Abstract
Cadherin is an adhesion molecule and a superfamily of calcium-mediated membrane glycoproteins. E-cadherin is the prototype of the class E-cadherin that links to catenins to form the cytoskeleton. Recent evidence has shown that E-cadherin not only acts as an adhesive, but also plays important roles in growth development and carcinogenesis. It has been recently viewed as an invasion as well as a growth suppressor gene. This review summarizes the recent discoveries on E-cadherin and its role in gastric cancer. In particular, our work on E-cadherin in gastric cancer, including its relation with Helicobacter pylori and clinical applications, are described in detail.
Keywords: E-cadherin, Gastric cancer
INTRODUCTION
Although decreasing in incidence, gastric cancer remains a major medical problem and is the second most common fatal cancer worldwide. In spite of intense interest and extensive investigations, its prognosis has not been improved significantly in recent years. The dramatic variations in the incidence of gastric cancer in different geographic areas and from one generation to the next have led to the hypothesis that the incidence of gastric cancer is determined largely by environmental rather than genetic factors. The identification and the subsequent classification of Helicobacter pylori (H pylori) infection as type I carcinogen by the WHO sparked a significant understanding in the etiology of gastric cancer. The current discovery of germline mutation at E-cadherin gene in familial gastric cancers and the findings of IL-1 polymorphisms with increased gastric cancer risk again turn the focus towards the studying of host genetics. The molecular studies of the host may give a definite proof of the etiological role of H pylori in inducing gastric carcinoma.
Only a small fraction of all cancers arise in individuals who carry a germline defect conferring genetic predisposition. Nevertheless, many genes that underlie inherited cancer syndromes have more widespread roles in sporadic cancers, as a result of somatic mutations that arise during tumor initiation or progression. Hence, in this review paper, we shall focus on the E-cadherin gene, one of the candidate genes in gastric cancer, and the work we have done on E-cadherin in gastric carcinogenesis.
E-CADHERIN
Cadherin is a superfamily of calcium-mediated membrane glycoproteins, with a molecular mass of 120 ku, forming one of the four classes of adhesion molecules[1-3]. Some common cadherins expressed by epithelial cells are E-cadherin, N-cadherin and P-cadherin. The intracellular domains of classical cadherins interact with β-catenin, γ-catenin (also called plakoglobin) and p120ctn to assemble the cytoplasmic cell adhesion complex (CCC) that is critical for the formation of extracellular cell-cell adhesion. β-catenin and γ-catenin bind directly to α-catenin, which links the CCC to the actin cytoskeleton[4,5]. The cadherins are responsible for the homotypic cell-cell adhesion. However, knowledge gained in the recent few decades has shown that E-cadherin not only acts as an adhesive, but also plays important roles in growth development and carcinogenesis.
E-CADHERIN AND CANCER
Role of E-cadherin in metastasis
E-cadherin is the prototype of the cadherin class. It is expressed in all epithelial cell types. Underexpression of the E-cadherin is found in gastric and other cancers, and correlates with infiltrative and metastatic ability[6]. It has been proposed that the loss of E-cadherin-mediated cell-cell adhesion is a prerequisite for tumor cell invasion and metastasis formation[7]. Re-establishing the functional cadherin complex, e.g. by forced expression of E-cadherin, results in a reversion from an invasive, mesenchymal, to a benign, epithelial phenotype of cultured tumor cells[7,8]. Hence, the E-cadherin gene is also called as an invasion suppressor gene.
Role of E-cadherin in oncogenesis
Recently, it has been postulated that the role of E-cadherin in carcinogenesis does not limit only to metastasis and invasion (Figure 1). It is now being increasingly recognized that there is also a possible role of E-cadherin in modulating intracellular signaling, and thus promoting tumor growth. There are several lines of evidence. Cadherin-mediated cell-cell adhesion can affect the Wnt-signaling pathway[9,10]. β-catenin (as well as γ-catenin) is usually sequestered by cadherins in the cadherin-catenin complex. Upon loss of E-cadherin function, non-sequestered, free β-catenin is usually phosphorylated by glycogen synthase kinase 3β (GSK-3β) in the adenomatous polyposis coli (APC)-axin-GSK-3β complex and subsequently degraded by the ubiquitin-proteasome pathway. In many cancer cells, loss of function of the tumor suppressor APC, mutations in β-catenin or inhibition of GSK-3β by the activated Wnt-signaling pathway leads to the stabilization of β-catenin in the cytoplasm. Subsequently, it translocates to the nucleus, where it binds to the members of the Tcf/Lef-1 family of transcription factors and modulates expression of Tcf/Lef-1-target genes, including the proto-oncogene c-myc and cyclin D1. In addition, recent studies on familial gastric cancer indicate that E-cadherin can also act at a much earlier stage during tumor development. Mutations of the E-cadherin gene were found in three familial gastric cancer kindred from New Zealand[11] and this observation was confirmed in the kindred of European origin[12]. These results demonstrate that loss of function of E-cadherin may play a role in susceptibility to initial tumor development in addition to its role as an inhibitor of tumor invasion.
Figure 1.
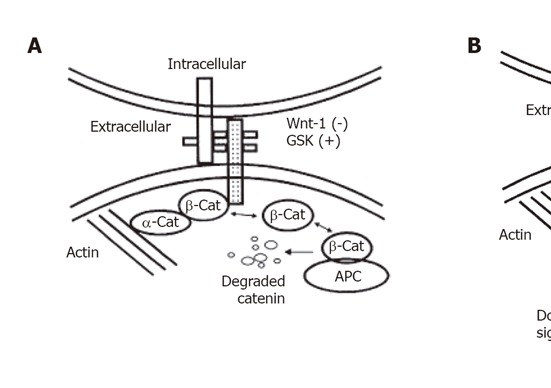
Illustrated interaction between the cadherin–catenin complex and the APC protein. (A) In the absence of Wnt-1 and the presence of glycogen synthase kinase (GSK), β-catenin (β-cat) is stabilized and bound to cadherin or APC protein. Cadherin acts as a negative regulator of β-catenin by regulating the amount of free β-catenin. The free cytoplasmic β-catenin is degraded. (B) In the presence of Wnt-1, GSK is antagonized and mutant APC or tyrosine phosphorylated β-catenin cannot bind to each other, cytosolic free β-catenin concentrations rise, which leads to down-stream cell signalling and may be involved in carcinogenesis.
EXPRESSION OF E-CADHERIN IN GASTRIC CANCER
The expression of E-cadherin has been studied by immunohistochemical method. Decreased expression has been observed in gastric cancer by various authors, ranging from 17%[13] to 92%[14], depending on the method and the definition used. The decreased expression of E-cadherin was mainly observed in diffuse type and less in intestinal type of gastric cancer. Direct correlation between E-cadherin and the grade of tumor differentiation has been observed in all these studies. In addition, it was shown in a study of 413 gastric cancers by Gabbert et al.[15] that patients with E-cadherin positive tumors had significantly better 3- and 5-year survival rates than patients with E-cadherin negative tumors. A similar trend of decrease in disease-free survival was also observed in other cancers exhibiting downregulation of E-cadherin[16].
We have also studied the change in the expression of the E-cadherin complex along the Correa’s cascade in gastric cancer by immunohistochemical staining[17]. We observed that the staining pattern, intensity and the percentage of cells with positive stains deceased along the Correa’s cascade; that is, the staining was strong and membranous in normal gastric epithelium, but gradually decreased in intensity and percentage, as well as the pattern was changed towards cytoplasm in chronic atrophic gastritis, intestinal metaplasia, dysplasia and eventually to adenocarcinoma.
However, methods to evaluate, qualitatively or quantitatively, protein expression in biopsies from human tumors may have serious limitations because of sampling from heterogeneous tissue, non-stoichiometric labeling and subjective evaluation.
MECHANISM OF INACTIVATION OF E-CADHERIN
Genetic inactivation
The E-cadherin gene can be genetically inactivated by a number of mechanisms. The first hit of a role for E-cadherin in tumor development, particularly in suppression of invasion, came from loss of heterozygosity studies on chromosome 16. Subsequently, mutations were reported in tumor samples in diffuse type gastric carcinomas[18], gynecologic cancers[19], and infiltrative lobular breast cancer[20,21]. In gastric cancer, E-cadherin mutations are common in diffuse type carcinomas, but not seen in intestinal type[18]. The specificity of types of cancer affected by mutations of the E-cadherin gene, despite the prevalence of reduced E-cadherin expression in many cancer types, suggests that E-cadherin mutations may be of particular importance in the development of these tumors. In addition, mutations in diffuse gastric cancer[22] have been detected early in tumor development, suggesting a role in tumor suppression, in addition to invasion suppression. Further evidence for this comes from the observations that mutations of E-cadherin have also been observed in several kindred exhibiting familial gastric cancer[11,12]. Furthermore, at least one kindred exhibited both diffuse gastric cancer and early-onset breast cancer[11].
Inactivation by hypermethylation
In recent years, it has become increasingly apparent that increased methylation within the promoter regions of genes plays a key role in the inactivation of many important genes during the development of cancer[23]. Subsequently, numerous reports of E-cadherin promoter methylation, associated with reduced E-cadherin expression, have been published. Hypermethylation at E-cadherin has also been shown to play a role in familial gastric cancer, acting as the second hit (the first hit is either mutation or loss of heterozygosity) in inactivation of E-cadherin[24].
We have previously shown that the immunostaining of E-cadherin decreases along the Correa’s cascade; therefore, we went on to study the underlying mechanism. We found that the frequency of methylation at E-cadherin also increases along the Correa’s cascade, with the highest frequency detected in the tumorous lesions and metastatic lesions[25]. The methylation frequency correlated with the tumor depth and node invasion. In addition, most of the decrease in immunostaining was accounted for by the presence of methylation at E-cadherin. More importantly, we also observed that H pylori infection was also associated with E-cadherin methylation.
In fact, the role of H pylori in the regulation of E-cadherin has been studied recently. It has been shown that H pylori infection is associated with downregulation of E-cadherin, probably by generating cell signaling events that counteract the normal function of protein kinase C[26,27]. The resulting increase in permeability mediated by the reduction in cell adhesion might allow H pylori antigens to reach the gastric lamina propria and activate the mucosal immune system, with resultant tissue damage.
In relation to the above findings, we therefore hypothesized that by eradicating H pylori, methylation at E-cadherin may disappear[28]. We performed a prospective randomized trial. Patients with dyspepsia and H pylori infection were randomized to receive H pylori eradication therapy (Group 1, n = 41) or no treatment (Group 2, n = 40) and were followed up prospectively. Gastric mucosae were taken for methylation assay at week 0 (before treatment) and week 6 (after treatment). Methylation was assessed using methylation-specific PCR. Methylation was detected in 46% (19/41) and 17% (7/41) at week 0 and 6, respectively in Group 1 (P = 0.004). 78.9% (15/19) specimens turned unmethylated after eradicating H pylori. Methylation was detected in 47.5% (19/40) and 52.5% (21/40) at weeks 0 and 6, respectively in Group 2 (P = 0.5). Archived specimens with intestinal metaplasia with H pylori infection (n = 22) and without the infection (n = 19) were also retrieved for methylation analysis. But methylation frequency did not differ in H pylori positive or negative intestinal metaplastic specimens (72.7% vs. 63%, P = 0.5). Therefore, we postulated that H pylori eradication therapy could reverse methylation in patients with chronic gastritis only and may halt the process of gastric carcinogenesis.
Postulated mechanism of helicobacter pylori infection for inducing methylation
Experimental data from in vitro studies support our contention that E-cadherin methylation might be related to H pylori infection. El-Omar et al [29] reported that interleukin-1β polymorphism that led to upregulation of interleukin-1β with H pylori infection was associated with increased risk of gastric cancer. Furthermore, Hmadcha et al [30] found that interleukin-1β, through the production of nitric oxide and the subsequent activation of DNA methyltransferase, might induce gene methylation. It is thus possible that H pylori induces methylation through the production of interleukin-1β and hence the downstream activation of nitric oxide and DNA methyltransferase, which subsequently led to an increased risk of E-cadherin methylation and hence gastric cancer[31].
OTHER REGULATORY MECHANISMS OF E-CADHERIN
Loss of E-cadherin function during tumor progression can also be caused by transcriptional repression binding to the CDH1-E box elements, e.g. by the repressors Snail[32,33] and Sip-1[34]. Tyrosine phosphorylation has also been previously implicated in the regulation of cadherin function: RTKs, such as EGFR, c-Met and FGFR, and the non-receptor tyrosine kinase, c-Src, phosphorylate E-cadherin, N-cadherin, β-catenin, -catenin and p120ctn, resulting in the disassembly of the cytoplasmic adhesion complex and a disruption of cadherin-mediated cell adhesion and cell scattering[35-37].
SOLUBLE E-CADHERIN
E-cadherin has a cleavage site near the transmembrane domain and artificially produces a soluble 80 ku amino-terminal fragment in the culture medium upon trypsin digestion in the presence of calcium[38]. This soluble E-cadherin fragment is considered to be a degradation product of the 120 ku intact E-cadherin generated by a calcium-dependent proteolytic action, and can be detected in the protein extract of tissue samples from peripheral blood and urine in normal subjects[39]. Serum soluble E-cadherin is reported to be increased in dermatological disorders (bullous pemphigoid, pemphigus vulgaris, psoriasis vulgaris), multi-organ failure, and various tumors like bladder cancer, prostate cancer, lung cancer and gastric cancer[39-43]. The role of soluble E-cadherin and its biological significance is still, at present, unclear. Recent studies indicate that, in inflammatory condition, serum soluble E-cadherin is induced by inflammatory mediators and cytokines[44]; whereas in cancerous diseases, soluble E-cadherin is increased by cleavage of tissue E-cadherin due to overexpressed proteases[45]. The potential of soluble E-cadherin to be a prognostic marker in cancerous diseases has been shown in bladder cancer[40] and gastric cancer[46].
We have examined the potential clinical application of E-cadherin. We first studied the correlation between E-cadherin immunostaining expression and the concentration in sera[47]. We found that normal strong membranous staining, e.g. in normal gastric epithelium, was associated with low levels of serum soluble E-cadherin, but a partially reduced; cytoplasmic staining, e.g. in intestinal type of gastric cancer, was associated with high levels; whereas complete absence of staining, e.g. in diffuse type of gastric cancer, was associated with low levels of soluble E-cadherin. We also found that soluble E-cadherin may be a potentially useful prognostic marker. High levels of soluble E-cadherin correlated with the depth of tumor invasion, as well as inoperability[48]. More importantly, levels higher than 10 000 ng/mL predicts survival less than 3 years in more than 90% of patients[47]. Currently, we are investigating the use of post-operative soluble E-cadherin levels to predict tumor recurrence in patients who have received curative surgery for gastric cancer.
CONCLUSION
Being an adhesion molecule, E-cadherin plays an important role in invasion and metastasis in almost all kinds of epithelial malignancies, and hence is named invasion suppressor gene. However, recent studies have shown that E-cadherin actually plays an early and important role in carcinogenesis and acts as a tumor suppressor gene, particularly in gastric cancer. Further thorough understanding of the role of E-cadherin and its association with extracellular environment and intracellular functions will be extremely important. Potentially, reversing methylation at E-cadherin in the gastric epithelium in patients with H pylori infection may halt the process of future development of gastric cancer. In addition, soluble E-cadherin may be a useful prognostic marker for gastric cancer.
Footnotes
S- Editor Guo SY L- Editor Elsevier HK E- Editor Li HY
References
- 1.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 2.Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- 3.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunwald GB. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993;5:797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 5.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 6.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 8.Vleminckx K, Vakaet L, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 9.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 10.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 11.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 12.Gayther SA, Gorringe KL, Ramus SJ, Huntsman D, Roviello F, Grehan N, Machado JC, Pinto E, Seruca R, Halling K, et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998;58:4086–4089. [PubMed] [Google Scholar]
- 13.Shimoyama Y, Hirohashi S. Expression of E- and P-cadherin in gastric carcinomas. Cancer Res. 1991;51:2185–2192. [PubMed] [Google Scholar]
- 14.Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- 15.Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, Hommel G. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996;69:184–189. doi: 10.1002/(SICI)1097-0215(19960621)69:3<184::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan AO, Wong BC, Lan HY, Loke SL, Chan WK, Hui WM, Yuen YH, Ng I, Hou L, Wong WM, et al. Deregulation of E-cadherin-catenin complex in precancerous lesions of gastric adenocarcinoma. J Gastroenterol Hepatol. 2003;18:534–539. doi: 10.1046/j.1440-1746.2003.02998.x. [DOI] [PubMed] [Google Scholar]
- 18.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- 19.Risinger JI, Berchuck A, Kohler MF, Boyd J. Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet. 1994;7:98–102. doi: 10.1038/ng0594-98. [DOI] [PubMed] [Google Scholar]
- 20.Kanai Y, Oda T, Tsuda H, Ochiai A, Hirohashi S. Point mutation of the E-cadherin gene in invasive lobular carcinoma of the breast. Jpn J Cancer Res. 1994;85:1035–1039. doi: 10.1111/j.1349-7006.1994.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muta H, Noguchi M, Kanai Y, Ochiai A, Nawata H, Hirohashi S. E-cadherin gene mutations in signet ring cell carcinoma of the stomach. Jpn J Cancer Res. 1996;87:843–848. doi: 10.1111/j.1349-7006.1996.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello JF, Plass C. Methylation matters. J Med Genet. 2001;38:285–303. doi: 10.1136/jmg.38.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 25.Chan AO, Lam SK, Wong BC, Wong WM, Yuen MF, Yeung YH, Hui WM, Rashid A, Kwong YL. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003;52:502–506. doi: 10.1136/gut.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terrés AM, Pajares JM, O'Toole D, Ahern S, Kelleher D. H pylori infection is associated with downregulation of E-cadherin, a molecule involved in epithelial cell adhesion and proliferation control. J Clin Pathol. 1998;51:410–412. doi: 10.1136/jcp.51.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terrés AM, Pajares JM, Hopkins AM, Murphy A, Moran A, Baird AW, Kelleher D. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect Immun. 1998;66:2943–2950. doi: 10.1128/iai.66.6.2943-2950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AO, Peng JZ, Lam SK, Wong WM, Yuen MF, Cheung HKL, Kwong YL, Rashid A, Hui WM, Wong BCY. Reversal of E-cadherin promoter hypermethylation status after Helicobacter pylori eradication - implication in gastric cancer chemoprevention. Gastroenterology. 2004;126(4):Suppl A–38: 333. [Google Scholar]
- 29.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 30.Hmadcha A, Bedoya FJ, Sobrino F, Pintado E. Methylation-dependent gene silencing induced by interleukin 1beta via nitric oxide production. J Exp Med. 1999;190:1595–1604. doi: 10.1084/jem.190.11.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan AO, Lam SK, Wong BC, Kwong YL, Rashid A. Gene methylation in non-neoplastic mucosa of gastric cancer: age or Helicobacter pylori related. Am J Pathol. 2003;163:370–31; author reply 370-31;. doi: 10.1016/s0002-9440(10)63663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 33.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 34.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 35.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 38.Damsky CH, Richa J, Solter D, Knudsen K, Buck CA. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983;34:455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- 39.Katayama M, Hirai S, Kamihagi K, Nakagawa K, Yasumoto M, Kato I. Soluble E-cadherin fragments increased in circulation of cancer patients. Br J Cancer. 1994;69:580–585. doi: 10.1038/bjc.1994.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffiths TR, Brotherick I, Bishop RI, White MD, McKenna DM, Horne CH, Shenton BK, Neal DE, Mellon JK. Cell adhesion molecules in bladder cancer: soluble serum E-cadherin correlates with predictors of recurrence. Br J Cancer. 1996;74:579–584. doi: 10.1038/bjc.1996.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gofuku J, Shiozaki H, Doki Y, Inoue M, Hirao M, Fukuchi N, Monden M. Characterization of soluble E-cadherin as a disease marker in gastric cancer patients. Br J Cancer. 1998;78:1095–1101. doi: 10.1038/bjc.1998.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuyoshi N, Tanaka T, Toda K, Okamoto H, Furukawa F, Imamura S. Soluble E-cadherin: a novel cutaneous disease marker. Br J Dermatol. 1995;132:745–749. doi: 10.1111/j.1365-2133.1995.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 43.Pittard AJ, Banks RE, Galley HF, Webster NR. Soluble E-cadherin concentrations in patients with systemic inflammatory response syndrome and multiorgan dysfunction syndrome. Br J Anaesth. 1996;76:629–631. doi: 10.1093/bja/76.5.629. [DOI] [PubMed] [Google Scholar]
- 44.Perry I, Tselepis C, Hoyland J, Iqbal TH, Scott D, Sanders SA, Cooper BT, Jankowski JA. Reduced cadherin/catenin complex expression in celiac disease can be reproduced in vitro by cytokine stimulation. Lab Invest. 1999;79:1489–1499. [PubMed] [Google Scholar]
- 45.Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 46.Juhasz M, Ebert MP, Schulz HU, Röcken C, Molnar B, Tulassay Z, Malfertheiner P. Dual role of serum soluble E-cadherin as a biological marker of metastatic development in gastric cancer. Scand J Gastroenterol. 2003;38:850–855. doi: 10.1080/00365520310003985. [DOI] [PubMed] [Google Scholar]
- 47.Chan AO, Chu KM, Lam SK, Wong BC, Kwok KF, Law S, Ko S, Hui WM, Yueng YH, Wong J. Soluble E-cadherin is an independent pretherapeutic factor for long-term survival in gastric cancer. J Clin Oncol. 2003;21:2288–2293. doi: 10.1200/JCO.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 48.Chan AO, Lam SK, Chu KM, Lam CM, Kwok E, Leung SY, Yuen ST, Law SY, Hui WM, Lai KC, et al. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48:808–811. doi: 10.1136/gut.48.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]


