Abstract
AIM: To determine which treatment modality - hepatectomy or percutaneous ablation - is more beneficial for patients with small hepatocellular carcinoma (HCC) (≤ 4 cm) in terms of long-term outcomes.
METHODS: A retrospective analysis of 149 patients with HCC ≤ 4 cm was conducted. Eighty-five patients underwent partial hepatectomy (anatomic in 47 and non-anatomic in 38) and 64 underwent percutaneous ablation (percutaneous ethanol injection in 37, radiofrequency ablation in 21, and microwave coagulation in 6). The median follow-up period was 69 mo.
RESULTS: Hepatectomy was associated with larger tumor size (P < 0.001), whereas percutaneous ablation was significantly associated with impaired hepatic functional reserve. Local recurrence was less frequent following hepatectomy (P < 0.0001). Survival was better following hepatectomy (median survival time: 122 mo) than following percutaneous ablation (median survival time: 66 mo; P = 0.0123). When tumor size was divided into ≤ 2 cm vs > 2 cm, the favorable effects of hepatectomy on long-term survival was seen only in patients with tumors >2 cm (P = 0.0001). The Cox proportional hazards regression model revealed that hepatectomy (P = 0.006) and tumors ≤ 2 cm (P = 0.017) were independently associated with better survival.
CONCLUSION: Hepatectomy provides both better local control and better long-term survival for patients with HCC ≤ 4 cm compared with percutaneous ablation. Of the patients with HCC ≤ 4 cm, those with tumors > 2 cm are good candidates for hepatectomy, provided that the hepatic functional reserve of the patient permits resection.
Keywords: Liver neoplasms, Hepatocellular carcinoma, Hepatectomy, Percutaneous ablation, Prognosis, Multivariate analysis
INTRODUCTION
Hepatectomy and percutaneous ablation are the treatments of choice for small hepatocellular carcinoma (HCC)[1,2]. Hepatectomy is recommended by surgeons, as this procedure removes portal venous thrombi in the adjacent liver[3], allows for better local control[4], and has better survival outcomes compared with percutaneous ablation[5,6]. In contrast, gastroenterologists and interventional radiologists advocate percutaneous ablation techniques including ethanol injection[7-9], microwave coagulation[10-12], and radiofrequency ablation[13-15], as these methods are not as invasive as hepatectomy[7-15], have a lower incidence of morbidity and mortality[7-13,15], are more feasible in patients with impaired hepatic functional reserve[7-15], and have similar survival outcomes compared with hepatectomy[7-9,15].
The aim of this study was to determine which treatment modality - hepatectomy or percutaneous ablation - is more beneficial for patients with small (≤ 4 cm) HCC in terms of long-term outcomes.
MATERIALS AND METHODS
Patients
From January 1990 to December 2002, 224 consecutive patients with HCC underwent either partial hepatectomy or percutaneous ablation therapy as an initial treatment at the Niigata University Medical and Dental Hospital. Of these patients, 149 with HCC measuring ≤ 4 cm formed the basis of this retrospective study; they included 104 men and 45 women with a median age of 64 years (range: 29-83 years). Only HCCs measuring ≤ 4 cm were included in the current study, because percutaneous ablation therapy was the treatment modality mainly recommended for such tumors in our hospital. All patients were Japanese.
Treatment modalities
Of the 149 patients, 85 underwent a hepatectomy for HCC in the Division of Digestive and General Surgery. Hepatectomy. Procedures included non-anatomic hepatectomy in 38 patients, monosegmentectomy in 14, bisegmentectomy in 15, right hepatectomy in 14, and left hepatectomy in 4. In our department, the type of hepatectomy procedure selected was primarily based on the disappearance rate of indocyanine green (KICG)[16,17], which is an indicator of hepatic functional reserve. The selection criteria were KICG ≥ 0.12 for hemihepatectomy, KICG ≥ 0.10 for bisegmentectomy, KICG ≥ 0.08 for monosegmentectomy, and KICG ≥ 0.06 for non-anatomic hepatectomy (including the enucleation of hepatic tumors).
The remaining 64 patients underwent a percutaneous ablation procedure for HCC in the Division of Gastroenterology and Hepatology. Hepatectomy was considered as a feasible option for 51 of these 64 patients and was offered as an alternative. However, all these patients preferred to undergo percutaneous ablation treatment. Hepatectomy was not considered feasible in the remaining 13 patients due to impaired hepatic functional reserve (KICG < 0.06); therefore, these patients were only offered percutaneous ablation treatment. Percutaneous ablation procedures included percutaneous ethanol injection (PEI) in 37 patients, radiofrequency ablation (RFA) in 21, and microwave coagulation therapy (MCT) in 6. In these patients, adequacy of the ablation was assured with dual-phase dynamic computed tomography (section thickness of 5 mm) within a month of the procedure. A microwave tissue coagulator (Microtaze® OT-110M; Alfresa-Pharma Co., Inc., Osaka, Japan) and an RF generator (Cool-tip® RF System, CMI Century Medical Co., Inc., Tokyo, Japan) were introduced in our hospital in 1995 and 2000, respectively.
Post-treatment follow-up
There were no mortalities 30 d post-treatment in the current study. Serum concentrations of alpha-fetoprotein were measured and abdominal ultrasonography and/or contrast-enhanced computed tomography was performed on all the patients approximately 1 mo after the treatment. Thereafter, patients were regularly monitored for recurrences in outpatient clinics every 3 mo by physical examination, laboratory tests, and imaging studies. When intrahepatic recurrences were detected, they were treated with either interventional radiological techniques, such as PEI, RFA, MCT, transarterial chemoembolization and hepatic arterial infusion, or repeat hepatectomy when indicated. Patients with disseminated recurrences and those in a debilitated state were treated with supportive care.
The follow-up period after the treatment was defined as the interval between the date of the initial treatment and that of the last follow-up, and ranged from 11 to 178 (median: 69) mo in the current study. The median follow-up period was 73 mo in patients who had undergone hepatectomy, and 61 mo in those who had undergone percutaneous ablation.
Definition of local recurrence after treatment
Local recurrence was defined as recurrences contiguous to resection margins in patients who had undergone hepatectomy, whereas in patients treated with percutaneous ablation, local recurrence was defined as recurrences contiguous to or within the ablated areas.
Laboratory examination
The following laboratory tests were performed before treatment: hepatitis B surface antigen; hepatitis C antibody; serum aspartate aminotransferase; serum alanine aminotransferase; the indocyanine green clearance test; and serum alpha-fetoprotein. Hepatitis B surface antigen and hepatitis C antibody in serum were detected by radioimmunoassay (Lumipulse II HBsAg; Fujirebio Co., Inc., Tokyo, Japan) and a second-generation enzyme-linked immunosorbent assay (Lumipulse II Ortho HCV; Ortho-Clinical Diagnostics Co., Inc., Tokyo, Japan), respectively. Indocyanine green (Diagnogreen; Daiichi Pharmaceutical Co., Inc., Tokyo, Japan) retention rate at 15 min after the injection of the dye (0.5 mg/kg) was used as an indicator of hepatic functional reserve, with a reference range of 10% or less[16-18]. Serum concentrations of alpha-fetoprotein were determined by enzyme immunoassay (Luminomaster AFP; Sankyo Yell Yakuhin Co., Ltd., Tokyo, Japan).
Pathologic examination
Resected specimens from all the patients who had undergone hepatectomy were submitted to the Department of Surgical Pathology in our hospital. Each specimen was examined to determine the number of hepatic tumors, tumor size, vascular invasion (gross or microscopic), and cirrhosis. Vascular invasion included both portal and hepatic venous invasion in the current study. Cirrhosis in the adjacent (non-tumorous) liver was diagnosed microscopically based on the presence of regenerative nodules surrounded by fibrous septa. The pathology of the liver was confirmed by fine-needle biopsy in all the patients undergoing percutaneous ablation. Among this group of patients, 41 patients had a fine-needle biopsy of the tumor.
Factors influencing outcomes after treatment
To determine the factors that may influence outcomes after the treatment, 13 conventional variables[19-22] were identified for univariate and multivariate analyses (Table 1). Vascular invasion and histologic grade were not chosen because they were often missed in patients undergoing percutaneous ablation.
Table 1.
Clinicopathologic characteristics of 149 patients with hepatocellular carcinoma according to treatment modality
| Variable | Hepatectomy | Percutaneous ablation |
| Age (yr) | ||
| ≤ 65 | 56 | 33 |
| > 65 | 29 | 31 |
| Gender | ||
| Male | 61 | 43 |
| Female | 24 | 21 |
| HBsAg | ||
| Negative | 61 | 54 |
| Positive | 24 | 10 |
| HCVAb | ||
| Negative | 38 | 11 |
| Positive | 47 | 53b |
| Cirrhosis | ||
| Absent | 38 | 13 |
| Present | 47 | 51b |
| Child-Pugh classification | ||
| A | 74 | 30 |
| B plus C | 11 | 34b |
| ICG R15 (%) | ||
| ≤ 10 | 52 | 10 |
| > 10 | 33 | 54b |
| AST(IU/L) | ||
| ≤ 50 | 48 | 17 |
| > 50 | 37 | 47b |
| ALT(IU/L) | ||
| ≤ 50 | 50 | 23 |
| > 50 | 35 | 41b |
| AFP (ng/mL) | ||
| ≤ 20 | 39 | 27 |
| > 20 | 46 | 37 |
| No. of hepatic tumors | ||
| Solitary | 67 | 41 |
| Multiple | 18 | 23 |
| Tumor size (cm) | ||
| ≤ 2 | 23 | 43 |
| > 2 | 62 | 21b |
ICG R15: indocyanine green retention rate at 15 min.
P < 0.01 between groups.
Statistical analyses
Medical records and survival data were obtained for all the patients. The causes of death were determined from the medical records. Deaths from other causes were treated as uncensored cases. The Kaplan-Meier method was used to estimate the cumulative incidences of events, and differences in these incidences were evaluated using the log rank test. The Cox proportional hazards regression model was performed to identify the factors that were independently associated with local recurrence and survival. In this model, a stepwise selection was used for variable selection with entry and removal limits of P < 0.1 and P > 0.15, respectively. The stability of each model was confirmed using a step-backward and step-forward fitting procedure, and variables identified as having an independent influence on local recurrence and survival were identical in both the procedures. Clinical features and pathologic tumor-related factors were compared between the two groups using Fisher’s exact test. All statistical evaluations were performed using the SPSS 11.5J software package (SPSS Japan Inc., Tokyo, Japan). All tests were two-sided and P values of < 0.05 were considered statistically significant.
RESULTS
Clinicopathologic characteristics according to treatment modality
A total of 125 hepatic tumors (median: one per patient; range: 1-14 tumors) were resected in patients undergoing hepatectomy, and a total of 100 tumors (median: one per patient; range: 1-5 tumors) were treated in patients undergoing percutaneous ablation.
Patients treated with hepatectomy had larger tumors than those treated with percutaneous ablation. Patients who had undergone percutaneous ablation were characterized by hepatitis C virus infection, cirrhosis, Child-Pugh classification B or C, an impaired indocyanine green retention rate at 15 min, and increased serum concentrations of aspartate aminotransferase and alanine aminotransferase, suggesting impaired hepatic functional reserve with active hepatitis in these patients (Table 1).
Factors influencing local recurrence
During the follow-up period, local recurrences developed in two patients who had undergone hepatectomy and 17 patients who had been treated with percutaneous ablation (10 treated with PEI and 7 treated with RFA). Univariate analysis revealed that treatment modality (P < 0.0001), indocyanine green retention rate at 15 min P = 0.0005), Child-Pugh classification (P = 0.0012), serum alpha-fetoprotein level (P = 0.0072), hepatitis C virus infection (P = 0.0374), and number of hepatic tumors (P = 0.0419) were risk factors for local recurrence. Of these six variables, multivariate analyses revealed that treatment modality and serum alpha-fetoprotein level were the only independently significant variables (Table 2).
Table 2.
Independent risk factors for local recurrence
| Variable | Relative risk | 95% CI | P value |
| Treatment modality Hepatectomy | 1.000 | 0.001 | |
| Percutaneous ablation | 13.442 | 3.102-58.254 | |
| AFP (ng/mL) | 0.014 | ||
| ≤ 20 | 1.000 | ||
| > 20 | 4.711 | 1.370-16.195 |
Factors influencing long-term survival
At the time of disease status assessment, 53 patients who had undergone hepatectomy were alive, and 32 had died. Thirty-four patients treated with percutaneous ablation were alive, and 30 had died. Univariate analysis revealed that Child-Pugh classification (P = 0.0010), serum alpha-fetoprotein level (P = 0.0052), treatment modality (P = 0.0123), serum aspartate aminotransferase (P = 0.0278), tumor size (P = 0.0454), and number of hepatic tumors (P = 0.0464) were significant prognostic factors of long-term survival. Of these six variables, multivariate analyses revealed that treatment modality and tumor size were the only independent significant variables (Table 3).
Table 3.
Independent factors influencing long-term survival
| Variable | Relative risk | 95% CI | P value |
| Treatment modality Hepatectomy | 1.000 | 0.006 | |
| Percutaneous ablation | 2.398 | 1.278-4.499 | |
| Tumor size (cm) | 0.017 | ||
| ≤ 2 | 1.000 | ||
| > 2 | 2.159 | 1.148-4.060 | |
| Child-Pugh classification | 0.050 | ||
| A | 1.000 | ||
| B + C | 1.773 | 1.000-3.142 | |
| AFP (ng/mL) | 0.072 | ||
| ≤ 20 | 1.000 | ||
| > 20 | 1.713 | 0.952-3.084 |
Outcomes after treatment according to tumor size
Local recurrence was significantly less frequent in patients who had undergone hepatectomy than in those who had undergone percutaneous ablation (P < 0.0001) (Figure 1). When the patients were divided into two groups according to tumor size (≤ 2 cm vs > 2 cm), hepatectomy was found to be the more effective treatment for controlling local recurrence, but only in patients with HCC >2 cm (P<0.0001) (Figure 2). Survival after the treatment was significantly better in patients who had undergone hepatectomy than in those treated with percutaneous ablation (P = 0.0123, Figure 3). Again, hepatectomy was the more effective treatment in terms of long-term survival, but only in patients with HCC >2 cm (P = 0.0001, Figure 4).
Figure 1.
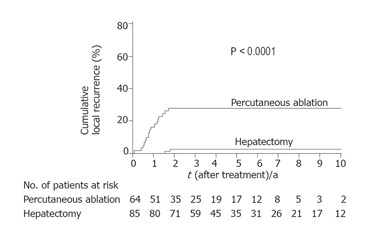
Kaplan-Meier estimates of local recurrence. The incidence of local recurrence reached a plateau of 28% at 20 mo after percutaneous ablation, and a plateau of 3% at 22 mo after hepatectomy.
Figure 2.
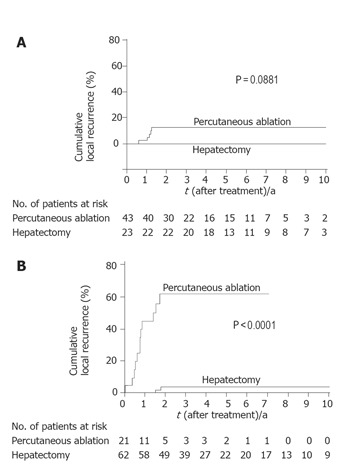
Kaplan-Meier estimates of local recurrence by tumor size. A: Among tumors ≤ 2 cm, the incidence of local recurrence reached a plateau of 12% at 15 mo after percutaneous ablation, whereas no recurrences had occurred after hepatectomy; B: among tumors > 2 cm, the incidence of local recurrence reached a plateau of 61% at 21 mo after percutaneous ablation, whereas it reached a plateau of 4% at 22 mo after hepatectomy.
Figure 3.
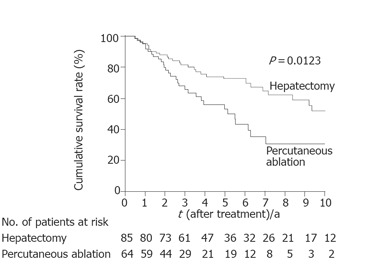
Kaplan-Meier estimates of survival. The median survival time was 122 mo with a 10-year survival rate of 53% in patients who had undergone hepatectomy. The median survival time was 66 mo with a 10-year survival rate of 31% in patients who had undergone percutaneous ablation.
Figure 4.
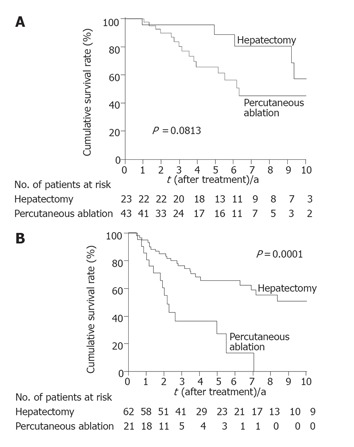
Kaplan-Meier estimates of survival by tumor size. A: Among patients with tumors ≤ 2 cm, survival was better following hepatectomy (median survival time of 122 mo; 10-year survival rate of 58%) than following percutaneous ablation (median survival time of 76 mo; 10-year survival rate of 45%); B: among patients with tumors >2 cm, survival was better following hepatectomy (median survival time of 126 mo; 10-year survival rate of 51%) than following percutaneous ablation (median survival time of 26 mo; 10-year survival rate of 0%).
Incidence of vascular invasion according to tumor size
Vascular invasion was more frequent in patients with tumors > 2 cm (16/62, 26%) than in those with tumors ≤ 2 cm (1/23, 4%; P = 0.033, Table 4).
Table 4.
Incidence of vascular invasion according to tumor size
Portal venous invasion was noted.
Portal venous invasion in 10 patients, hepatic venous invasion in 3, and both portal and hepatic venous invasion in 3.
DISCUSSION
Selecting the correct treatment modality to suit individual patients with HCC remains a matter of debate[1,4,5]. This prompted us to conduct the current study, which has revealed that hepatectomy provided better outcomes for patients with HCC ≤4 cm than percutaneous ablation. This may partly be due to the fact that hepatic functional reserve was better in patients who had undergone hepatectomy. Despite this, we found that the incidence of local recurrence decreased and long-term survival increased independently after hepatectomy, indicating that this is an oncologically reliable treatment modality for HCC ≤ 4 cm.
Local recurrence was found to be more frequent after percutaneous ablation than after hepatectomy. Prospective, randomized controlled trials for PEI treatment of HCC ≤ 4 cm[23-25] demonstrated that cumulative 2-year local recurrence rates ranged from 33% to 45%. These rates are comparable with the 28% incidence of local recurrence after percutaneous ablation detected in the current study. Local recurrences after percutaneous ablation may be attributable to insufficient ablation of the primary tumor and/or the presence of portal or hepatic venous tumor thrombi in the adjacent liver[26,27]. Hepatectomy removes a rim of non-neoplastic liver parenchyma (a resection margin) together with the primary tumor, and thus eradicates both the primary tumor and venous tumor thrombi within the resection margin. This may explain better the outcomes following hepatectomy. In the current study, the beneficial effect of hepatectomy was prominent in a subgroup of patients with tumors >2 cm (Figures 2B and 4B), suggesting that these patients are good candidates for hepatectomy. Tumors > 2 cm had a higher incidence of vascular invasion than tumors ≤ 2 cm (Table 4). Taken together, these findings suggest that the beneficial effect of hepatectomy is due to the clearance of venous tumor thrombi in the adjacent liver in addition to the complete removal of the primary tumor.
HCC mainly spreads via the portal and hepatic veins. Vascular invasion is an established adverse prognostic factor of HCC[19-21,28-30], and the incidence of vascular invasion increases as the tumor enlarges[30-33]. The current study confirmed this, with vascular invasion being more frequent in tumors >2 cm. Recent authors have suggested that tumors >2 cm are independently associated with local failure after RFA[34,35]. Considering that vascular invasion was less frequent in tumors ≤2 cm in our patients, percutaneous ablation appears to be an appropriate treatment modality for HCCs ≤2 cm. Despite this, outcomes for patients with HCCs ≤2 cm were better following hepatectomy than following percutaneous ablation. Due to the small sample size of this study, however, these differences were only marginally significant. We believe that hepatectomy may also be an appropriate treatment modality for HCCs ≤2 cm, provided that the patient is robust and that the hepatic functional reserve of the patient is at a level permitted for the resection.
Recent evidence suggests that high pre-treatment serum alpha-fetoprotein levels are associated with both the presence of portal venous invasion and intrahepatic recurrences after the treatment in HCC[26,36-41]. Serum alpha-fetoprotein levels were independently associated with local recurrences in the current study. The above findings suggest that high serum alpha-fetoprotein levels predict the presence of portal venous invasion, which may lead to treatment failure, in patients with HCC.
The current study has some limitations. First, it was a retrospective analysis of a small number of patients; second, the follow-up period in 64 patients was <60 mo; and third, percutaneous ablation therapy included three different treatment modalities. However, we believe that these limitations do not significantly influence the outcome of the study, as the marked differences between each group appear to overcome these biases.
In conclusion, hepatectomy provides both better local control and better long-term survival for patients with HCC ≤ 4 cm than percutaneous ablation, probably because hepatectomy eradicates both the primary tumor and venous tumor thrombi within the hepatectomy margin. Among the patients with HCC ≤ 4 cm, those with tumors >2 cm are good candidates for hepatectomy, provided that the level of hepatic functional reserve of the patient is suitable for resection.
Footnotes
S- Editor Guo SY and Pan BR L- Editor Elsevier HK E- Editor Bi L
References
- 1.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura H, Matsuyama Y, Miyagawa Y, Ishida K, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032–1038. doi: 10.1046/j.1365-2168.1999.01185.x. [DOI] [PubMed] [Google Scholar]
- 4.Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, Costa M. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62–7; discussion 67-8. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–79; discussion 790-79;. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 8.Kotoh K, Sakai H, Sakamoto S, Nakayama S, Satoh M, Morotomi I, Nawata H. The effect of percutaneous ethanol injection therapy on small solitary hepatocellular carcinoma is comparable to that of hepatectomy. Am J Gastroenterol. 1994;89:194–198. [PubMed] [Google Scholar]
- 9.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023–1028. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- 10.Sato M, Watanabe Y, Ueda S, Iseki S, Abe Y, Sato N, Kimura S, Okubo K, Onji M. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110:1507–1514. doi: 10.1053/gast.1996.v110.pm8613057. [DOI] [PubMed] [Google Scholar]
- 11.Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694–1702. [PubMed] [Google Scholar]
- 13.Allgaier HP, Deibert P, Zuber I, Olschewski M, Blum HE. Percutaneous radiofrequency interstitial thermal ablation of small hepatocellular carcinoma. Lancet. 1999;353:1676–1677. doi: 10.1016/S0140-6736(99)00368-2. [DOI] [PubMed] [Google Scholar]
- 14.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 15.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 16.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki S, Sugiyama Y, Iga T, Hanano M, Sanjo K, Beppu T, Idezuki Y. Pharmacokinetic study on the hepatic uptake of indocyanine green in cirrhotic patients. Am J Gastroenterol. 1985;80:801–806. [PubMed] [Google Scholar]
- 18.Moody FG, Rikkers LF, Aldrete JS. Estimation of the functional reserve of human liver. Ann Surg. 1974;180:592–598. doi: 10.1097/00000658-197410000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual, 6th edition. New York: Springer-Verlag; 2002. pp. 131–138. [Google Scholar]
- 20.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 21.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 22.Wakai T, Shirai Y, Nomura T, Nagakura S, Hatakeyama K. Computed tomographic features of hepatocellular carcinoma predict long-term survival after hepatic resection. Eur J Surg Oncol. 2002;28:235–242. doi: 10.1053/ejso.2001.1241. [DOI] [PubMed] [Google Scholar]
- 23.Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology. 1998;27:67–72. doi: 10.1002/hep.510270112. [DOI] [PubMed] [Google Scholar]
- 24.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 25.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 27.Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, Niwa Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68:1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::aid-cncr2820680711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Wakai T, Shirai Y, Yokoyama N, Nagakura S, Hatakeyama K. Hepatitis viral status affects the pattern of intrahepatic recurrence after resection for hepatocellular carcinoma. Eur J Surg Oncol. 2003;29:266–271. doi: 10.1053/ejso.2002.1395. [DOI] [PubMed] [Google Scholar]
- 29.Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Hsia CY, Wu CW. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 31.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 32.Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022–2031. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2022::AID-CNCR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–32; discussion 232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 34.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 35.Yu HC, Cheng JS, Lai KH, Lin CP, Lo GH, Lin CK, Hsu PI, Chan HH, Lo CC, Tsai WL, et al. Factors for early tumor recurrence of single small hepatocellular carcinoma after percutaneous radiofrequency ablation therapy. World J Gastroenterol. 2005;11:1439–1444. doi: 10.3748/wjg.v11.i10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128–1134. doi: 10.1053/jhep.2001.29202. [DOI] [PubMed] [Google Scholar]
- 37.Pompili M, Rapaccini GL, de Luca F, Caturelli E, Astone A, Siena DA, Villani MR, Grattagliano A, Cedrone A, Gasbarrini G. Risk factors for intrahepatic recurrence of hepatocellular carcinoma in cirrhotic patients treated by percutaneous ethanol injection. Cancer. 1997;79:1501–1508. doi: 10.1002/(sici)1097-0142(19970415)79:8<1501::aid-cncr9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 38.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137–1140. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoyagi Y, Oguro M, Yanagi M, Mita Y, Suda T, Suzuki Y, Hata K, Ichii K, Asakura H. Clinical significance of simultaneous determinations of alpha-fetoprotein and des-gamma-carboxy prothrombin in monitoring recurrence in patients with hepatocellular carcinoma. Cancer. 1996;77:1781–1786. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1781::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Aoyagi Y, Isokawa O, Suda T, Watanabe M, Suzuki Y, Asakura H. The fucosylation index of alpha-fetoprotein as a possible prognostic indicator for patients with hepatocellular carcinoma. Cancer. 1998;83:2076–2082. [PubMed] [Google Scholar]
- 41.Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, Dela Torre A, Cho KC, Contractor D, Korogodsky M. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–764. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]


