de Hemptinne B, Gemmel F, Van Vlierberghe H, Van de Wiele C. Radiolabeled lipiodol therapy for hepatocellular carcinoma in patients awaiting liver transplantation: pathology of the explant livers and clinical outcome. Cancer Biother Radiopharm. 2005;20:209–214. doi: 10.1089/cbr.2005.20.209. [DOI] [PubMed] [Google Scholar]
- 30.Yamashiki N, Tateishi R, Yoshida H, Shiina S, Teratani T, Sato S, Mine N, Kondo Y, Kawabe T, Omata M. Ablation therapy in containing extension of hepatocellular carcinoma: a simulative analysis of dropout from the waiting list for liver transplantation. Liver Transpl. 2005;11:508–514. doi: 10.1002/lt.20392. [DOI] [PubMed] [Google Scholar]
- 31.Johnson EW, Holck PS, Levy AE, Yeh MM, Yeung RS. The role of tumor ablation in bridging patients to liver transplantation. Arch Surg. 2004;139:825–829; discussion 829-830;. doi: 10.1001/archsurg.139.8.825. [DOI] [PubMed] [Google Scholar]
- 32.Huynh H. Overexpression of tumour suppressor retinoblastoma 2 protein (pRb2/p130) in hepatocellular carcinoma. Carcinogenesis. 2004;25:1485–1494. doi: 10.1093/carcin/bgh154. [DOI] [PubMed] [Google Scholar]
- 33.Liu LX, Jiang HC, Liu ZH, Zhu AL, Zhou J, Zhang WH, Wang XQ, Wu M. Gene expression profiles of hepatoma cell line BEL-7402. Hepatogastroenterology. 2003;50:1496–1501. [PubMed] [Google Scholar]
- 34.Figueras J, Ibañez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, Mir J, Loinaz C, Herrera L, López-Cillero P, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl. 2001;7:877–883. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]


