Abstract
AIM: There is strong evidence that interleukin-11 (IL-11) is involved in the regulation of tumor progression, cellular growth and differentiation. Recently, interleukin-11 receptor (IL-11R) has been detected on some cancer cells. In this study, we investigated the expression of IL-11 and IL-11R in colorectal adenocarcinoma.
METHODS: To elucidate the involvement of IL-11 and IL-11Rα in human intestinal adenocarcinomas, we examined 115 cases of surgically resected human colonic adenocarcinoma and 11 cases of adenoma by immunohistochemistry and Western blotting.
RESULTS: Among 115 cases of adenocarcinoma, 100 cases (87.0%) showed positive staining in the cytoplasm of carcinoma cells for the IL-11, and 87 cases (75.6%) were positive for the IL-11Rα. Six cases (54.5%) and four cases (36.4%) of 11 adenomas were positive for IL-11 and IL-11Rα, respectively. The expression of IL-11Rα correlated with the histological differentiation (P = 0.033503), the depth of tumor invasion (P = 0.006395), Dukes’ classification (P = 0.015648) and lymphatic invasion (P = 0.003865). However, the expression of IL-11Rα was not correlated with the venous invasion and the presence of lymph node metastasis. The expression of IL-11 was not correlated with any clinicopathological factors. In Western blot analysis, two human colorectal carcinoma cell lines and four tissues of surgically resected human carcinoma expressed both IL-11 and IL-11Rα proteins.
CONCLUSION: IL-11 and IL-11Rα are highly expressed in human colorectal adenocarcinoma and the IL-11Rα expression is correlated with clinicopathological factors. These findings suggest that the expression of IL-11Rα is an important factor for the invasion of human colorectal adenocarcinoma.
Keywords: IL-11, IL-11 receptor α, Colorectal cancer
INTRODUCTION
Colorectal cancer is one of the most common neoplasms and is a significant cause of morbidity and mortality[1,2]. Consequently, colorectal carcinoma is one of the best characterized examples of multistep progression in which activation of oncogenes, such as the K-ras gene[3], and inactivation of tumor suppressor genes, such as APC, DCC and p53[4-7] take place. The prognosis of colorectal cancer patients is based on the depth of tumor cell invasion and the presence of lymph node metastasis. Recently, it has been suggested that the occurrence and progression of cancer is related to the activation of some cytokines and/or an intracellular signaling pathway. But the development and progressive mechanism of the colorectal cancer is not fully understood.
IL-11 was cloned from the primate stromal cell line PU-34 and was initially considered to be a hematopoietic cytokine[8,9]. It was found later to have effects on non-hematopoietic systems, and to act on many different cells and tissues, which included stimulation of megakaryocyte maturation, platelet production, and growth stimulation of CD34+ hematopoietic progenitor cells[8,9]. This cytokine has also been shown to mediate inhibition of adipogenesis[10] and stimulation of osteoclasts[11], and acts as a cytoprotective agent of gut mucosa during treatment with radiation and chemotherapy and in models of inflammatory bowel disease[12-15].
The IL-11 receptor (IL-11R) is a member of the gp130-dependent receptor group, which includes the receptors for interleukin-6 (IL-6), leukemia inhibitory factor (LIF), ciliary neurotrophic factor and oncostatin M, and it has been associated with various unique biological actions[16]. IL-11-induced signaling is mediated by the formation of a receptor complex, composed of two molecules each of IL-11, two subunits of the IL-11Rα, and gp130. An important signaling system activated by the IL-11Rα and other members of this receptor family is the PI3 kinase pathway, MAP kinase pathway and the Janus kinase signaling transducer and activator of transcription (Jak-STAT) pathway[17].
Recent studies have shown that IL-11R expression was not only present in megakaryocytes, osteoclasts and colon epithelium but also in breast, ovarian and prostate cancers[18-20]. This suggested that it was theoretically possible that IL-11 could affect the growth of tumor cells. In contrast, one study showed that IL-11 may be involved in the normal growth control in the intestinal epithelium, which suggests that the inhibition by IL-11 is lost during carcinogenic transformation[21]. In addition, another study showed that IL-11 appears unlikely to stimulate the growth of the most common solid tumors[22]. Since, IL-11 could become a therapeutically important compound for providing supportive care for patients who receive cancer chemotherapy, it is important to establish that IL-11 does not stimulate growth of tumor cells.
In this study, we investigated the expression of IL-11 and IL-11Rα in human colorectal adenocarcinoma and compared that with the clinicopathological factors to elucidate the relationship of IL-11 and IL-11R in colorectal carcinoma.
MATERIALS AND METHODS
Human colorectal tissues and cell lines
Human colorectal tissues were obtained from fresh surgical samples or paraffin-embedded blocks as described previously. All specimens were obtained from patients operated at Nagasaki University Hospital between 1998 and 2003. We examined 115 cases of human colorectal adenocarcinoma and 11 cases of adenoma as benign lesions with moderate dysplasia. Each tumor was assigned a histological type according to the Japanese Classification of Colorectal Carcinoma by the World Health Organization classification[23] and a depth grading of infiltration according to the TNM staging system by the American Joint Commission on Cancer[24]. 24 mucosal carcinomas (Tis), 11 submucosal infiltrative carcinomas (T1), 10 carcinomas invading proprial muscle layers (T2), 48 carcinomas reaching the subserosa (T3), and 22 carcinomas penetrating the serosal surface (T4) were examined. Histologically, 57 were well-differentiated adenocarcinomas (wel), 46 were moderately-differentiated adenocarcinomas (mod), 5 were poorly differentiated adenocarcinomas (por), and 7 were mucinous carcinomas (muc) (Table 1).
Table 1.
Expression of IL-11 and IL-11Rα in colorectal carcinoma, (%)
| n | IL-11 | IL-11Rα | |||||
| - | + | ++ | - | + | ++ | ||
| Normal mucosa | 1P = 0.001883, vs carcinoma | 1P = 0.00196, vs carcinoma | |||||
| 10 | 5 (50.0) | 3 (30.0) | 2 (20.0) | 6 (60.0) | 4 (40.0) | 0 (0.0) | |
| Adenoma | 1P = 0.023291, vs carcinoma | 1P = 0.000833, vs carcinoma | |||||
| 11 | 5 (45.5) | 2 (18.2) | 4 (36.4) | 7 (63.6) | 4 (36.4) | 0 (0.0) | |
| Total carcinoma | 115 | 15 (13.0) | 26 (22.6) | 74 (64.3) | 28 (24.3) | 31 (27.0) | 56 (48.7) |
| Histological differentiation | NS | 1P = 0.033503 | |||||
| Wel | 57 | 8 (14.0) | 15 (26.3) | 34 (59.6) | 17 (29.8) | 16 (28.1) | 24 (42.1) |
| Mod | 46 | 5 (10.9) | 8 (17.4) | 33 (71.7) | 6 (13.0) | 14 (30.4) | 26 (56.5) |
| Por | 5 | 0 (0.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) | 0 (0.0) | 4 (80.0) |
| Muc | 7 | 2 (28.6) | 2 (28.6) | 3 (42.9) | 4 (57.1) | 1 (14.3) | 2 (28.6) |
| Depth of tumor invasions | NS | 1P = 0.006395 | |||||
| Tis | 24 | 4 (16.7) | 6 (25.0) | 14 (58.3) | 8 (33.3) | 10 (41.7) | 6 (25.0) |
| T1 | 11 | 1 (9.1) | 5 (45.5) | 5 (45.5) | 6 (54.5) | 2 (18.2) | 3 (27.3) |
| T2 | 10 | 1 (10.0) | 1 (10.0) | 8 (80.0) | 2 (20.0) | 3 (30.0) | 5 (50.0) |
| T3 | 48 | 7 (14.6) | 9 (18.8) | 32 (66.7) | 8 (16.7) | 10 (20.8) | 30 (62.5) |
| T4 | 22 | 2 (9.1) | 5 (22.7) | 15 (68.2) | 4 (18.2) | 6 (27.3) | 12 (54.5) |
| Dukes’ classification | NS | 1P = 0.015648 | |||||
| A | 44 | 6 (13.6) | 13 (29.5) | 25 (56.8) | 16 (36.4) | 15 (34.1) | 13 (29.5) |
| B | 35 | 4 (11.4) | 6 (17.1) | 25 (71.4) | 4 (11.4) | 8 (22.9) | 23 (65.7) |
| C | 32 | 4 (12.5) | 6 (18.8) | 22 (68.8) | 7 (21.9) | 7 (21.9) | 18 (56.3) |
| D | 4 | 1 (25.0) | 1 (25.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | 2 (50.0) |
| Lymphatic invasion | NS | 1P = 0.003865 | |||||
| Present | 76 | 11 (14.5) | 14 (18.4) | 51 (67.1) | 15 (19.7) | 16 (21.1) | 45 (59.2) |
| Absent | 39 | 4 (10.3) | 12 (30.8) | 23 (59.0) | 13 (33.3) | 15 (38.5) | 11 (28.2) |
| Venous invasion | NS | NS | |||||
| Present | 44 | 5 (11.4) | 10 (22.7) | 29 (65.9) | 8 (18.2) | 11 (25.0) | 25 (56.8) |
| Absent | 71 | 10 (14.1) | 16 (22.5) | 45 (63.4) | 20 (28.2) | 20 (28.2) | 31 (43.7) |
| Lymphnode metastasis | NS | NS | |||||
| Present | 35 | 5 (14.3) | 6 (17.1) | 24 (68.6) | 8 (22.9) | 7 (20.0) | 20 (57.1) |
| Absent | 80 | 10(12.5) | 20 (25.0) | 50 (62.5) | 20 (25.0) | 24 (30.0) | 36 (45.0) |
NS: Not significant,
statistical analyses are performed by Spearman’s correlation coefficient by rank test or Mann-Whitney’s U test methods.
Colo320DM (adenocarcinoma) and DLD-1 (adenocarcinoma) cell lines derived from human colorectal cancer were obtained from the Human Health Resources Bank (Osaka, Japan). All cell lines were incubated at 37 ºC in a humidified incubator containing 5% CO2 and 95% air. Colo320DM was maintained in RPMI 1640 (Invitrogen Corp., Carlsbad, CA, USA) supplemented with heat-inactivated 10% fetal calf serum (Invitrogen Corp.). DLD-1 was maintained in DMEM/F-12 (Invitrogen Corp.) supplemented with 10% fetal calf serum.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissues were cut into 4 µm sections, deparaffinized in xylene and rehydrated in phosphate-buffered saline. Deparaffinized sections were preincubated with normal bovine serum to prevent nonspecific binding, and then incubated overnight at 4 ºC with an optimal dilution (0.1 µg/mL) of a primary polyclonal rabbit antibody against human IL-11 (H-169) and IL-11Rα (N-20). Each antibody was bought from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The slides for IL-11 were then sequentially incubated with a horseradish-conjugated goat antirabbit immunoglobulin G antibody. And the reaction products were resolved using a diaminobenzidine (DAB; DAKO, Carpinteria, CA, USA). The slides for IL-11Rα were sequentially incubated with an alkaline phosphatase-conjugated goat antirabbit immunoglobulin G antibody, and the reaction products were resolved using a mixture of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride (BCIP/NBT; DAKO). Primary antibodies preabsorbed with excess recombinant IL-11 and IL-11Rα peptides (Santa Cruz Biotechnology, Inc.), respectively, were used as negative controls. Prostatic cancer tissues[19] served as the internal positive control for IL-11 and IL-11Rα immunostaining. Analysis of the immunohistochemical staining was performed independently by two investigators (K. Yamazumi and T. Nakayama). IL-11 and IL-11Rα expression was classified into three categories depending on the percentage of cells stained: -, 0 to 10% positive cells; +, 10 to 50% positive tumor cells; and ++, > 50% positive tumor cells.
Western blot analysis
Western blot analysis for IL-11 and IL-11Rα expressio was performed on four human colorectal carcinoma tissues, normal mucosal tissues and two colorectal carcinoma cell lines. Human colorectal tissues were obtained within 1 hour of surgery and immediately frozen. The tissues were then suspended in RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate and 0.05% SDS, pH 7.4), broken into pieces on ice and subjected to three freeze-thaw cycles. The insoluble cell debris was removed by centrifugation at 14 000 r/min at 0 °C for 10 min. The supernatant was collected and the protein concentration was quantified using a protein assay reagent (Bio-Rad Laboratories, Hercules, CA). The proteins (20 μg) were separated by polyacrylamide gel electrophoresis (PAGE) under denaturing and reducing conditions, and then transferred to a Hybond ECL nitrocellulose membrane (Amersham Biosciences, Buckinghamshire, UK). The membranes were rinsed in TBS, blocked with 5% low-fat dried milk in TBS containing 0.1% Tween 20 (TBS-T), and then incubated for 1 h at room temperature with a 1 μg /mL dilution of the anti-human IL-11 or IL-11 receptor α antibody (Santa Cruz Biotechnology, Inc.). After extensive washing of the membranes with TBS-T, they were incubated for 1 h with a 1: 1 000 dilution of the horseradish-peroxidase-conjugated donkey anti-rabbit immunoglobulin G (Santa Cruz Biotechnology, Inc.) in TBS-T containing 3% low-fat dried milk. The membranes were washed and developed with a horseradish peroxidase chemiluminescence detection reagent (ECL Plus System, Amersham Bioscience), and then exposed to Hyperfilm ECL (Amersham Bioscience).
Statistical analysis
The Stat View II program (Abacus Concepts, Inc., Berkeley, CA) was used for statistical analyses. Analyses comparing the degrees of IL-11 or IL-11Rα expression were performed by Spearman’s correlation coefficient by rank test or Mann-Whitney’s U test methods.
RESULTS
IL-11 was localized in the cytoplasm of the carcinoma cells (Figure 1A), and IL-11Rα was localized both in the membrane and the cytoplasm, mainly in the membrane (Figure 1B). No staining with anti-IL-11 or anti-IL-11Rα was seen in the negative controls for tissue staining. Although normal epithelium and stromal tissue around tumors expressed IL-11 and IL-11Rα, the expression in these tissues were more faint than that in the carcinoma cells.
Figure 1.
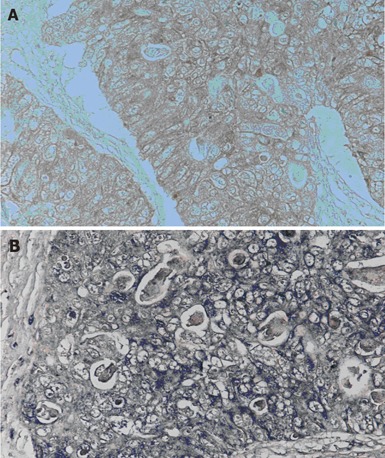
Immunohistochemical staining for the IL-11 and IL-11Rα in human colorectal carcinoma. (A) is for IL-11, and (B) is for IL-11Rα. IL-11 and IL-11Rα show strong cytoplasmic and membranous expression.
Immunohistochemical results are summarized in the Table 1. Among 115 cases of adenocarcinoma, 100 cases (87.0%) showed positive staining for the IL-11, and 87 cases (75.6%) were positive for the IL-11Rα. Among 10 cases of normal colorectal mucosa, 5 cases (50.0%) expressed IL-11 and 4 cases (40.0%) were positive for IL-11Rα. And there were significant differences between IL-11 and IL-11Rα expression and in between normal mucosa and adenocarcinoma (P = 0.001883, P = 0.00196, respectively). Among 11 cases of adenoma, 6 cases (54.5%) were expressed IL-11 and 4 cases (57.1%) of adenoma were positive for IL-11Rα, and there were significant differences of IL-11 or IL-11Rα expressions between adenoma and adenocarcinoma (P = 0.023291, P = 0.000833, respectively).
IL-11 and IL-11Rα were expressed more intensely in the invasive than the superficial parts of the carcinoma. The expression of IL-11Rα correlated significantly with histological differentiation (P = 0.000833), depth of tumor invasion (P = 0.006395), Dukes’ classification (P = 0.015648) and lymphatic invasion (P = 0.003865)(Table 1). However, there was no correlation with lymph node metastasis and venous invasion. On the other hand, there was no correlation between IL-11 expression and clinicopathological factors.
IL-11 and IL-11Rα expression was also detected in two colorectal carcinoma cell lines and all of four human carcinoma tissues by Western blot analysis (Figure 2). The IL-11 protein of 19.1 Kd was clearly detected in all of the two cell lines and four carcinoma tissues. The IL-11Rα expression was detected in the two cell lines and four carcinoma tissues at various levels. IL-11 and IL-11Rα proteins were also expressed in normal mucosa but were more intense in the cancer tissue than in the normal mucosa (Figure 2).
Figure 2.
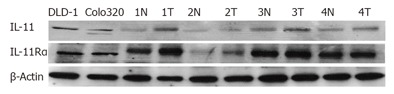
Demonstration of IL-11 and IL-11Rα in colorectal carcinoma cells and tissues by Western blotting. All of colorectal carcinoma cells, carcinoma tissues and normal mucosa expressed both IL-11 and IL-11Rα. (N: normal mucosa, T: human colorectal cancer tissue).
DISCUSSION
IL-11 is well known as a hematopoietic cytokine. However, the expression of IL-11 has also been observed in many types of cells, such as fibroblasts, chondrocytes, synovial epithelium, bronchial epithelium and colonic epithelium[15,25,26], and it has been shown that IL-11 has various actions on hepatic, stromal, epithelial, neural and osteoclastic cells[27]. Recent studies have shown that IL-11R was not only expressed in megakaryocytes, osteoclasts and colon epithelium but also in breast cancer, ovarian cancer and prostate cancer[12-14]. In this study, we have demonstrated for the first time that IL-11 and IL-11Rα are expressed in the human colorectal mucosa, colorectal cancer tissue and cell lines. Both IL-11 and IL-11Rα were expressed simultaneously in the carcinoma cell. Therefore, it is suggested that the tumor cell may respond to the IL-11 in an autocrine and/or paracrine fashion to promote the invasion of tumors.
There were statistically significant correlations between the expression of IL-11Rα and histological differentiation, tumor invasion and lymphatic invasion. These results suggest that IL-11Rα is involved in the regulation of cellular differentiation and tumor progression in human colorectal carcinoma. Recently, the expression of IL-11Rα has been reported in carcinoma of the breast, ovary and prostate[12-14]. We suggest that IL-11 might play an important role in the progression of carcinomas.
IL-11 is known as the activator of the JAK/STAT pathway through GP130[11]. A recent study showed that STAT3 signaling directly regulates tumor invasion and metastasis[28]. And other GP130-dependent cytokines, such as IL-6 and LIF, have been reported to have connections with several tumors[29-33]. Especially, the tumor-promoting and invasion activity of IL-6 has been well studied[30-32]. IL-11 also shares the GP130 connection, and in one report it was noted that a complex of the soluble IL-11 receptor and IL-11 act as an IL-6-type cytokine[34]. Thus, it is suggested that IL-11 may stimulate colorectal carcinoma cell growth and invasion through activation of the GP130/STAT-3 signaling pathway.
Recently, it was suggested that IL-11 could become a therapeutically important molecule in supportive care of cancer patients who receive chemotherapy[35], and another study showed that IL-11 appears unlikely to stimulate the growth of the most common solid tumors[16]. However, in this study, we have demonstrated that IL-11 may up-regulate the activity of colorectal carcinoma cell growth and/or invasion, so it is necessary to pay very careful attention to the therapeutic use of IL-11.
In conclusion, we have demonstrated that IL-11Rα is an important factor involved in the invasion of human colorectal adenocarcinoma. However, the roles of IL-11 and IL-11R in human colorectal carcinoma remain unclear and await further study.
ACKNOWLEDGEMENTS
We are grateful to Mr. Toshiyuki Kawada (Nagasaki University Graduate School of Biomedical Sciences) for his excellent immunohistochemical and molecular biological assistance.
Footnotes
S- Editor Pravada J and Guo SY L- Editor Elsevier HK E- Editor Cao L
References
- 1.Steinberg SM, Barwick KW, Stablein DM. Importance of tumor pathology and morphology in patients with surgically resected colon cancer. Findings from the Gastrointestinal Tumor Study Group. Cancer. 1986;58:1340–1345. doi: 10.1002/1097-0142(19860915)58:6<1340::aid-cncr2820580626>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Baisse B, Bouzourene H, Saraga EP, Bosman FT, Benhattar J. Intratumor genetic heterogeneity in advanced human colorectal adenocarcinoma. Int J Cancer. 2001;93:346–352. doi: 10.1002/ijc.1343. [DOI] [PubMed] [Google Scholar]
- 3.Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 4.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 6.Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF, Nakamura Y, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244:217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- 7.Dix B, Robbins P, Carrello S, House A, Iacopetta B. Comparison of p53 gene mutation and protein overexpression in colorectal carcinomas. Br J Cancer. 1994;70:585–590. doi: 10.1038/bjc.1994.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teramura M, Kobayashi S, Yoshinaga K, Iwabe K, Mizoguchi H. Effect of interleukin 11 on normal and pathological thrombopoiesis. Cancer Chemother Pharmacol. 1996;38 Suppl:S99–102. doi: 10.1007/s002800051048. [DOI] [PubMed] [Google Scholar]
- 9.Yang YC. Interleukin 11: an overview. Stem Cells. 1993;11:474–486. doi: 10.1002/stem.5530110617. [DOI] [PubMed] [Google Scholar]
- 10.Keller DC, Du XX, Srour EF, Hoffman R, Williams DA. Interleukin-11 inhibits adipogenesis and stimulates myelopoiesis in human long-term marrow cultures. Blood. 1993;82:1428–1435. [PubMed] [Google Scholar]
- 11.Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest. 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keith JC, Albert L, Sonis ST, Pfeiffer CJ, Schaub RG. IL-11, a pleiotropic cytokine: exciting new effects of IL-11 on gastrointestinal mucosal biology. Stem Cells. 1994;12 Suppl 1:79–89; discussion 89-90. [PubMed] [Google Scholar]
- 13.Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer. 1995;62:356–361. doi: 10.1002/ijc.2910620321. [DOI] [PubMed] [Google Scholar]
- 14.Orazi A, Du X, Yang Z, Kashai M, Williams DA. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest. 1996;75:33–42. [PubMed] [Google Scholar]
- 15.Du X, Liu Q, Yang Z, Orazi A, Rescorla FJ, Grosfeld JL, Williams DA. Protective effects of interleukin-11 in a murine model of ischemic bowel necrosis. Am J Physiol. 1997;272:G545–G552. doi: 10.1152/ajpgi.1997.272.3.G545. [DOI] [PubMed] [Google Scholar]
- 16.Curtis DJ, Hilton DJ, Roberts B, Murray L, Nicola N, Begley CG. Recombinant soluble interleukin-11 (IL-11) receptor alpha-chain can act as an IL-11 antagonist. Blood. 1997;90:4403–4412. [PubMed] [Google Scholar]
- 17.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell CL, Guardiani R, Ollari C, Nelson BE, Quesenberry PJ, Savarese TM. Interleukin-11 receptor expression in primary ovarian carcinomas. Gynecol Oncol. 2001;80:121–127. doi: 10.1006/gyno.2000.6064. [DOI] [PubMed] [Google Scholar]
- 19.Campbell CL, Jiang Z, Savarese DM, Savarese TM. Increased expression of the interleukin-11 receptor and evidence of STAT3 activation in prostate carcinoma. Am J Pathol. 2001;158:25–32. doi: 10.1016/S0002-9440(10)63940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotiriou C, Lacroix M, Lespagnard L, Larsimont D, Paesmans M, Body JJ. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett. 2001;169:87–95. doi: 10.1016/s0304-3835(01)00524-9. [DOI] [PubMed] [Google Scholar]
- 21.Booth C, Potten CS. Effects of IL-11 on the growth of intestinal epithelial cells in vitro. Cell Prolif. 1995;28:581–594. doi: 10.1111/j.1365-2184.1995.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 22.Soda H, Raymond E, Sharma S, Lawrence R, Cerna C, Gomez L, Schaub R, Von Hoff DD, Izbicka E. Recombinant human interleukin-11 is unlikely to stimulate the growth of the most common solid tumors. Anticancer Drugs. 1999;10:97–101. doi: 10.1097/00001813-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Jass JR, Sabin LH. Histological typing of intestinal tumors. World Health Organization International Histological Classification of Tumors. 2nd ed. Berlin, Germany: Springer-Verlag; 1989. [Google Scholar]
- 24.Greene FL, Page DL, Fleming ID (eds) Cancer staging handbook: From the American Joint commit tee on cancer Cancer staging manual. 6th ed. New York, USA: Springer-Verlag; 2002. pp. 127–129. [Google Scholar]
- 25.Minshall E, Chakir J, Laviolette M, Molet S, Zhu Z, Olivenstein R, Elias JA, Hamid Q. IL-11 expression is increased in severe asthma: association with epithelial cells and eosinophils. J Allergy Clin Immunol. 2000;105:232–238. doi: 10.1016/s0091-6749(00)90070-8. [DOI] [PubMed] [Google Scholar]
- 26.Maier R, Ganu V, Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem. 1993;268:21527–21532. [PubMed] [Google Scholar]
- 27.Morris JC, Neben S, Bennett F, Finnerty H, Long A, Beier DR, Kovacic S, McCoy JM, DiBlasio-Smith E, La Vallie ER, et al. Molecular cloning and characterization of murine interleukin-11. Exp Hematol. 1996;24:1369–1376. [PubMed] [Google Scholar]
- 28.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie RC, Szepietowski J. Cutaneous leukemia inhibitory factor and its potential role in the development of skin tumors. Dermatol Surg. 2004;30:279–290. doi: 10.1111/j.1524-4725.2004.30087.x. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto M, Hattori K, Oyasu R. Interleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitro. Int J Cancer. 1997;72:149–154. doi: 10.1002/(sici)1097-0215(19970703)72:1<149::aid-ijc21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Yu CY, Wang L, Khaletskiy A, Farrar WL, Larner A, Colburn NH, Li JJ. STAT3 activation is required for interleukin-6 induced transformation in tumor-promotion sensitive mouse skin epithelial cells. Oncogene. 2002;21:3949–3960. doi: 10.1038/sj.onc.1205499. [DOI] [PubMed] [Google Scholar]
- 32.Liu XH, Kirschenbaum A, Lu M, Yao S, Klausner A, Preston C, Holland JF, Levine AC. Prostaglandin E(2) stimulates prostatic intraepithelial neoplasia cell growth through activation of the interleukin-6/GP130/STAT-3 signaling pathway. Biochem Biophys Res Commun. 2002;290:249–255. doi: 10.1006/bbrc.2001.6188. [DOI] [PubMed] [Google Scholar]
- 33.Wojciechowska-Lacka A, Matecka-Nowak M, Adamiak E, Lacki JK, Cerkaska-Gluszak B. Serum levels of interleukin-10 and interleukin-6 in patients with lung cancer. Neoplasma. 1996;43:155–158. [PubMed] [Google Scholar]
- 34.Baumann H, Wang Y, Morella KK, Lai CF, Dams H, Hilton DJ, Hawley RG, Mackiewicz A. Complex of the soluble IL-11 receptor and IL-11 acts as IL-6-type cytokine in hepatic and nonhepatic cells. J Immunol. 1996;157:284–290. [PubMed] [Google Scholar]
- 35.Ault P, Kantarjian H, Welch MA, Giles F, Rios MB, Cortes J. Interleukin 11 May improve thrombocytopenia associated with imatinib mesylate therapy in chronic Myelogenous leukemia. Leuk Res. 2004;28:613–618. doi: 10.1016/j.leukres.2003.11.003. [DOI] [PubMed] [Google Scholar]


