Abstract
We report an extremely rare case where a mesenchymal differentiation, especially embryonal sarcoma, was demonstrated in cholangiocarcinoma. At autopsy, a yellowish-white tumor (15 cm x 12 cm) was found in the right hepatic lobe, and there were several daughter nodules in both hepatic lobes. Histologically, most of the main tumor and all of the daughter nodules examined showed sarcomatous changes (spindle cells, pleomorphic cells and hyalization). Histologic examination of a part of the main tumor disclosed a focus of adenocarcinoma within the tumor. The frequent transitions between the adenocarcinomatous areas and the sarcomatous areas suggested that sarcomatous transformation occurred in the cholangiocarcinoma and then spread rapidly. Immunohistochemically, the adenocarcinomatous elements were positive for cytokeratin, carcinoembryonic antigen (CEA) and epithelial membrane antigen, and negative in the sarcomatous cells. Vimentin was positive only in the sarcomatous elements. The findings of the present case support the view that carcinosarcomas represent carcinomas that develop sarcomatous elements via metaplasia of the epithelial element.
Keywords: Cholangiocarcinoma, Carcinosarcoma, Mesenchymal differentiation
INTRODUCTION
Cholangiocarcinoma is usually a moderately to well differentiated adenocarcinoma with considerable demoplastic reactions. Nakajima et al[1] reported that 92 of 102 cases of cholangiocarcinoma were mucin-producing adenocarcinomas, and the remaining were adenosquamous (3 cases), squamous (3 cases), mucinous (1 case), or anaplastic carcinoma (3 cases). Only brief descriptions of sarcomatous changes and sarcomatous variants of cholangiocarcinoma can be found in a few studies or reviews dealing with a large series of cholangiocarcinoma cases[1,2] . However, detailed clinicopathological studies of sarcomatous changes in cholangiocarcinoma have not been reported in English and Japanese literature to the best of our knowledge.
Because we recently encountered an autopsy case of cholangiocarcinoma showing sarcomatous changes, we report this case to emphasize the autopsy findings, as well as the histogenesis of sarcomatous changes in cholangiocarcinoma.
CASE REPORT
A 74-year old Japanese woman was admitted to our hospital complaining of pain in the right hypochondrium and underwent a cholecystectomy. The main laboratory data were as follows: red blood cells 378 × 104/mm3, white blood cells 5200/mm3, serum total protein 8.0 g/dL, total bilirubin 0.8 mg/dL, serum glutamic-oxaloacetic transaminase 46 IU/L, serum glutamic-pyruvic transaminase 58 IU/L, lactic acid dehydrogenase 416 IU/L, alkaline phosphatase 476 IU/L, γ-glutamyl transpeptidase 119 IU/L, and C- reactive protein 2.9 mg/dL. Carcinoembryonic antigen (CEA) was 51.5 ng/mL, but serum α-fetoprotein (AFP), a protein induced by vitamin K absence or antagonists (PIVKA-II) and carbohydrase antigen 19-9 (CA19-9) were within the normal range. Hepatitis B core (HBc) antibody, hepatitis C antibody (HCV-Ab), and human immunodeficiency virus (HIV) were all negative. A computed tomography scan of the abdomen revealed a low-density mass with renal invasion in all segments of the right hepatic lobe, without lymph node swelling or dilatation of the intrahepatic bile ducts. Magnetic resonance imaging revealed hypointensity on the T1-weighted images and heterogeneous hyperintensity on the T2-weighted images (Figures 1A and B). Angiography showed a malignant blush in the right lobe (not shown). A sonographically guided hepatic tumor biopsy showed the proliferation of spindle cells. An immunohistochemical study showed that α-smooth muscle actin (SMA) was positive, and keratin, vimentin, desmin, CEA, and S-100 protein were negative, thus leiomyosarcoma was suspected (Figure 2). Her general condition gradually worsened and she died of hepatic failure and disseminated intravascular coagulation (DIC) after two months.
Figure 1.
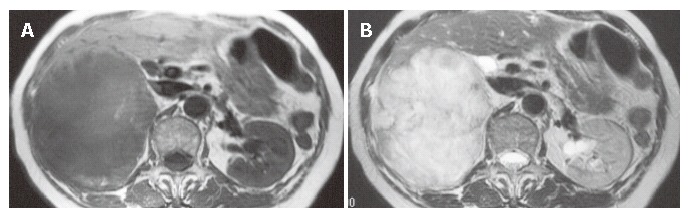
Magnetic resonance imaging showing hypointense areas in the right lobe of the liver on a T1-weighted image (A) and an inhomogeneously hyperintense mass on a T2-weighted image (B).
Figure 2.
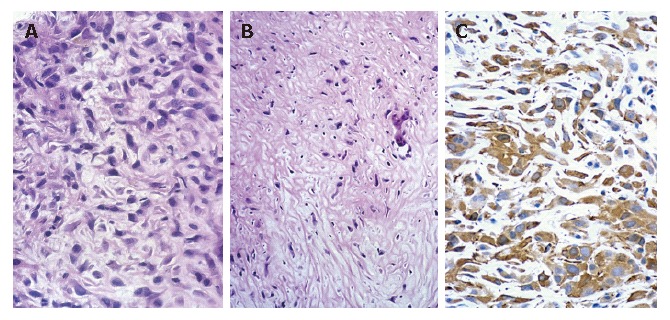
Needle biopsy showing a sarcomatous area consisting of interlacing bundles of atypical spindle cells [hematoxylin-eosin stain; magnification x 200 (A), x 100 (B)] and immunohistochemical staining showing positive α-SMA (C).
Autopsy findings
The gross findings were a yellowish-white tumor (15 cm × 12 cm) with blurred borders in the right hepatic lobe (Figure 3) where the right branch of the portal vein and right kidney were occluded.
Figure 3.
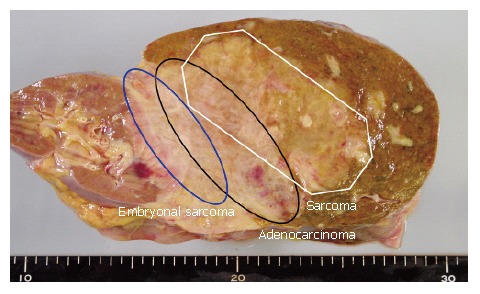
Gross appearance of hepatic tumor.
Microscopically, the majority of the main tumor and all daughter nodules examined showed a sarcomatous appearance. Elongated cells were arranged in bundles, occasionally interlacing. These histologic features were similar to those of fibrosarcoma or leiomyosarcoma. That is, these areas were composed of nonadhesive spindle-shaped or fusiform cells, and to a lesser degree, pleomorphic giant or multinuclear cells, the majority of the latter showing bizarre nuclei and prominent nucleoli (Figure 4). These sarcomatous areas looked like malignant leiomyosarcoma. There were many foci of coagulative necrosis within these sarcomatous areas. In addition, there was a well-differentiated tubular adenocarcinoma within the tumor (Figure 5). There were direct transitions between adenocarcinomatous elements and sarcomatous elements. However, the transitions were unclear. A hydropsy-like part was recognized at the side edge of the tumor, and tumor cells floating in mucinous cytoplasm were evidence of undifferentiated (embryonal) sarcoma (Figure 6).
Figure 4.
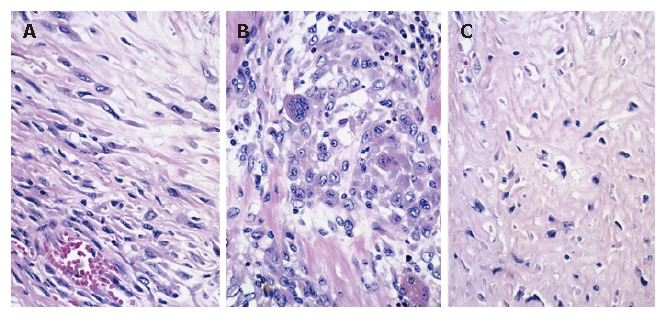
Spindle cells (A), pleomorphic areas (B), or hyalization (C) in leiomyosarcoma (hematoxylin-eosin stain; magnification x 160).
Figure 5.
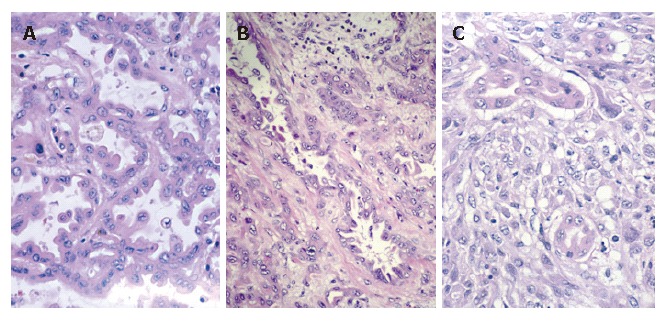
Cholangiocarcinoma focus (A) (hematoxylin-eosin stain; magnification x160), component surrounded by fibrosis (B) (hematoxylin-eosin stain; magnification x100), intimately mixed carcinomatous and sarcomatous components (C) (hematoxylin-eosin stain; magnification x 160).
Figure 6.
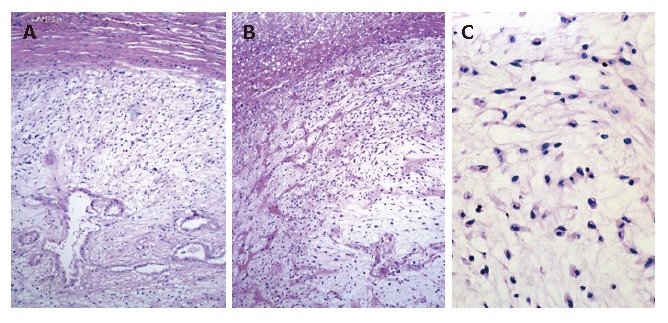
Sarcomatous portion of the tumor consisting of undifferentiated (embryonal) sarcoma [hematoxylin-eosin stain; magnification x 25 (A, B), x 160 (C)].
These two elements on the representative section of tumor were mapped (Figure 3), showing adenocarcino-matous elements within the tumor and a considerable amount of sarcomatous elements surrounding this carcinoma. There were no histologic elements suggestive of hepatocellular carcinoma.
The nontumorous hepatic tissue showed nonspecific reactive changes with mild fibrous enlargement of the portal tracts, but not liver cirrhosis. There were no regenerative nodules throughout the liver, and HBsAg was not detected in the liver by orcein stain.
Primary antisera to keratin, vimentin, desmin, AFP, CEA, and S-100 protein were obtained from DAKO Corporation. Keratin was weakly positive in the adenocarcinoma cells. There was positive staining for CEA at the part of tubular adenocarcinoma. Vimentin was expressed only in the sarcomatous cells. There were no positive reactions for AFP, desmin, S-100 protein, or CA19-9 in the tumor cells.
It was diagnosed as cholangiocarcinoma and carcino-sarcoma with mesenchymal differentiation that specialized and coexisted in a lobe corollary tumor.
DISCUSSION
Sarcomatous transformation of primary cancer of the liver in adults is most common in hepatocellular carcinoma (HCC), especially after anticancer chemotherapy or transarterial embolization therapy[3]. In our patient, there were no lesions resembling HCC, and AFP was negative in the tumor, so that the derivation of sarcomatous cells from HCC was excluded.
Histologic mapping of a whole section of the main tumor showed adenocarcinomatous and sarcomatous elements within the main tumor. Considering the mapping, the sarcomatous elements would be interpreted as the result of the metaplastic transformation of pre-existing adenocarcinoma cells[4].
In our case, direct transitions between sarcoma and adenocarcinoma in the main tumor suggested that the sarcomatous changes might have developed secondarily from pre-existing cholangiocarcinoma. Most of the daughter nodules showed such sarcomatous changes, suggesting that sarcomatous elements grow and spread more rapidly than the adenocarcinomatous elements.
Thompson et al[5] stated that the carcinomatous and sarcomatous components may be monoclonal in origin and derived from single stem cells. Genetic analysis is one possible solution to this problem, as reported in esophageal carcinosarcoma[6].
What is more, various other hypotheses have been proposed to explain the biphasic appearance of sarcomatoid carcinomas[7,8]. Briefly, the explanations include the collision theory of independent neoplastic growth from multipotent stem cell origins, epithelial to mesenchymal conversion by epithelial-stromal interaction, and a combination of the two. The salient features in our case were the presence of dysplasia and adenocarcinoma in situ, morphological “transition” between carcinomatous and sarcomatous tissues in relation to expansion of invasion, and the detection of sarcomatous characteristics by immunohistochemistry in the epithelial component, which strongly support the histogenesis of epithelial to mesenchymal conversion. Gentile et al[9] reported that the presence of productive retroviral infection in the sarcomatous cells is related with tumor progression from the carcinomatous to the sarcomatous phase. The examination I performed confirmed that it was negative for viruses, but it was related with an unknown virus. However, why sarcomatous changes develop is a mystery.
In summary, sarcomatoid carcinoma derived from cholangiocarcinoma is an extremely rare tumor composed of mixed malignant epithelial and mesenchymal cells, with only 12 cases[10-12] reported to date who died of liver failure due to extensive metastatic growth of sarcomatoid carcinoma despite postoperative chemotherapy. The histologic features, stage, and outcome of the reported cases indicate that this neoplasm generally pursues a highly aggressive and malignant biological course with rapid growth and wide local infiltration, leading to a poor prognosis. Radical surgery with adjuvant chemotherapy, and close follow-up are necessary for the management of this disease.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
References
- 1.Nakajima T, Kondo Y, Miyazaki M, Okui K. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum Pathol. 1988;19:1228–1234. doi: 10.1016/s0046-8177(88)80156-4. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K, Nakashima T. Primary carcinoma of the liver. 4th ed. In: Berk JE, editor. Bockus gastroenterology. Philadelphia: WB Saunders; 1985. pp. 3361–3364. [Google Scholar]
- 3.Kakizoe S, Kojiro M, Nakashima T. Hepatocellular carcinoma with sarcomatous change. Clinicopathologic and immunohistochemical studies of 14 autopsy cases. Cancer. 1987;59:310–316. doi: 10.1002/1097-0142(19870115)59:2<310::aid-cncr2820590224>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima T, Kondo Y. A clinicopathologic study of intrahepatic cholangiocarcinoma containing a component of squamous cell carcinoma. Cancer. 1990;65:1401–1404. doi: 10.1002/1097-0142(19900315)65:6<1401::aid-cncr2820650626>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996;20:277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Iwaya T, Maesawa C, Tamura G, Sato N, Ikeda K, Sasaki A, Othuka K, Ishida K, Saito K, Satodate R. Esophageal carcinosarcoma: a genetic analysis. Gastroenterology. 1997;113:973–977. doi: 10.1016/s0016-5085(97)70194-x. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, Bostwick DG. Carcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 cases. J Urol. 1998;159:1497–1503. doi: 10.1097/00005392-199805000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Guarino M, Tricomi P, Giordano F, Cristofori E. Sarcomatoid carcinomas: pathological and histopathogenetic considerations. Pathology. 1996;28:298–305. doi: 10.1080/00313029600169224. [DOI] [PubMed] [Google Scholar]
- 9.Gentile R, Castellaneta A. Carcinosarcoma of the colon, one or two tumors? Pathologica. 1997;89:62–68. [PubMed] [Google Scholar]
- 10.Nomura K, Aizawa S, Ushigome S. Carcinosarcoma of the liver. Arch Pathol Lab Med. 2000;124:888–890. doi: 10.5858/2000-124-0888-COTL. [DOI] [PubMed] [Google Scholar]
- 11.Eriguchi N, Aoyagi S, Hara M, Okuda K, Fukuda S, Tamae T, Kanazawa N. Malignant sarcomatoid tumor of the liver: report of a case. Surg Today. 2001;31:170–173. doi: 10.1007/s005950170205. [DOI] [PubMed] [Google Scholar]
- 12.Wang XW, Liang P, Li HY. Primary hepatic carcinosarcoma: a case report. Chin Med J (Engl) 2004;117:1586–1587. [PubMed] [Google Scholar]


