Abstract
AIM: To study the expression of vascular endothelial growth factor A (VEGF - A) and VEGF - C and to determine whether the presence of VEGF - A and VEGF - C was associated with the clinicopathologic characteristics of pancreatic cancer.
METHODS: VEGF - A and VEGF - C mRNA transcripts were examined by Northern blot in 6 human pancreatic cancer cell lines and 8 normal pancreatic tissues and 8 pancreatic carcinoma specimens. The expression of VEGF - A and VEGF - C proteins was examined by Western blot in the tested cell lines and by immunohistochemical stain in 50 pancreatic carcinoma samples.
RESULTS: VEGF - A and VEGF - C mRNA transcripts were present in all the 6 human pancreatic cancer cell lines. Immunoblotting revealed the presence of VEGF - A and VEGF - C proteins in all the cell lines. Northern blot analysis of total RNA revealed 3.0-fold and 3.6 - fold increase in VEGF - A and VEGF - C mRNA transcript in the cancer samples, respectively. Immunohistochemical analysis confirmed the expression of VEGF - A and VEGF - C in cancer cells within the tumor mass. Immunohistochemical analysis of 50 pancreatic cancer tissue samples revealed the presence of VEGF-A and VEGF - C immunoreactivity in 50% and 80% of the cancer tissue samples, respectively. The presence of VEGF-A in these cells was associated with larger tumor size and enhanced local spread (χ2 = 6.690, P = 0.035<0.05) but was not associated with decreased patient survival. However, the presence of VEGF-C in the cancer cells was associated with increased lymph node metastasis (χ2 = 5.710, P = 0.017<0.05), but was not associated with decreased patient survival. There was no correlation between the expression of VEGF - A and VEGF - C in the same cancer cells.
CONCLUSION: VEGF - A and VEGF-C are commonly overexpressed in human pancreatic cancer and may contribute to tumor growth and lymph node metastasis. There is no relationship between the expression of VEGF - A and VEGF - C in pancreatic cancer.
Keywords: Pancreatic cancer, Vascular endothelial growth factor - A, Vascular endothelial growth factor - C, Survival
INTRODUCTION
Though the mortality rate of pancreatic cancer has decreased and the survival rate of pancreatic cancer patients is improved after surgery, the 1-year survival rate after diagnosis of pancreatic cancer remains below 20% and the 5-year survival rate is only 3%[1,2]. One reason for this poor prognosis is the propensity of pancreatic cancer to metastasize. Pancreatic carcinoma is characterized by its aggressive local invasion of adjacent structures, perineural invasion and early lymph node and liver metastasis[3-5]. Lymph node metastasis and blood vessel invasion are the definitive factors of the prognosis after resection[6,7].
Angiogenesis plays an important role in the development, growth and metastasis of carcinoma in mammals[8]. It is also important to consider the possible impact of lymphangiogenesis (development of lymphatic vessels) on cancer growth and metastasis [4]. In fact, tumor spread is dependent on both the angiogenic and lymphangiogenic systems. Many carcinomas metastasize through the lymphatic system, whereas others spread mainly hematogenously.
VEGF-A has a potent mitotic activity specific to vascular endothelial cells[10], suggesting that VEGF-A contributes to the pathologic angiogenesis associated with solid tumors. Recently VEGF -B to -E showing homology to VEGF-A have been identified[11-13]. Among these, VEGF -C possessing approximately 30% identity with VEGF, has been shown to induce specific lymphatic endothelial proliferation and hyperplasia of the lymphatic vasculature by binding to its specific receptor flt-4 [11,12,14-16]. VEGF-C may also be an important factor regulating the mutual paracrine relationship between tumor cells and lymphatic endothelial cells not only in primary tumor but also in adjacent normal tissue.
We have previously reported that VEGF-C and its receptor are overexpressed in pancreatic cancer[17]. We now report the coexpression of VEGF -A and VEGF - C in human pancreatic cancer.
MATERIALS AND METHODS
Cell culture
ASPC-1, CAPAN-1, MIA-PaCa-2 and PANC-1 human pancreatic cancer cells were obtained from the American Type Culture (Rockville, MD, USA). COLO-357 and T3M4 human pancreatic cancer cells were a gift from R. S. Metzger at Duke University. Cells were grown in monolayer culture containing humidified 50ml/L CO2 and 95% air at 37°C. ASPC-1, CAPAN-1 and T3M4 cells were grown in RPMI-1640 medium. COLO-357, MIA-PaCa-2 and PANC-1 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM). Madia contained 10% fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin.
Tissues for Northern blot analysis
Normal pancreatic tissue and pancreatic cancer tissue samples were frozen in liquid nitrogen and stored at -80°C for RNA extraction.
Tissues for immunohistochemistry
Pancreatic cancer tissue samples were obtained from 50 patients (35 men and 15 women; mean age, 64.3 ± 10.8 years; range, 48-81 years) undergoing surgery for pancreatic cancer. The tumors were classified as previously described[18]. Histologically, there were 15 well, 31 moderately and 4 poorly differentiated adenocarcinomas. There were 0 stage I, 3 stageII, 9 stage III and 38 stage IV tumors. Tissue samples were fixed in 10% paraformaldehyde solution for 18-24 h and embedded in paraffin.
Cloning and labeling of VEGF-A and VEGF- C probes
Reverse-transcription polymerase chain reaction (RT-PCR) was carried out using RT-PCR kit according to the manufacturer’s instructions. Briefly, total RNA was extracted from human placenta using the acid guanidinium thiocyanate method. The prepared RNA (1 µg) was mixed with reverse transcription reagents in a total volume of 20 µL, incubated for 30 min at 42 °C into first-strand cDNA. One µL cDNA was used for PCR amplification. The upstream primer for VEGF-A was 5’-CTC ACC TGC TTC TGA GTT GC-3’, and the downstream primer was 5’-TTC TCT GCC TCC ACA ATG G-3’ with an expected size of 319 bp. The upstream primer for VEGF-C was 5’-CGGGATCCACGGCTTATGCAAGCAAA-3’, and the downstream primer was 5’-CGGAATTCAACACAGTTTTCCATAATAGA-3 with an expected size of 1167 bp. Amplification conditions were denaturation at 94 °C for 5 min, followed by 32 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min. The PCR products were electrophoresed on 1.5% agarose gels and the target bands containing the specific cDNA were cut down and purified using an ultraclean DNA purification kit (Mo Bio Laboratories, Solana Beach, CA, USA.) according to the manufacturer’s instructions. The accuracy of the RT-PCR products was confirmed by sequencing of cloned cDNA. The prepared cDNA probes were labeled by PCR-DIG labeling mix according to the manufacturer’s instructions.
Northern blot analysis
Total RNA was extracted from human tissue samples and human pancreatic cancer cell lines using the acid guanidinium thiocyanate method. RNA was fractionated on 0.8% agarose/2.2% formaldehyde gels, electrotransferred onto nylon membranes and cross-linked by ultraviolet irradiation[19]. The blots were prehybridized in hybridization buffer for 1 h at 42 °C, hybridized with the indicated DIG-labeled cDNA probes overnight at 42°C, and washed twice for 5 min with 2×SSC/SDS (0.1%) at room temperature, twice for 15 min with 0.1×SSC/SDS (0.1%) at 68°C. DIG-labeled nucleic acids were detected with CSPD according to the manufacturer’s instructions at room temperature. Briefly, after rinsed in washing buffer, the membrane was incubated for 30 min in blocking solution and anti-DIG-AP conjugate, respectively. Then the membrane was washed twice in washing buffer for 15 min and in detection buffer for 5 min and incubated for 5 min in CSPD solution. The damp membrane was sealed in a hybridization bag, incubated for 15 min at 37 °C and exposed to radiographic film for 15 min at room temperature. Equivalent loading of RNA in each lane was confirmed by hybridizing the total RNA with a β-actin cDNA. All cDNAs were labeled with DIG as above. Densitometric analysis of the signals were performed with a gel imaging system.
Immunoblotting
Human pancreatic cancer cells were solubilized in lysis buffer containing 50 mmol Tris, 150 mmol NaCl, 1 mmol ethyleneglycoltetraacetic acid, 1% NP40, 1% sodium deoxycholate, 1 mmol sodium banadate, 50 mmol sodium flupride, 2 mmol EDTA (pH 8.0), 100 µg/mL benzamidine, 50 µg/mL aprotinin, 10 µg/mL leupeptin, 10 µg/mL pepstatin A, and 1 mmol phenylmethylsulfonyl fluoride. Proteins were subjected to sodium dodecyl sulfatepolyacrylamide gel electrophoresis and transferred to Immobilon P membrane. Membranes were incubated for 90 min with highly specific anti-VEGF-A or anti-VEGF-C polyclonal antibody, then washed and incubated with a horseradish peroxidase-coupled secondary goat anti-rabbit antibody for 60 min. After washing, antibodies were visualized by enhanced chemiluminescence. Equivalent loading of protein in each lane was confirmed by hybridizing the total protein with β-actin.
Immunohistochemistry
The same anti-VEGF-A and anti-VEGF-C polyclonal antibodies were used for immunohistochemical analysis. Paraffin-embedded sections (4 µm) of pancreatic cancer and noncancerous pancreatic tissues were subjected to immunostaining using the streptavidin-peroxidase technique. After deparaffinization, antigen retrieval was performed by immersing the sections in 10 mmol citrate buffer (pH 6.0) and heated twice in a microwave oven (95 °C) for 5 min each time. Endogenous peroxidase activity was blocked by incubation with 1% hydrogen peroxide in distilled water for 10 min. Tissue sections were incubated with normal donkey serum for 20 min at room temperature and then incubated with polyclonal anti-VEGF-A antibody (2 µg/mL) or anti-VEGF-C antibody (2 µg/mL) in normal donkey serum for 2 h at room temperature. Bound antibodies were detected with biotinylated goat anti-rabbit IgG secondary antibody and streptavidin-peroxidase complex, using diaminobenzidine tetrahvdrochloride as the substrate. The sections were counterstained with Mayer’s hematoxylin and incubated with nonimmunized rabbit IgG or without primary antibodies, which did not yield positive immunoreactivity. Scoring was carried out by two independent observers blinded to the patient’s status. Positive staining was defined as the presence of VEGF-A and VEGF-C immunoreactivity in at least 10% of the cancer cells.
Statistical analysis
Differences in distribution of VEGF-A or VEGF-C were determined using the x2 test. Kaplan-Meier survival analysis was used to estimate survival time and log-rank test was used to compare differences in survival time between VEGF-A positive and negative groups or between VEGF-C positive and negative groups[20]. P < 0.05 was considered statistically significant.
RESULTS
Expression of VEGF-A and VEGF-C in human pancreatic cancer cell lines
Northern blot analysis of total RNA isolated from pancreatic cancer cell lines indicated that the 6 cell lines expressed the 4.1 kb VEGF-A transcript and 2.4 kb VEGF-C transcript (Figures 1A and 1B). Immunoblotting with a highly specific anti-VEGF-A or anti-VEGF-C antibody revealed an approximately Mr 43000 band (occasionally also a Mr 41000 band) of VEGF-A protein or Mr 55000 band (occasionally proteolytic forms of Mr 41000 molecules) of VEGF-C protein in all the cell lines (Figures 1A and 1B). Both Mr 43000 band and Mr 41000 band corresponded to the VEGF165 isoform homodimer.
Figure 1.
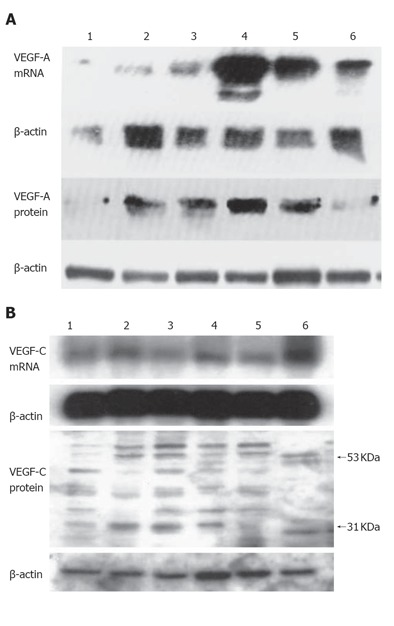
Expression of VEGF-A (A) and VEGF-C (B) in six human pancreatic cancer cell lines. Lane 1: COLO-357; lane 2: MIA-PaCa-2; leane 3: PANC-1; lane 4: T3M4; lane 5: ASPC-1; lane 6: CAPAN-1.
Expression of VEGF-A and VEGF-C mRNA in human pancreas
Northern blot analysis of total RNA isolated from human pancreatic tissue revealed the 4.1 kb VEGF-A transcript and the 2.4 kb VEGF-C transcript in all normal pancreatic tissue samples and cancer tissue samples (Figure 2). Densitometric analysis of the autoradiograms indicated that the levels of 4.1 kb VEGF-A transcript and 2.4 kb VEGF-C transcript were approximately 3.0-fold and 3.6-fold higher in pancreatic cancer tissues in comparison with normal pancreatic tissues.
Figure 2.
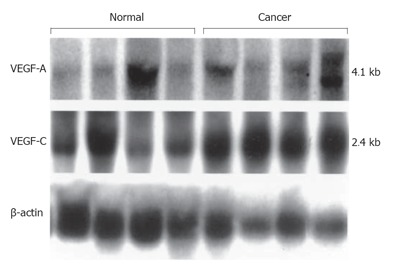
Northern blot analysis of VEGF-A and VEGF-C expression in human pancreatic tissues.
Expression of VEGF-A and VEGF-C protein in human pancreatic tissue
The same anti-VEGF-A or anti-VEGF-C antibody was used to localize VEGF-A or VEGF-C immunohistochemically. In the normal pancreatic tissue, moderate VEGF-A and VEGF-C immunoreactivity was present in the cytoplasm of endocrine islet cells, a weak immunoreactivity in some ductal cells within the small ductules and in a few acinar cells (data not shown). In the pancreatic cancertissue, moderate to strong VEGF-A and VEGF-C immunoreactivity was present in the cytoplasm of many cancer cells (Figures 3A and 3B). Among the 50 pancreatic cancer cases, 25 (50%) were positive for VEGF-A and 40 (80%) for VEGF-C immnunoreactivity in the cytoplasm of cancer cells.
Figure 3.
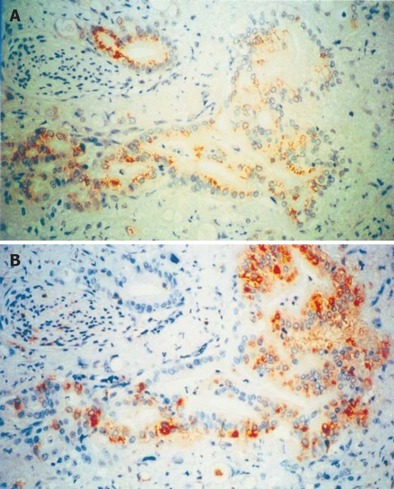
Immunohistochemical analysis of VEGF-A (A) and VEGF-C (B) expression in human pancreatic cancer tissues. In the pancreatic cancers, moderate to strong VEGF-A and VEGF-C immunoreactivity was present in the cytoplasm of cancer cells, ×400.
Correlation between VEGF-A and VEGF-C expression and patient survival
χ2 analysis indicated that the presence of VEGF-A in cancer cells was associated with a statistically significant increase in tumor size and local extension (T category). However, there was no correlation between the presence of VEGF-A in cancer cells and lymph node metastasis or distant metastasis or tumor stage or histological grade of cancers (Table 1). The presence of VEGF-C in cancer cells was associated with a statistically significant increase in the presence of lymph node metastasis (N category), but there was no correlation between the presence of VEGF-C in cancer cells and other factors (Table 1). The mean survival time of the VEGF-A negative and positive groups was 20.80 ± 15.00 months and 16.04 ± 9.00 months respectively. Thus, the survival time of patients with VEGF-A positive tumors was shorter. However, Kaplan-Meier analysis and log-rank test indicated that there was no significant difference in survival between these two groups (Figure 4A). The mean survival time of VEGF-C negative and positive groups was 30.7 ± 14.00 months and 15.4 ± 10.00 months respectively. The survival time of patients with VEGF-C positive tumors was shorter. But Kaplan-Meier analysis and log-rank test indicated that there was no significant difference in survival between these two groups (Figure 4B).
Table 1.
VEGF-A and VEGF-C expression in relation to the clinicopathologic characteristics of 50 patients with pancreatic cancer
| VEGF-A | VEGF-C | |||||
| + | - | P | + | - | P | |
| Age (yr) | 64 ± 10 | 62 ± 9 | 65 ± 10 | 62 ± 8 | ||
| Gender | ||||||
| Male | 18 | 17 | 28 | 7 | ||
| Female | 7 | 8 | 12 | 3 | ||
| TNM | ||||||
| T1 | 1 | 8 | 0.035 | 8 | 1 | 0.701 |
| T2 | 9 | 7 | 13 | 3 | ||
| T3 | 15 | 10 | 19 | 6 | ||
| N0 | 6 | 5 | 0.733 | 6 | 5 | 0.017 |
| N1 | 19 | 20 | 34 | 5 | ||
| M0 | 20 | 23 | 0.221 | 35 | 8 | 0.541 |
| M1 | 5 | 2 | 5 | 2 | ||
| Stage | ||||||
| I | 0 | 0 | 0.801 | 0 | 0 | 0.416 |
| II | 1 | 2 | 2 | 1 | ||
| III | 5 | 4 | 6 | 3 | ||
| IV | 19 | 19 | 32 | 6 | ||
| Differentiated degree | ||||||
| well | 10 | 5 | 0.290 | 14 | 1 | 0.304 |
| moderate | 13 | 18 | 23 | 8 | ||
| poor | 2 | 2 | 3 | 1 | ||
TNM: tumor node metastasis.
Figure 4.
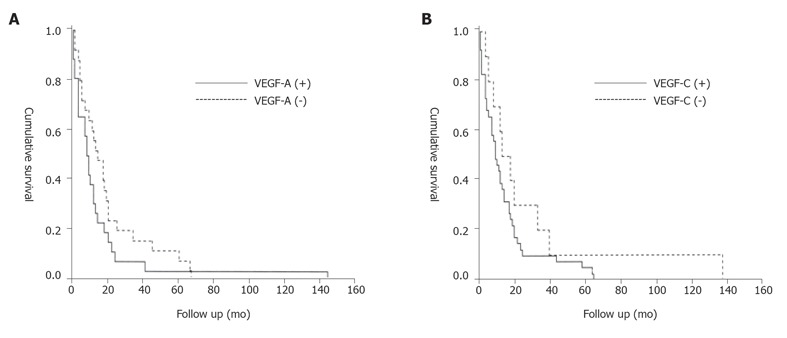
Survival curve. A: Cumulative survival (Kaplan-Meier) plot of the postoperative survival period of patients whose pancreatic tumors exhibited cytoplasmic VEGF-A immunostaining (solid line) versus those whose tumors were negative for VEGF-A (broken line), (P = 0.184); B: Cumulative survival (Kaplan-Meier) plot of the postoperative survival period of patients whose pancreatic tumors exhibited cytoplasmic VEGF-C immunostaining (solid line) versus those whose tumors were negative for VEGF-C (broken line), (P = 0.159).
Correlation between VEGF-A and VEGF-C expression in pancreatic cancer
There was no correlation between VEGF-A and VEGF-C expression in pancreatic cancer (Table 2).
Table 2.
Correlation between VEGF-A and VEGF-C expression in 50 patients with pancreatic cancer
|
VEGF(-A) |
P | ||
| + | - | ||
| + 21 | 19 | ||
| VEGF-C | 0.480 | ||
| - 4 | 6 | ||
DISCUSSION
Recently, VEGF -B to -E showing homology to VEGF-A have been identified[11-13]. Among these, VEGF-C has a high degree (approximately 30%) of homology to VEGF(-A)[11,12,14-16]. VEGF-A plays an important role in tumor growth and metastasis[21-23]. VEGF-C is a ligand for VEGF-A receptor 3 (flt-4) expressed mainly in endothelia of lymphatic vessels and induces specific lymphatic endothelial proliferation and hyperplasia of the lymphatic vasculature[11,24].
Expression of VEGF-A and VEGF-C was detected in normal and cancer tissues in the present study. Overexpression of VEGF-A has been reported in mammary, colorectal, liver, gastric and pancreatic malignancies[21,22,25-27]. Expression of VEGF-A and its receptor KDR correlates with vascularity, proliferation and metastasis of colonic cancer [22] and with vessel count and tumor stage of gastric cancer [26] as well as with growth of pancreatic cancer. Recent studies demonstrated that expression of VEGF-C in various human tumors, including lymphoma, melanoma, fibrosarcoma, squamous cell carcinoma, breast caner, gastric cancer, colorectal cancer, pancreatic cancer and hepatocellular carcinoma[17,28-34]. VEGF-C is expressed only in lymph node-positive breast cancer[29]. Gastric cancer patients with a high expression of VEGF-C protein have a much poorer prognosis than those with a low VEGF-C expression [14]. VEGF-C promotes lymph node metastasis of rectal cancer [30]. Expression of VEGF-C and its receptor flt-4 correlates with lymphangiogenic process and metastasis of pancreatic cancer[17].
The present study showed that VEGF-A and VEGF-C expression was abnormal in pancreatic cancer in vivo. Northern blot analysis revealed a single 4.1 kb VEGF-A mRNA transcript and a single 2.4 kb VEGF-C mRNA transcript in all normal and pancreatic cancer tissue specimens, as well as 3.0-fold and 3.6-fold increase of VEGF-A and VEGF-C mRNA transcripts in pancreatic cancer tissue specimens, indicating that both VEGF-A and VEGF-C are overexpressed in pancreatic cancer. The distribution of VEGF-A and VEGF-C in normal and cancerous tissue specimens was different. In the normal pancreas, VEGF-A and VEGF-C ware relatively abundant in endocine islets, less frequently present in ductal cells and only occasionally present in acinar cells. In contrast, VEGF-A and VEGF-C were abundant in ductal-like cancer cells of pancreas. VEGF-A is a specific mitogen toward endothelial cells and VEGF-C is a specific mitogen to lymphatic endothelial cells, suggesting that VEGF-A has a potential to act in a paracrine manner on endothelial cells within the pancreatic tumor mass, leading to enhanced angiogenesis and tumor growth, while VEGF-C has a potential to act in a paracrine manner on lymphatic endothelial cells within the pancreatic tumor mass, leading to enhanced invasion to lymphatic vessels and lymph node metastasis. Cancer with active lymphangiogenesis could be predisposed to metastatic spread via the lymphatic system, resulting in a poor survival rate. It appears that tumor cells need to penetrate the lymphatic endothelium twice to translocate into the interstitium of a lymph node. Because lymphatic vessels have no tight junctions or continuous basal laminae, their penetration may only require tumor cell adhesion to the endothelium and transmigration through intracellular gaps between lymphatic endothelial cells. VEGF-A can increase microvascular permeability by enhancing the transmigration activity of vesicular-vacuolar organelles[32-34]. VEGF-C and flt-4 may be components of a paracrine signaling network between cancer cells and the endothelium and may be involved in modifying the permeabilities of both blood and lymphatic vessels and metastasis [35].
Univariate analysis of the immunohistochemical data demonstrated that the presence of VEGF-A and VEGF-C in pancreatic cancer cells was associated with tumor size and lymph node metastasis respectively, indicating that VEGF-A and VEGF-C contribute to pancreatic tumor growth and lymphatic metastasis in vivo. Though the survival time was shorter in patients with VEGF-A and VEGF-C positive tumors, this correlation was not statistically significant. Niedergethmann et al [36] and Kuwahara et al [37] reported that the survival time of patients with pancreatic ductal carcinoma showing high expression of VEGF-A is significantly shorter than that of those with low or no expression of VEGF-A. Kurahara et al [38] reported that VEGF-C and VEGF-D expression in tumor cells is significantly associated with the 5-year survival rate of patients with pancreatic head cancer. In our study, VEGF-A or VEGF-C did not emerge as an independent prognostic parameter. Moreover, our previous study showed that the survival time of patients with VEGF-C positiove tumors is shorter[17]. Because the majority of patients in the present study had no detectable metastatic disease at the time of surgery, whether VEGF-A or VEGF-C expression correlates with enhanced propensity of pancreatic cancer to metastasize remains unknown. Further studies are needed.
Footnotes
Supported by grant from Ministry of Education of China, No. 2002247
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Li HY
References
- 1.Zhang ZD. Cancer of the pancreas. In: Wu ZH, Wu ZD, Zheng S, An H, ed , et al., editors. Wai Ke Xue. 6th ed. Beijing: Renmin Weisheng Press; 2003. pp. 608–613. [Google Scholar]
- 2.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Nagakawa T, Konishi I, Higashino Y, Ueno K, Ohta T, Kayahara M, Ueda N, Maeda K, Miyazaki I. The spread and prognosis of carcinoma in the region of the pancreatic head. Jpn J Surg. 1989;19:510–518. doi: 10.1007/BF02471656. [DOI] [PubMed] [Google Scholar]
- 4.Nagakawa T, Kayahara M, Ueno K, Ohta T, Konishi I, Ueda N, Miyazaki I. A clinicopathologic study on neural invasion in cancer of the pancreatic head. Cancer. 1992;69:930–935. doi: 10.1002/1097-0142(19920215)69:4<930::aid-cncr2820690416>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–14; discussion 120-14;. doi: 10.1016/0002-9610(91)90371-j. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Ohashi O, Saitoh Y. [Studies on TNM classification of carcinoma of the exocrine pancreas] Gan To Kagaku Ryoho. 1998;25:143–149. [PubMed] [Google Scholar]
- 8.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 9.van Netten JP, Cann SA, van der Westhuizen NG. Angiogenesis and tumor growth. N Engl J Med. 1996;334:920–91; author reply 921. [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson U, Alitalo K. Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr Top Microbiol Immunol. 1999;237:41–57. doi: 10.1007/978-3-642-59953-8_3. [DOI] [PubMed] [Google Scholar]
- 12.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 14.Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 15.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 16.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22:285–292. doi: 10.1097/00006676-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Inoue Y, Kiyohara Y, Ikeda S. [TNM classification of malignant melanoma and non-melanoma skin cancer] Gan To Kagaku Ryoho. 1997;24:2163–2169. [PubMed] [Google Scholar]
- 19.Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992;90:1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toi M, Hoshina S, Takayanagi T, Tominaga T. Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res. 1994;85:1045–1049. doi: 10.1111/j.1349-7006.1994.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 23.Olson TA, Mohanraj D, Carson LF, Ramakrishnan S. Vascular permeability factor gene expression in normal and neoplastic human ovaries. Cancer Res. 1994;54:276–280. [PubMed] [Google Scholar]
- 24.Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mise M, Arii S, Higashituji H, Furutani M, Niwano M, Harada T, Ishigami S, Toda Y, Nakayama H, Fukumoto M, et al. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455–464. doi: 10.1053/jhep.1996.v23.pm0008617424. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2:1679–1684. [PubMed] [Google Scholar]
- 27.Itakura J, Ishiwata T, Shen B, Kornmann M, Korc M. Concomitant over-expression of vascular endothelial growth factor and its receptors in pancreatic cancer. Int J Cancer. 2000;85:27–34. doi: 10.1002/(sici)1097-0215(20000101)85:1<27::aid-ijc5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Salven P, Lymboussaki A, Heikkilä P, Jääskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol. 1998;153:103–108. doi: 10.1016/S0002-9440(10)65550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurebayashi J, Otsuki T, Kunisue H, Mikami Y, Tanaka K, Yamamoto S, Sonoo H. Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J Cancer Res. 1999;90:977–981. doi: 10.1111/j.1349-7006.1999.tb00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami M, Yanai Y, Hata F, Hirata K. Vascular endothelial growth factor C promotes lymph node metastasis in a rectal cancer orthotopic model. Surg Today. 2005;35:131–138. doi: 10.1007/s00595-004-2896-0. [DOI] [PubMed] [Google Scholar]
- 31.Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H. Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol. 2004;40:13–20. doi: 10.1016/s1368-8375(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 32.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 33.Peng L, Wang SX, Zhang FR, Tang RF, Zhang M. Expression of VEGF-C in hepatocellular carcinoma tissue. Zhengzhou Daxue Xuebao (Yixueban) 2005;40:302–305. [Google Scholar]
- 34.O'Morchoe CC, O'Morchoe PJ. Differences in lymphatic and blood capillary permeability: ultrastructural-functional correlations. Lymphology. 1987;20:205–209. [PubMed] [Google Scholar]
- 35.Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crusells J, Fletcher CD, de Waal RM, Kaipainen A, Alitalo K. Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol. 1998;153:395–403. doi: 10.1016/S0002-9440(10)65583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122–129. doi: 10.1097/00006676-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26:344–349. doi: 10.1097/00006676-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T. Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: its relationship to lymph node metastasis. Clin Cancer Res. 2004;10:8413–8420. doi: 10.1158/1078-0432.CCR-04-0379. [DOI] [PubMed] [Google Scholar]


