Abstract
AIM: To investigate the correlation between ASCA and presence of mucosal S. cerevisiae DNA in a population of CD, ulcerative colitis (UC) patients and controls.
METHODS: S. cerevisiae-specific primers and a fluorescent probe were designed for a 5’ exonuclease real time PCR (TaqManTM) assay, which is a homogenous system using a fluorescent-labelled probe for the detection of PCR product in real time. We analyzed the relation of the PCR results with the ASCA findings in a group of 76 inflammatory bowel disease (IBD) patients (31 CD, 45 UC) and 22 healthy controls (HC).
RESULTS: ASCA (IgA or IgG) were positive in 19 (61%) patients with CD, 12 (27%) with UC and none of the HC. PCR amplification was inhibited and excluded from the final results in 10 (22%) UC patients, 7 (22%) CD patients, and 6 (30%) HC. In only 15 of the mucosal samples, S. cerevisiae DNA was detected by real time PCR, including 7 (29%) in CD, 7 (19%) in UC, 1 (6%) in HC. In 4 CD and in 4 UC patients, ASCA and mucosal S. cerevisiae were positive. Mucosal S. cerevisiae was present in combination with negative ASCA IgA and IgG in 3 UC, and 3 CD patients.
CONCLUSION: We conclude that since the presence of S. cerevisiae in colonic mucosal biopsy specimens is very rare, ASCA is unlikely to be explained by continuous exposure to S. cerevisiae in the mucosa. Therefore, ASCA formation must occur earlier in life and levels remain relatively stable thereafter in immunological susceptible persons.
Keywords: ASCA, Saccharomyces cerevisiae, mucosa, IBD
INTRODUCTION
Inflammatory bowel disease (IBD) is characterized by a chronic relapsing intestinal inflammation. The causes of IBD are unknown but genetic, environmental, immunological, and microbial factors may be involved. Results from studies on animals suggest that the intestinal flora participates in the initiation and perpetuation of IBD[1].
Saccharomyces cerevisiae is the most common species of the genus Saccharomyces. It is used in baker’s yeast and readily finds its way into our foods[2]. Several case-series describe serious S. cerevisiae infections in immune-compromised patients, usually following treatment with broad-spectrum antibiotics[3-6]. Opportunistic infections by viral and fungal agents have been described as occurring in rare cases of ulcerative colitis (UC). Only one case describes diarrhea associated with S. cerevisiae cultured in the stool specimen of an UC patient[7]. So far, no studies have been published concerning the presence of S. cerevisiae in the intestinal tissue of patients with IBD. S. cerevisiae specific primers and a fluorescent probe were designed for a 5’ exonuclease real time PCR (TaqManTM) assay. This method provides high specificity and sensitivity for detecting S. cerevisiae DNA but is hampered by the difficulties arising from DNA extraction of Saccharomyces species .
In 1988, the presence of anti-Saccharomyces cerevisiae antibodies (ASCA) in patients with Crohn’s disease (CD) was firstly described[8]. The ASCA test for diagnosing CD has a sensitivity of 72% and a specificity of 82%[9-12]. The underlying cause of generating S. cerevisiae antigens supporting the specific antibody response in CD is still unknown. ASCA are thought to result from a specific antibody response to the S. cerevisiae cell wall mannan (phosphopeptidomannans). It is unknown whether this is a direct response towards the yeast itself or an epiphenomena with a similar immunologic response towards another antigen. It is postulated that the yeast wall cell mannan may mimic a high mannose-containing molecule towards which the antibody is directed inducing a hypersensitivity reaction during inflammation[12,13].
MATERIALS AND METHODS
Patients
Seventy-six patients with IBD (45 UC, 31 CD) and 22 healthy age- and sex-matched controls regularly visiting the Departments of Gastroenterology from the VU University Medical Center, Amsterdam, the Netherlands, a third line referral center, and St Anna Hospital, Geldrop, the Netherlands, a regional hospital, were enrolled in the study. IBD patients with clinical complaints compatible with active inflammation of the mucosa were screened for the study, but both IBD patients with active inflammation as well as quiescent disease assessed by macroscopic endoscopic findings were included in the study. The diagnosis of CD and UC was based on standard endoscopic, histological, and radiographic features[14]. Disease localization and activity, demographical data and medication were documented. Fifty-four percent of UC patients and 42% of CD patients was treated with immunosuppressive medication (Table 1). None of the patients was treated with probiotics containing S. boulardii. However, they were not requested to use a diet low of S. cerevisiae either.
Table 1.
Medication at time of harvest of biopsy specimens
| Medication | No of patients (%) | ||
| UC | Prednisone | 12/37 | 32 |
| AZA | 11/37 | 30 | |
| ASA | 8/37 | 22 | |
| Ciclosporine | 4/37 | 11 | |
| No medication | 12/37 | 32 | |
| Any immunomodulating drug | 20/37 | 54 | |
| CD | Prednisone | 6/24 | 25 |
| AZA | 5/24 | 21 | |
| ASA | 2/24 | 8 | |
| Ciproxin | 1/24 | 4 | |
| Anti-TNF | 2/24 | 8 | |
| No medication | 13/24 | 54 | |
| Any immunomodulating drug | 10/24 | 42 | |
During sigmoidoscopy, 2 mucosal biopsy specimens were obtained from the sigmoid and directly snap frozen in liquid nitrogen and then stored at -18 oC until further analysis. In addition, blood samples were drawn for detection of ASCA antibodies.
This study was approved by the Medical Ethical Committee of the VU University Medical Center, Amsterdam, the Netherlands.
ASCA ELISA
ASCA IgA and IgG were determined by commercially available ELISA kits (Inova, Uniprom Diagnostics BV, Krimpen aan de IIssel, the Netherlands). The antigen consists of phosphopeptidomannan (PPM) extracted from S. cerevisiae. ASCA ELISAs were performed according to the manufacturer’s instructions. ASCA results were expressed as arbitrary units with a cut-off for positivity of 25 U/mL as advised by the manufacturer and described elsewhere[15]. Serum was considered positive if either IgA or IgG or both were positive. Serum was considered negative if both IgA and IgG ASCA were negative.
DNA isolation
Biopsy specimens were pretreated with 200 µL of 5 g/L collagenase A (Roche Diagnostics, Almere, Nederland B.V.) and incubated at 37°C overnight with continuous shaking. Subsequently, each biopsy was divided into two equal cell fractions. One fraction was spiked with 20 colony forming units/µL S. cerevisiae . Both fractions were incubated for 90 min at 37 0C with 600 µL of sorbitol buffer and 200 U of lyticase (Sigma-Aldrich, Steinheim, Germany), prior to the isolation of chromosomal DNA with the DNeasyTM Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The end volume after the extraction of the DNA from the biopsy specimen was 100 µL. The control fraction of the biopsy specimen contained 20 CFU equivalents/µL (elution concentration).
S. cerevisiae specific primers and a fluorescent probe for a 5’ exonuclease RT PCR (TaqManTM) assay were designed with the Primer Express software package (Applied Biosystems, Foster City, CA, USA). This assay is a homogenous system with a fluorescent double-labeled probe for the quantitative detection of PCR product. This provides a rapid automated combined PCR amplification and detection system with no post-amplification manipulation of amplicons, thereby considerably reducing the risk of contamination. Coupled with a quick, simple DNA extraction method, this protocol allows for rapid specification of clinical isolates. Amplification reactions were carried out in a total volume of 25 µL. Reaction mixtures contained 1x universal master mix (Applied Biosystems), 300 nmol of Saccharomyces forward primer (5’- GAA ATG CCA CCG TGA ATG C), 300 nmol of Saccharomyces reverse primer (5’-CTT TGG TGG TGA TCC TCT ATG ATT G) and 100 nmol of labeled probe (FAM - TGG CAC CAT GAA CCC TAG CGT CGT T - TAMRA), and 5 µL of DNA. To prevent inhibition, 5 g/L bovine serum albumin (BSA) (Sigma–Aldrich, Steinheim, Germany) was added to the PCR mixtures. Amplification was carried out after an incubation for 2 min at 50 °C and for 10 min at 95 °C, followed by 45 cycles at 95 °C for 15 s, and at 60 °C for 1 min. These reactions were performed on an ABI Prism 7000 Sequence Detection System (SDS) (Applied Biosystems, Figure 1).
Figure 1.
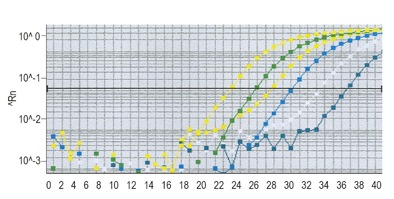
Annealing cycles of S. cerevisae
Statistics
SDS software was used for analysis of the data obtained from RT-PCR results, and t-test with separate variance estimates were used to test differences between patients with CD, UC and controls.
RESULTS
ASCA (IgA or IgG) were identified in 19 (61%) CD patients, 12 (27%) UC patients and none of the HC. Of the patients, 42% of CD patients and 54% of UC patients were using immunosuppressive medication (Table 2).
Table 2.
RT-PCR and ASCA results in relation to medication
| Medication | RT-PCR + | (%) | RT-PCR - | (%) | ASCA + | (%) | ASCA - | (%) | |
| UC | Prednisone | 6/7 | 86 | 6/30 | 20 | 5/12 | 42 | 7/25 | 28 |
| AZA | 1/7 | 14 | 10/30 | 33 | 2/12 | 17 | 9/25 | 36 | |
| ASA | 3/7 | 43 | 5/30 | 17 | 0 | 8/25 | 32 | ||
| Ciclosporine | 1/7 | 14 | 3/30 | 10 | 1/12 | 8 | 3/25 | 12 | |
| No medication | 0 | 12/30 | 40 | 7/12 | 58 | 6/25 | 24 | ||
| CD | Prednisone | 2/7 | 29 | 4/17 | 24 | 4/16 | 25 | 2/8 | 25 |
| AZA | 2/7 | 29 | 3/17 | 18 | 2/16 | 13 | 3/8 | 38 | |
| ASA | 1/7 | 14 | 1/17 | 6 | 1/16 | 6 | 1/8 | 13 | |
| Ciproxin | 1/7 | 14 | 0 | 1/16 | 6 | 0 | |||
| Anti-TNF | 2/7 | 29 | 0 | 2/16 | 13 | 0 | |||
| No medication | 2/7 | 29 | 11/17 | 65 | 8/16 | 50 | 5/8 | 63 |
To determine the sensitivity of real time PCR, S. cerevisiae cells were cultured and the amount of colony forming units (CFU) were counted. After DNA isolation a dynamic range, based on the related amount of DNA (CFU equivalents) was made from 10 to 105 (Figure 2).
Figure 2.
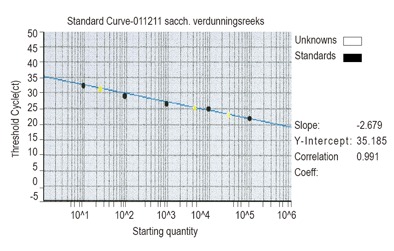
Dilution series of S. cerevisiae
Specificity of the PCR for S. cerevisiae was examined by comparing the results of pure S. cerevisiae DNA and S. cerevisiae DNA mixed with DNA isolated from 10 different bacteria (Bacteroides vulgatus, Bifidobaterium adolescentis, Clostridium difficile, Lactobacillus acidophilus, Proprionibacterium acnes, Actinomyces viscosus, Enterococus faecalis, Fusobacterium nucleatum, Escherichia coli, Streptococcus pyogenus, Listeria monocytogenes, Listeria ivanovii), belonging to the normal gut flora and 6 different Saccharomyces species (S. boulardii, S. unisporus, S. kluyveri, S. pastorianus, S. paradoxus, S. servazzii). In addition, this DNA mixture neither influenced the sensitivity nor the specificity of the S. cerevisiae amplification (data not shown). Among the 15 mucosal biopsies, S. cerevisiae DNA was detected in 7 (29%) CD patients, 7 (19%) UC patients, 1 (6%) HC. The amount of S. cerevisiae DNA was low and varied. Positive values were to be found between 37-38 CFU equivalents. Inhibition during amplification occurred in 8 (18%) UC patients, 7 (22%) CD patients and 6 (30%) HC.
Furthermore, 25% (4/16) CD patients and 33% (4/12) UC patients showed positivity for both ASCA and mucosal S. cerevisiae DNA. In 12% (3/25) UC and 38% (3/8) CD patients, mucosal S. cerevisiae DNA was present in combination with negative ASCA IgA and IgG (Tables 3 and 4).
Table 3.
ASCA and RT-PCR detection of S. cerevisiae in CD patients
| ASCA positive | ASCA negative | Total | |
| RT-PCR positive | 4 | 3 | 7 |
| RT-PCR negative | 12 | 5 | 17 |
| Total | 16 | 8 | 24 |
Table 4.
ASCA and RT-PCR detection of S. cerevisiae in UC patients
| ASCA positive | ASCA negative | Total | |
| RT-PCR positive | 4 | 3 | 7 |
| RT-PCR negative | 8 | 22 | 30 |
| Total | 12 | 25 | 37 |
DISCUSSION
ASCA (IgA or IgG) were positive in 19 (61%) CD patients, 12 (27%) UC patients and none of the HC. Out of the 15 mucosal biopsies, RT PCR demonstrated the presence of S. cerevisiae DNA in 7 (29%) CD patients, 7 (19%) UC patients, and 1 (6%) HC. No significant correlation could be found between ASCA and the presence of S. cerevisae DNA isolated from mucosal biopsies.
Frequencies of ASCA reported in literatures range from 39-76% for CD patients, 5-15% for UC patients to 0-5 % in controls[8,11,12,16-23]. In this study ASCA positivity in the UC group was high (27%) as compared to earlier ASCA studies from our group and reported frequencies in literature.
The presence of ASCA appears not to be related to disease activity[23]. Therefore, we assume that our selection of patients (clinical complaints compatible with active disease) is not likely to influence ASCA status or the presence of S. cerevisiae. Forty-two percent of CD patients and 54% of UC patients were using immunosuppressive agents in this cohort (Table 1). This high percentage of patients using immunosuppressive agents reflects the overall disease activity of our patient population, being relatively severe. However, use of immunosuppressive agents does not explain the high frequency of ASCA in our UC population since most ASCA-positive UC patients did not use this type of medication. Therefore, it can be hypothesized that healing of the mucosa with the use of immunosuppressive agents will lead to decreasing ASCA levels, as has been reported in the treatment of children with active CD[22]. ASCA were detected with well-validated commercially available kits that have been described in previous studies[9].
Mucosal permeability appears increased in active CD as a consequence of direct effects of pro-inflammatory molecules and transmigrating neutrophils. However, considerable controversy exists regarding a primary genetically determined defect in epithelial barrier function[24-26].
In a small study[27], intestinal permeability, tested by a cellobiose/mannitol test, was raised in 6 (37%) of the CD patients and in 11% of their relatives. This altered intestinal permeability was unrelated to sex, age, disease activity, localization, duration, treatment schedule, as well as to serum ASCA positivity, a rather stringent subdivision in such a small patient group. Although an interesting finding, this makes interpretation difficult. Furthermore, genetic polymorphisms were not taken into account.
ASCA is not related to mucosal disintegrity, because ASCA is independent of disease activity[28]. Elevated serum levels of ASCA did not primarily seem to result from a defect of the gut barrier[29]. These data fit in with the hypothesis that a relationship between antibody responses toward microbial antigens and complicated small bowel diseases reflect the interplay between a genetically susceptible host and relevant luminal flora, as has been suggested before, relating intestinal permeability to environmental factors, and ASCA generation to genetic predisposition[23].
To our knowledge, this is the first study describing the presence of S. cerevisiae DNA in intestinal mucosa of IBD patients by using RT-PCR.The presence of ASCA antibodies could not be correlated with mucosal S. cerevisiae DNA in biopsy specimens taken during sigmoidoscopy although the RT-PCR test is highly sensitive and specific. Every individual sample was checked for inhibition by a simultaneous spiked amplification. Inhibition during amplification occurred in 8 (18%) UC patients, 7 (22%) CD patients and 6 (30%) HC. Extraction of DNA from yeast requires special enzymes to remove the cell wall[30]. Theoretically, excluding a number of patients from the total patients due to inhibition of the RT-PCR amplification may influence the results, particularly taking into account the relatively small study population. However, amplification problems were equally distributed in all groups. Furthermore, one could hypothesize that the presence of S. cerevisiae is higher in ileal mucosa than in the sigmoid since higher ASCA levels were detected in CD patients with ileal localization of disease[28]. Preliminary RT-PCR data from our group show comparable numbers of S. cerevisiae in the left and right side of the colon consistent with a equally distributed presence of S. cerevisiae throughout the (distal) intestinal tract (Akol, personal communication). Interbiopsy variability between multiple biopsies taken at the same localization was low. Therefore, our results were unlikely to be biased by neither the number of biopsy specimens nor the localization from where the biopsies were obtained.
The presence of mucosal S. cerevisiae DNA with concomitant negative serum ASCA IgA and IgG in one UC and two CD patients can not be explained by the intestinal leakage hypothesis. This finding corroborates the hypothesis that ASCA formation is an epiphenomenon (in genetically susceptible individuals) rather than the result of antigenic challenge by intestinal presence of S. cerevisiae in leaking mucosa. On the contrary, we postulate that since the presence of S. cerevisiae DNA in mucosal biopsy specimens is very rare, presence of ASCA can not be explained by antigenic exposure to S. cerevisiae.
Another hypothesis is that everyone encounters S. cerevisiae early in life. In some patients, the (transitory) S. cerevisiae presence may lead to ASCA formation, particularly in people prone to develop CD. Only in a small percentage of people, life-long colonisation may be the result, comparable to what has been documented in other species such as Clostridium difficile. Although intra-individual, longitudinal determination values of ASCA were not tested in this study, ASCA formation may be initiated early in life and perpetuate thereafter as described in adult populations[31]. In contrast, ASCA levels decrease after treating active inflammation in children with CD[22,32,33]. These differences between stable ASCA values in an adult population and decreasing ASCA values in a paediatric population have recently been described for a population with coeliac disease[34], pointing at differences in antigen handling/immunologic response in children and adults.
In conclusion, our study demonstrates no any correlation between the presence of ASCA antibodies and mucosal S. cerevisiae DNA. Although the study population was small, this finding underscores the hypothesis that ASCA antibodies are not solely formed as a reaction on the mannan from the yeast cell wall but rather is an epiphenomenon with a similar immunologic response towards another, yet unidentified antigen.
ACKNOWLEDGEMENTS
Xander Huijsdens is kindly acknowledged for designing the primer-probe combinations for S. cerevisiae. Furthermore, we thank Teun Boekhout from the CBS in Utrecht for providing the Candida species for testing the specificity of the PCR. We also thank Uniprom Diagnostics BV, Krimpen aan de IIssel, the Netherlands for providing the ASCA kits.
Footnotes
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M
References
- 1.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 2.Floch MH. Saccharomyces: is it a probiotic or a pathogen and what is the significance of an elevated anti-S. cerevisiae antibody. J Clin Gastroenterol. 2003;36:5–6. doi: 10.1097/00004836-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Reuther GA, Rodgers DJ. AOA continuing medical education. J Am Osteopath Assoc. 1992;92:1404, 1411–1417. [PubMed] [Google Scholar]
- 4.Cesaro S, Chinello P, Rossi L, Zanesco L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Support Care Cancer. 2000;8:504–505. doi: 10.1007/s005200000123. [DOI] [PubMed] [Google Scholar]
- 5.Riquelme AJ, Calvo MA, Guzmán AM, Depix MS, García P, Pérez C, Arrese M, Labarca JA. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol. 2003;36:41–43. doi: 10.1097/00004836-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Fiore NF, Conway JH, West KW, Kleiman MB. Saccharomyces cerevisiae infections in children. Pediatr Infect Dis J. 1998;17:1177–1179. doi: 10.1097/00006454-199812000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Candelli M, Nista EC, Nestola M, Armuzzi A, Silveri NG, Gasbarrini G, Gasbarrini A. Saccharomyces cerevisiae-associated diarrhea in an immunocompetent patient with ulcerative colitis. J Clin Gastroenterol. 2003;36:39–40. doi: 10.1097/00004836-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Main J, McKenzie H, Yeaman GR, Kerr MA, Robson D, Pennington CR, Parratt D. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105–1106. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linskens RK, Mallant-Hent RC, Groothuismink ZM, Bakker-Jonges LE, van de Merwe JP, Hooijkaas H, von Blomberg BM, Meuwissen SG. Evaluation of serological markers to differentiate between ulcerative colitis and Crohn's disease: pANCA, ASCA and agglutinating antibodies to anaerobic coccoid rods. Eur J Gastroenterol Hepatol. 2002;14:1013–1018. doi: 10.1097/00042737-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Sendid B, Quinton JF, Charrier G, Goulet O, Cortot A, Grandbastien B, Poulain D, Colombel JF. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn's disease. Am J Gastroenterol. 1998;93:1306–1310. doi: 10.1111/j.1572-0241.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 11.Seibold F, Stich O, Hufnagl R, Kamil S, Scheurlen M. Anti-Saccharomyces cerevisiae antibodies in inflammatory bowel disease: a family study. Scand J Gastroenterol. 2001;36:196–201. doi: 10.1080/003655201750065960. [DOI] [PubMed] [Google Scholar]
- 12.Annese V, Andreoli A, Andriulli A, Dinca R, Gionchetti P, Latiano A, Lombardi G, Piepoli A, Poulain D, Sendid B, et al. Familial expression of anti-Saccharomyces cerevisiae Mannan antibodies in Crohn's disease and ulcerative colitis: a GISC study. Am J Gastroenterol. 2001;96:2407–2412. doi: 10.1111/j.1572-0241.2001.04043.x. [DOI] [PubMed] [Google Scholar]
- 13.Oshitani N, Hato F, Suzuki K, Sawa Y, Matsumoto T, Maeda K, Higuchi K, Kitagawa S, Arakawa T. Cross-reactivity of yeast antigens in human colon and peripheral leukocytes. J Pathol. 2003;199:361–367. doi: 10.1002/path.1276. [DOI] [PubMed] [Google Scholar]
- 14.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16-9. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 15.Klebl FH, Bataille F, Hofstädter F, Herfarth H, Schölmerich J, Rogler G. Optimising the diagnostic value of anti-Saccharomyces cerevisiae-antibodies (ASCA) in Crohn's disease. Int J Colorectal Dis. 2004;19:319–324. doi: 10.1007/s00384-003-0557-1. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990;31:536–538. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 18.Koutroubakis IE, Petinaki E, Mouzas IA, Vlachonikolis IG, Anagnostopoulou E, Castanas E, Maniatis AN, Kouroumalis EA. Anti-Saccharomyces cerevisiae mannan antibodies and antineutrophil cytoplasmic autoantibodies in Greek patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:449–454. doi: 10.1111/j.1572-0241.2001.03524.x. [DOI] [PubMed] [Google Scholar]
- 19.Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan SR, Colombel JF, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton CL, Yang H, Li Z, Rotter JI, Targan SR, Braun J. Familial expression of anti-Saccharomyces cerevisiae mannan antibodies in affected and unaffected relatives of patients with Crohn's disease. Gut. 2000;46:58–63. doi: 10.1136/gut.46.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sendid B, Colombel JF, Jacquinot PM, Faille C, Fruit J, Cortot A, Lucidarme D, Camus D, Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996;3:219–226. doi: 10.1128/cdli.3.2.219-226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–829. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 23.Vermeire S, Peeters M, Vlietinck R, Joossens S, Den Hond E, Bulteel V, Bossuyt X, Geypens B, Rutgeerts P. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis. 2001;7:8–15. doi: 10.1097/00054725-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hollander D. Permeability in Crohn's disease: altered barrier functions in healthy relatives. Gastroenterology. 1993;104:1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 25.Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 26.Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 27.Secondulfo M, de Magistris L, Fiandra R, Caserta L, Belletta M, Tartaglione MT, Riegler G, Biagi F, Corazza GR, Carratù R. Intestinal permeability in Crohn's disease patients and their first degree relatives. Dig Liver Dis. 2001;33:680–685. doi: 10.1016/s1590-8658(01)80045-1. [DOI] [PubMed] [Google Scholar]
- 28.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Harrer M, Reinisch W, Dejaco C, Kratzer V, Gmeiner M, Miehsler W, Norman GL, Gangl A, Vogelsang H. Do high serum levels of anti-Saccharomyces cerevisiae antibodies result from a leakiness of the gut barrier in Crohn's disease. Eur J Gastroenterol Hepatol. 2003;15:1281–1285. doi: 10.1097/00042737-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Harju S, Fedosyuk H, Peterson KR. Rapid isolation of yeast genomic DNA: Bust n' Grab. BMC Biotechnol. 2004;4:8. doi: 10.1186/1472-6750-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teml A, Kratzer V, Schneider B, Lochs H, Norman GL, Gangl A, Vogelsang H, Reinisch W. Anti-Saccharomyces cerevisiae antibodies: a stable marker for Crohn's disease during steroid and 5-aminosalicylic acid treatment. Am J Gastroenterol. 2003;98:2226–2231. doi: 10.1111/j.1572-0241.2003.07673.x. [DOI] [PubMed] [Google Scholar]
- 32.Canani RB, Romano MT, Greco L, Terrin G, Sferlazzas C, Barabino A, Fontana M, Roggero P, Guariso G, De Angelis G, et al. Effects of disease activity on anti-Saccharomyces cerevisiae antibodies: implications for diagnosis and follow-up of children with Crohn's disease. Inflamm Bowel Dis. 2004;10:234–239. doi: 10.1097/00054725-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Desir B, Amre DK, Lu SE, Ohman-Strickland P, Dubinsky M, Fisher R, Seidman EG. Utility of serum antibodies in determining clinical course in pediatric Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:139–146. doi: 10.1016/s1542-3565(03)00321-5. [DOI] [PubMed] [Google Scholar]
- 34.Granito A, Zauli D, Muratori P, Muratori L, Grassi A, Bortolotti R, Petrolini N, Veronesi L, Gionchetti P, Bianchi FB, et al. Anti-Saccharomyces cerevisiae and perinuclear anti-neutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment Pharmacol Ther. 2005;21:881–887. doi: 10.1111/j.1365-2036.2005.02417.x. [DOI] [PubMed] [Google Scholar]


