Abstract
AIM: To assess the efficacy and safety of a compound containing alginic acid plus antacid (Topaal®) compared to equal-strength antacid (Nacid®) in patients with endoscopy-negative reflux disease (ENRD).
METHODS: A total of 121 patients with ENRD were randomized to receive Topaal® (65 patients) or Nacid® (56 patients) for 6 weeks, with a consultation every 3 weeks. The primary end-point assessment was the change in the severity of heartburn as evaluated using a visual analog scale (VAS) at 6 weeks. The secondary end-point assessments were the VAS at 3 weeks, the change of frequency of the reflux symptom, the change of quality of life and the adverse effects.
RESULTS: Demographics of randomized subjects in each treatment group were comparable except that the Topaal® group included more males. The baseline characteristics between the groups were similar. After 6 weeks of treatment, the reduction of VAS of heartburn was more prominent in the Topaal® group (-6.29 cm vs -4.11 cm). At the 3rd week, Topaal® group showed greater reduction of VAS for heartburn (P = 0.0016), regurgitation (P = 0.0006), vomiting (P = 0.0373), and belching (P <0.0001). The patients of the Topaal® group had lower frequency of heartburn (P = 0.0015) and pain (P = 0.0163) at the end of the 6-week treatment period. From the doctor’s point of view, the Topaal® group also showed significant reduction in the severity of heartburn (P = 0.0020), regurgitation (P = 0.0081), vomiting (P = 0.0182), and belching (P = 0.0018) at the end of the treatment. The improvement of the quality of life was more remarkable in the Topaal® group at the end of the 6-week treatment period (P < 0.0001). For the adverse effect, there was no difference in both the groups.
CONCLUSION: Topaal® is more effective than Nacid® for the treatment of symptoms presented by patients with ENRD.
Keywords: Alginic acid, Endoscopy-negative reflux disease
INTRODUCTION
The incidence of gastroesophageal reflux disease (GERD) in Taiwan has been increasing. According to the end-oscopic surveillance reports, the rate of erosive esophagitis in Taiwan has increased from 2.4% to 14.5% in the past 20 years[1,2].
GERD is a disorder in which the gastric contents are refluxed into the esophagus, causing irritation and injury to the esophageal mucosa[3]. The typical symptoms of GERD include heartburn and regurgitation. Less common symptoms or symptoms suggestive of more aggressive reflux disease include dysphagia, odynophagia, or gastrointestinal bleeding. Atypical complaints include chest pain, hoarseness, sore throat, chronic cough, and asthma[3]. It has been shown that defective lower esophageal sphincter (LES) is the major mechanism underlying in most patients with reflux[4].
Endoscopy is the most common diagnostic tool to grade and to define GERD. However, only one-third of patients with GERD have esophageal mucosal erosion or ulceration[3]. Patients who experience typical heartburn despite that no evident mucosal lesions found at endoscopy are defined to have endoscopy-negative reflux disease (ENRD)[5]. Compared with patients who have reflux-related erosive esophagitis, those with ENRD are more likely to be younger, of lower body weight, and without a hiatal hernia[6]. Approximately 50% of those with ENRD have abnormal intra-esophageal acid exposure[6]. Although persons with ENRD experience similar decrements in their quality of life as those for individuals with erosive esophagitis, their complaints were often ignored if the diagnosis and treatment are based only on endoscopic finding. It has been proposed that the analysis of symptoms is probably the most useful method for the diagnosis of GERD[7]. The pharmacological treatment of ENRD comprises two therapeutic classes: (1) local antacids or anti-secretory drugs including histamine receptor and proton pump inhibitors (PPI)[8], which act by decreasing the acidity of the reflux contents; (2) alginates which act by decreasing the gastric reflux into the esophagus and by protecting the esophageal mucosa[9]. PPI were shown to provide symptom relief in patients with ENRD[8,10] but these treatments are not curative and not cheap. A cohort study of patients with ENRD has shown that after a median time of 10 years following the original diagnosis, 75% of patients are on prolonged antisecretory therapy because of recurrent symptoms/lesions of gastro-esophageal reflux disease[11]. The study confirms that ENRD is a protracted disease, which requires long-term medical therapy in most of the patients. Besides, Taiwan’s reimbursement system covers the PPI only when patients have endoscopic evidence of erosive esophagitis. Local antacid and alginates are more convenient and cheap options for patients with ENRD[9]. However, there were few studies comparing the efficacy of these two classes of drug on the treatment of ENRD. We therefore carried out a prospective, randomized trial comparing the efficacy and tolerance of sodium alginate (Topaal®) vs antacid (Nacid®) in the treatment of patients with ENRD.
MATERIALS AND METHODS
Selection of patients
Disease definition: All patients who presented with the classic symptoms including heartburn and/or regurgitation, but who did not have either Barrett's esophagus or definite endoscopic esophageal mucosal breaks (esophageal mucosal erosion or ulceration) were referred to have ENRD[7,10].
Entry procedures: The patients prior to the participation in this study signed the informed consent document, which has been approved by an ethical review board of National Taiwan University Hospital.
This study enrolled outpatients of both sexes, who were aged between 18 and 75 years, and were diagnosed to have ENRD. The included patients were asked to discontinue antacid, metoclopramide, cisapride, H2 blocker and PPI for at least 3 days before entering the study. Those patients who had a history of intolerance or allergy to alginic acid and antacid, endoscopic evidence of esophagitis, history of partial or total gastrectomy, or had esophageal stricture, pregnancy or lactation were excluded from the enrollment.
Study endpoints
Primary efficacy endpoint: The primary efficacy end-point was the change in the severity of heartburn as evaluated by using a visual analog scale (VAS) at the 6th week of treatment compared to baseline in the intention-to-treat (ITT) population. The VAS[12,13] is a 100-mm straight line with anchors (0 cm indicated no symptom and 10 cm indicated terrible) placed at both poles. Patients were asked to place a mark somewhere along the line that best described the actual status of their symptoms. The ITT population was defined as randomized patients who received at least one dose of study medication, had a baseline value and at least one post-baseline efficacy assessment. In this analysis, data from the Topaal® group were compared with data from Nacid® group.
Secondary efficacy endpoints: There were three secon-dary endpoints evaluated in this trial: (1) the change in the severity of reflux symptoms including heartburn, regurgitation, dysphagia, epigastric pain, nausea, vomiting and belching by VAS at the 3rd week of treatment compared to baseline; (2) the change in the frequency of heartburn, regurgitation, pain, and sleeping disturbance according to patient’s diary; and (3) the change in the quality of life from doctor’s point of view. All of these secondary efficacy endpoints were assessed in the ITT population.
During the 7th day before the first visit and the entire study period, each patient had to record the frequency (number of episodes per day) on the patient’s diary. Each patient was asked to record the symptom of reflux disease at the moment it happened. At least a 4-d record in a week was necessary for this evaluation.
The patient’s quality of life judged by the investigator’s point of view was graded by modified visick grading[11] on each visit as the following scores: no symptoms (= 1), mild symptoms easily controlled (= 2), moderate symptoms not controlled but not interfering daily life (= 3), moderate symptoms interfering daily life (= 4), and symptoms as bad or worse (= 5).
Safety endpoint: The incidence of adverse drug reaction (ADR), whether reported spontaneously, elicited by questioning, or observed by the investigators, of both Topaal® and Nacid® groups was calculated in the safety population. Safety population was defined as patients who received at least one dose of study medication after randomization. The ADR was recorded and graded according to the WHO definition[14]. The investigators also assessed the causal relationship of any adverse event to the study medication by using the ADR probability scale which was generated from Naranjo et al [15].
All routine laboratory variables (hematology and serum chemistry) were recorded at each visit. Every assessment was compared with baseline values.
Allocation of patients
This was a prospective, randomized, open-label and active-controlled study. Doctors prescribed the test drugs according to the random number sheet, which is generated by SAS. Eligible patients were allocated the next sequential patient number based on preprinted numbers on the study drug labels.
Patients with symptomatic GERD will underwent endoscopy. Randomization occurred within 7 days of the baseline endoscopy. Patients with ENRD were enrolled into the study and then randomly allocated to treat with either Topaal® or Nacid®. No other medical treatment for reflux esophagitis such as other antacid, metoclopramide, cisapride, H2 blocker and PPI was allowed.
The duration of treatment was 6 wk and each patient returned for assessment every 3 wk. At randomization (visit 1) and visit 2, all patients will received one plastic bottle of study medication. Every bottle contains 200 chewable tablets of Topaal® or Nacid®, including 4-day supply in the event of a delay in the scheduled appointment. The dosage of Topaal® and Nacid® is two chewable tablets t.i.d. and h.s.
At the initial assessment, the medical history, past history, life-style (smoking, drinking, coffee consumption) and baseline demographic data, hematologic and serum biochemistry were recorded. A physical examination will be by performed.
Treatment medications
Drug information: Topaal® contains 200 mg alginic acid, 30 mg colloidal aluminum hydroxide and 40 mg magnesium hydrocarbonate per tablet. Nacid® contains 500 mg Mg6Al2(OH)16CO3 4H2O per tablet.
Topaal® is an alginate-based raft-forming formulation[16]comprising alginic acid and antacid. In the presence of gastric acid, alginates precipitate and form a gel[16]. The bicarbonate inside is converted to carbon dioxide, which is then entrapped within the gel precipitate, converting it into foams floating on the surface of the gastric contents, much like a raft on water. The “raft” can act as a physical barrier to reduce reflux episodes.
Statistical analysis
Comparisons of frequency employed the χ2 test of Fisher’s exact test, the test of linear trend for the ordered variables, Student’s t-test of non-parametric Wilcoxon test, and variance analysis for the repeated VAS, all adjusted to the initial values. Two-sample t test was used to compare the continuous data such as height, weight, age, the number of heartburn, the number of sleeping disturbance etc., and the 95%CI for the difference were calculated. Statistical significance was assessed at the 5% level. The principle analysis was carried out on an ITT basis.
A listing of patients with withdrawal as well as those with premature termination of study drugs, along with the date and reasons for the termination was provided.
RESULTS
Disposition of patients
The trial was conducted from June 10, 2003 (first patient screened) to December 24, 2004 (last patient completed). Figure 1 gives an overview of the disposition of the patient population for the treatment phases. A total of 134 patients were randomized to the treatment phase; 69 patients were randomized to Topaal® and 65 were randomized to Nacid®. Of the 134 randomized patients, a total of 112 patients completed the study.
Figure 1.
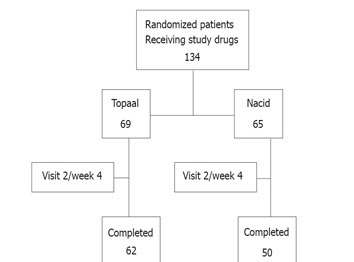
Disposition of patients in the treatment phase.
The safety population included 134 patients (69 in the Topaal® group, 65 in the Nacid® group) who received at least one dose of study medication after randomization. The ITT population consisted of all randomized subjects who received at least one dose of study treatment and who had a baseline and at least one post-baseline efficacy assessment. Three of the sixty-five patients in Nacid® group dropped out for safety/efficacy reason; six in Nacid® group and four in the Topaal® group dropped out for administrative reasons (e.g., lost to follow-up, moved out of the area, etc.). Therefore, 65 patients in the Topaal® group and 56 patients in the Nacid® group were included in the efficacy population.
Demographics and baseline characteristics
Demographic characteristics of randomized subjects in each treatment group are summarized in Table 1. The mean age of the patients in both treatment groups was about 42 years. There were more female than male patients participating in the study in both groups. Although there were significant differences in gender ratio between groups (36.23% male in the Topaal® group vs 16.92 % male in the Nacid® group), no evidence showed that the gender difference would have an effect on the pharmacokinetics or efficacy of study medications. The body mass index (BMI), pulse rate and diastolic blood pressure were all comparable between treatment groups. Although the patients in the Topaal® group seem to have higher systolic blood pressure than the patients in the Nacid® group (119.7± 14.2 mmHg in the Topaal® group vs 114.1 ± 13.5 mmHg in the Nacid® group), those values were all clinically considered to be within normal limits.
Table 1.
Demographics between groups- (safety populations)
| All (134) | Topaal (-69) | Nacid (-65) | P | |
| Sex | ||||
| Male:Female | 36:98 | 25:44 | 11:54 | 0.01861 |
| Age (yr) | 41.9 ± 13.4 | 41.6 ± 14.8 | 42.4 ± 11.8 | 0.73002 |
| Body mass index(kg/m2) | 23.1 ± 3.8 | 23.4 ± 3.8 | 22.8 ± 3.8 | 0.35472 |
| Pulse rate (bpm) | 75.2 ± 6.8 | 75.3 ± 7.0 | 75.1 ± 6.7 | 0.90752 |
| SBP (mmHg) | 117.0 ± 14.1 | 119.7 ± 14.2 | 114.1 ± 13.5 | 0.02112 |
| DBP (mmHg) | 75.0 ± 1.01 | 76.6 ± 9.5 | 73.2 ± 10.6 | 0.05162 |
| History of heartburn (%) | 0.96583 | |||
| 6–12 mo | 75 (56.4) | 39 (56.5) | 36 (56.3) | |
| 1–5 yr | 45 (33.8) | 23 (33.3) | 22 (34.4) | |
| > 5 yr | 13 (9.8) | 7 (10.1) | 6 (9.4) | |
| Smoking (%) | 0.40111 | |||
| Yes | 14 (10.5) | 9 (13.0) | 5 (7.7) | |
| No | 120 (89.5) | 60 (87.0) | 60 (92.3) | |
| Drinking (%) | 1.00001 | |||
| Yes | 5 (3.7) | 3 (4.4) | 2 (3.1) | |
| No | 129 (96.3) | 66 (95.6) | 63 (96.9) | |
| Coffee (%) | 0.08811 | |||
| Yes | 28 (20.9) | 10 (14.5) | 18 (27.7) | |
| No | 106 (79.1) | 59 (85.5) | 47 (72.3) | |
| VAS (cm) | 0.83882 | |||
| Heartburn | 7.5 ± 3.4 | 7.6 ± 3.0 | 7.4 ± 3.7 | |
| Regurgitation | 8.2 ± 3.0 | 8.1 ± 3.1 | 8.2 ± 2.9 | |
| Dysphagia | 1.7 ± 3.2 | 1.4 ± 3.0 | 2.0 ± 3.5 | |
| Epigastric pain | 5.4 ± 4.1 | 4.6 ± 4.1 | 6.2 ± 3.9 | |
| Nausea | 3.5 ± 3.8 | 3.3 ± 3.8 | 3.7 ± 3.8 | |
| Vomiting | 1.1 ± 2.7 | 1.2 ± 2.7 | 1.0 ± 2.6 | |
| Belching | 6.2 ± 4.0 | 5.9 ± 3.9 | 6.4 ± 4.1 | |
| Quality of life (%) | 0.84893 | |||
| Mild symptoms | 4 (3.0) | 3 (4.4) | 1 (1.5) | |
| Moderate w/o interfering | 33 (24.6) | 15 (21.7) | 18 (27.7) | |
| Moderate with interfering | 66 (49.3) | 36 (52.2) | 30 (46.2) | |
| Symptom as bad/worse | 31 (23.1) | 15 (21.7) | 16 (24.6) |
1Fisher’s exact test; 2ANOVA model; 3Mantel–Henszel test; VAS: visual analog scale.
Table 1 also summarizes patients’ baseline symptom analysis that included VAS, the reflux symptoms from doctor’s point of view, and quality of life from doctor’s point of view. More than 56% of the patients in each group had suffered from the reflux symptom of heartburn for at least 6-12 mo. No significant difference in other characteristics including life-style was noted. The baseline characteristics of all randomized subjects were compatible between the two treatment groups, and no statistically significant difference between treatment groups was detected, except for the mean VAS of epigastric pain (P = 0.0218).
EFFICACY EVALUATION
The change in the severity of the heartburn as evaluated using a VAS at 6 wk, using LOCF data from ITT popul-ation, was the primary efficacy endpoint of this trial. At baseline, the mean VAS of heartburn was 7.52 cm in the Topaal® group and 7.43 cm in the Nacid® group. At the end of the 6-week treatment period, the mean VAS of heartburn was 1.20 cm in the Topaal® group and 3.36 cm in the Nacid® group. Overall, the mean change from baseline in VAS of heartburn over the 6-weeks treatment was -6.29 cm (-6.85 to -5.74 cm) in the Topaal® group, and -4.11 cm in the Nacid® group (-4.71 to -3.51 cm). The mean difference between the two treatment groups was -2.19 cm (-3.01 to -1.37 cm) and the difference was statistically significant (P < 0.0001) as detailed in Table 2.
Table 2.
The change in the VAS of heartburn (ITT population)
| Unit: cm | Topaal Mean (SE) | Nacid Mean (SE) | Difference | P |
| Visit 1/baseline Observed data | 7.52 (0.42) | 7.43 (0.45) | 0.09 | 0.8774 |
| Visit 3/week 7Observed data | 1.15 (0.30) | 3.06 (0.34) | –1.91 | < 0.0001 |
| Change from baseline | –6.39 (0.28) | –4.44 (0.31) | –1.95 | < 0.0001 |
ANCOVA model: Dependent variable = (baseline value) + treatment.
The first secondary endpoint was to compare the change of VAS at 3 wk (Table 3). There were significant differences in the mean change of VAS for heartburn (P = 0.0016), regurgitation (P = 0.0006), vomiting (P=0.0373) and belching (P < 0.0001). No significant differences were found in the mean change of VAS for nausea, epigastric pain, and dysphagia. The results suggested that Topaal® is superior to Nacid® for the improvement of the predominant symptoms of ENRD such as heartburn and regurgitation.
Table 3.
Summary of the changes in the VAS at 3 weeks (ITT population)
| Unit: cm |
Topaal (65) |
Nacid (56) |
P-value | |||
| Mean | (SE) | Mean | (SE) | Difference | ||
| Heartburn | ||||||
| Observed data | 2.98 | 0.37 | 4.43 | 0.4 | –1.44 | 0.0091 |
| Change from baseline | –4.52 | 0.31 | –3.03 | 0.34 | –1.49 | 0.0016 |
| Regurgitation | ||||||
| Observed data | 3.52 | 0.37 | 5.32 | 0.4 | –1.80 | 0.0012 |
| Change from baseline | –4.55 | 0.33 | –2.84 | 0.36 | –1.72 | 0.0006 |
| Dysphagia | ||||||
| Observed data | 0.65 | 0.27 | 1.25 | 0.29 | –0.60 | 0.1326 |
| Change from baseline | –0.97 | 0.19 | –0.68 | 0.2 | –0.28 | 0.3138 |
| Epigastric pain | ||||||
| Observed data | 2.08 | 0.42 | 3.88 | 0.45 | –1.80 | 0.0039 |
| Change from baseline | –2.93 | 0.33 | –2.10 | 0.36 | –0.84 | 0.0925 |
| Nausea | ||||||
| Observed data | 1.25 | 0.32 | 1.89 | 0.34 | –0.65 | 0.1705 |
| Change from baseline | –2.10 | 0.25 | –1.51 | 0.27 | –0.59 | 0.1137 |
| Vomiting | ||||||
| Observed data | 0.31 | 0.18 | 0.61 | 0.2 | –0.30 | 0.2662 |
| Change from baseline | –0.92 | 0.12 | –0.55 | 0.13 | –0.37 | 0.0373 |
| Belching | ||||||
| Observed data | 2.54 | 0.4 | 5.14 | 0.43 | –2.60 | <0.0001 |
| Change from baseline | –3.64 | 0.32 | –1.40 | 0.34 | –2.23 | <0.0001 |
ANCOVA model: Dependent variable = (baseline value) + treatment.
The second secondary endpoint was to compare the frequency of heartburn, regurgitation, pain, and sleep disturbance according to patient’s diary every week during treatment period (Figure 2). Patients taking Topaal® showed consistently and significantly lower frequency of heartburn than those who were taking Nacid® throughout the entire 6-week period (P = 0.0015). Patients taking Topaal® also had fewer episodes of pain than those who were taking Nacid® from the 4th week to the end of the study. Both Topaal® and Nacid® decreased the frequency of regurgitation during the study period compared to baseline, but the difference between treatments was not significant. No difference was observed for the change in the frequency of sleeping disturbance.
Figure 2.
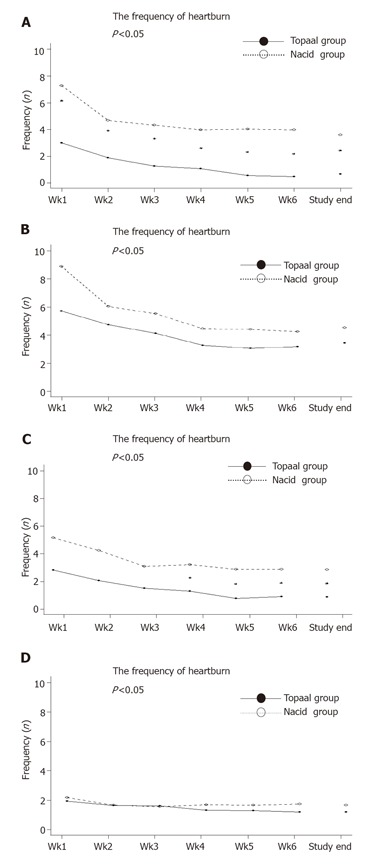
The frequency of heartburn, regurgitation, pain and sleep disturbance.
The third and fourth secondary endpoints were to compare the change in the severity of the reflux symptoms (Table 4) and the quality of life from doctor’s point of view (Table 5). There were no significant differences in the severity of the reflux symptoms and the quality of life from doctor’s point of view at baseline. At the end of the 6-week treatment period, patients who took Topaal® showed better improvement in the severity of heartburn (P = 0.0020), regurgitation (P = 0.0081), vomiting (P = 0.0182), and belching (P = 0.0018) than those who took Nacid®. There were no significant differences in the improvement of dysphagia (P = 0.7551), epigastric pain (P = 0.2648), and nausea (P = 0.0577).
Table 4.
The change in the severity of the reflux symptom
| Population: intent-to-treat | Topaal (%) | Overall (%) | Nacid (%) | P-value |
| Heartburn | 0.00191 | |||
| Change from baseline | ||||
| –1 | 4 (3.3) | 1 (1.5) | 3 (5.4) | |
| 0 | 28 (23.1) | 8 (12.3) | 20 (35.7) | |
| 1 | 42 (34.7) | 21 (32.3) | 21 (37.5) | |
| 2 | 30 (24.8) | 23 (35.4) | 7 (12.5) | |
| 3 | 17 (14.1) | 12 (18.5) | 5 ( 8.9) | |
| Regurgitation | 0.06251 | |||
| Change from baseline | ||||
| –1 | 2 (1.6) | 1 (1.5) | 1 ( 1.8) | |
| 0 | 30 (24.8) | 12 (18.5) | 18 (32.1) | |
| 1 | 42 (34.7) | 21 (32.3) | 21 (37.5) | |
| 2 | 36 (29.8) | 21 (32.3) | 15 (26.8) | |
| 3 | 11 ( 9.1) | 10 (15.4) | 1 (1.8) | |
| Dysphagia | 0.25591 | |||
| Change from baseline | ||||
| –1 | 4 (3.3) | 2 (3.1) | 2 (3.8) | |
| 0 | 90 (74.4) | 50 (79.6) | 40 (71.4) | |
| 1 | 16 (13.2) | 5 ( 7.5) | 11 ( 19.6) | |
| 2 | 10 ( 8.3) | 7 (10.8) | 3 ( 5.3) | |
| 3 | 1 ( 0.8) | 1 ( 1.5) | 0 ( 0.0) | |
| Epigastric pain | 0.25171 | |||
| Change from baseline | ||||
| –2 | 3 (2.5) | 1 (1.5) | 2 (3.8) | |
| –1 | 4 ( 3.3) | 3 ( 4.6) | 1 ( 1.8) | |
| 0 | 57 (47.1) | 30 (46.2) | 27 (48.2) | |
| 1 | 33 (27.3) | 15 ( 23.1) | 18 ( 32.1) | |
| 2 | 19 (15.7) | 11 (16.9) | 8 ( 14.3) | |
| 3 | 5 (4.1) | 5 (7.7) | 0 ( 0.0) | |
| Nausea | 0.22101 | |||
| Change from baseline | ||||
| –1 | 1 (0.8) | 0 (0.00%) | 1 (1.8) | |
| 0 | 70 (57.9) | 33 (50.8) | 37 (66.1) | |
| 1 | 23 (19.0) | 15 (23.1) | 8 (14.3) | |
| 2 | 25 (20.7) | 15 (23.1) | 10 (17.9) | |
| 3 | 2 (1.7) | 2 (3.1) | 0 (0.0) | |
| Vomiting | 0.18881 | |||
| Change from baseline | ||||
| –1 | 1 (0.8) | 0 (0.0) | 1 (1.8) | |
| 0 | 100 (82.6) | 50 (79.6) | 50 (81.3) | |
| 1 | 9 (7.4) | 6 (9.2) | 3 ( 5.4) | |
| 2 | 9 (7.4) | 7 (10.8) | 2 ( 3.6) | |
| 3 | 2 (1.7) | 2 (3.1) | 0 ( 0.0) | |
| Belching | 0.03661 | |||
| Change from baseline | ||||
| –1 | 8 (6.6) | 2 (3.1) | 6 (10.7) | |
| 0 | 48 (39.7) | 21 (32.3) | 27 (48.2) | |
| 1 | 42 (34.7) | 25 (38.5) | 17 (30.4) | |
| 2 | 19 (15.7) | 13 (20.0) | 6 (10.7) | |
| 3 | 4 (3.3) | 4 ( 6.2) | 0 (0.0) |
χ2 test.
Table 5.
Summary of the change of quality of life
| ITT population | Overall (%) | Topaal (%) | Nacid (%) | P-value |
| Visit 1/baseline | ||||
| Observed data | 0.9298 | |||
| No symptom | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Moderate symptom | 4 (3.3) | 3 (4.6) | 1 (1.8) | |
| Moderate symptom w/o interfering | 29 (24.0) | 13 (20.0) | 16 (28.6) | |
| Moderate symptom with interfering | 61 (50.4) | 35 (53.9) | 26 (46.4) | |
| Symptom as bad/worse | 27 (22.3) | 14 (21.5) | 13 (23.2) | |
| Study end | ||||
| Observed data | <0.0001 | |||
| No symptom | 24 (19.8) | 19 (29.2) | 5 (8.9) | |
| Moderate symptom | 51 (42.2) | 34 (52.3) | 17 (30.4) | |
| Moderate symptom w/o interfering | 27 (22.3) | 8 (12.3) | 19 (33.9) | |
| Moderate symptom with interfering | 15 (12.4) | 3 (4.6) | 12 (21.4) | |
| Symptom as bad/worse | 4 (3.3) | 1 (1.5) | 3 (5.4) | |
| Decrease from baseline | <0.0001 | |||
| –1 | 1 (0.8) | 0 (0.0) | 1 (1.8) | |
| 0 | 24 (19.8) | 7 (10.8) | 17 (30.4) | |
| 1 | 36 (29.8) | 16 (24.6) | 20 (35.7) | |
| 2 | 36 (29.8) | 22 (33.9) | 14 (25.0) | |
| 3 | 16 (13.2) | 13 (20.0) | 3 (5.4) | |
| 4 | 8 (6.6) | 7 (10.8) | 1 (1.8) |
1Mantel–Henszel χ2 test.
For the quality of life from doctor’s point of view, there were no significant differences at baseline. About 50% of the patients in each group suffered from moderate reflux symptoms interfering with the quality of life before the treatment. At the end of treatment period, patients who took Topaal® had greater alleviations of symptoms (P < 0.0001) and greater reduction of symptom score from baseline (P < 0.0001) than those who took Nacid®. The results suggested that Topaal is more efficient than Nacid for improving the quality of life of ENRD patients.
SAFETY EVALUATION
The ADRs of Topaal® and Nacid® were the safety end-points of this trial. In this analysis, data from the Topaal® group were compared with data from Nacid® group.
Adverse events which occurred during the course of the study were recorded. Seven patients had at least one adverse event after entering the study, three (4.35 %) patients in the Topaal® group and four (6.15 %) patients in the Nacid® group. The most commonly adverse events reported by Topaal®-treated patients were constipation (2 patients, 2.90 %). For Nacid® group, two patients (3.08 %) experienced diarrhea and two patients (3.08 %) reported constipation. No statistical significant difference was found between the two groups in the incidence of adverse event. All of these adverse events were resolved and graded as mild or moderate in severity. None of them was considered by the investigators to be definitely related to treatment. No dosage modification of medication was applied for the three adverse events in Topaal®-treated patients, whereas two of the four adverse events in Nacid®-treated patients led to dose reduction of medication. No serious adverse event was reported during the entire study period. The results suggest that Topaal® had a similar safety profile as Nacid®.
DISCUSSION
The design of this study is based on symptomatic analysis. This is an original, though not blind, trial that compares the therapeutic effects of an alginate to an antacid on ENRD patients. For the best interest of the patients, a placebo-controlled study was not considered in our design. The investigators’ and the patients’ assessments of efficacy over the study period are similar and in favor of the alginate. After 6-week treatment, Topaal® was found to be more effective than Nacid® in reducing VAS of five symptoms including heartburn, regurgitation, vomiting, and belching in patients with ERND. Patients in Topaal® group had fewer episodes of heartburn and epigastric pain at the end of treatment. Also, from doctor’s point of view, Topaal® is more effective than Nacid® in reducing symptoms of reflux and in improving the quality of life in patients with ERND. Our result is consistent with that of other trials that compared the effects of alginate-based formulation with antacids on patients with reflux esophagitis[17] and volunteers[18],though their patient groups were more heterogenous.
According to our data, the symptom-relieving effect in the Topaal® group was faster than that of Nacid®. Patients taking Topaal® had major reduction in VAS scores of heartburn and regurgitation from the 1st week of study, and patients taking Nacid® did not feel the difference in symptoms until the 2nd week of study. Alginic acid is different from antacids in the mechanism of efficacy. The alginic acid is converted to sodium alginate by the small amount of antacid contained in the formulation. This salt floats atop of the esophagogastric junction and provides a barrier when reflux occurs[16]. The physical property of Topaal® might explain why it worked faster and better than Nacid® in relieving the symptoms of reflux. On the other hand, our results did not show differences in improving the sleep disturbance in patients of both groups. We did not assess the incidence of night refluxer or asthma in the enrolled patients who might have greater disturbance in sleeping. We deduced that the incidence of night refluxer maybe low because both study groups had low VAS score of sleep disturbance at the baseline. Though alginate products have been shown to be effective in relieving symptoms of reflux, it has not been proved that the alginates promote healing of erosive esophagitis. In this way, alginate-based formulation is a reasonable option for patients with ENRD.
Goves et al[19] compared the effectiveness of PPI, with alginates for heartburn relief in dyspeptic patients. This design showed that PPI was superior to alginates in relieving the heartburn of patients at 4 weeks of treatment. This is not surprising for they included patients with severe esophagitis, and omeprazole had the dramatic antisecretory actions. However, considering ENRD is a protracted disease, alginate is a better option for long-term and on-demand use in these patients for cost-effectiveness.
For adverse events, Topaal® group had a compatible safety profile as Nacid® group. Alginates have been shown to be safe when used in pregnant women[20] and children[21] In our patients treated with Topaal®, the most commonly reported adverse events were constipation (2.90 %).
In conclusion, Topaal® is more effective than Nacid® in symptomatic control of patient with ENRD. For the safety measures, the results supported that Topaal® group had a compatible safety profile as Nacid® group.
Footnotes
S- Editor Guo SY L- Editor Elsevier HK E- Editor Wu M
References
- 1.Chen PC, Wu CS, Chang-Chien CS, Liaw YF. [Comparison of Olympus GIF-P2 and GIF-K panendoscopy] Taiwan Yi Xue Hui Za Zhi. 1979;78:136–140. [PubMed] [Google Scholar]
- 2.Yeh C, Hsu CT, Ho AS, Sampliner RE, Fass R. Erosive esophagitis and Barrett's esophagus in Taiwan: a higher frequency than expected. Dig Dis Sci. 1997;42:702–706. doi: 10.1023/a:1018835324210. [DOI] [PubMed] [Google Scholar]
- 3.Wesdorp IC. Reflux oesophagitis: a review. Postgrad Med J. 1986;62 Suppl 2:43–55. [PubMed] [Google Scholar]
- 4.Dent J, Holloway RH, Toouli J, Dodds WJ. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988;29:1020–1028. doi: 10.1136/gut.29.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlando RC. Mechanisms of reflux-induced epithelial injuries in the esophagus. Am J Med. 2000;108 Suppl 4a:104S–108S. doi: 10.1016/s0002-9343(99)00348-4. [DOI] [PubMed] [Google Scholar]
- 6.Chey WD. Endoscopy-negative reflux disease: concepts and clinical practice. Am J Med. 2004;117 Suppl 5A:36S–43S. doi: 10.1016/j.amjmed.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson R, Dent J, Bolling-Sternevald E, Johnsson F, Junghard O, Lauritsen K, Riley S, Lundell L. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D, Talley NJ, Lauritsen K, Moum B, Lind T, Tunturi-Hihnala H, Venables T, Green J, Bigard MA, Mössner J, et al. The role of acid suppression in patients with endoscopy-negative reflux disease: the effect of treatment with esomeprazole or omeprazole. Aliment Pharmacol Ther. 2004;20:413–421. doi: 10.1111/j.1365-2036.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, Vernisse B, Agostini H. Randomized, multicentre comparison of sodium alginate and cisapride in the symptomatic treatment of uncomplicated gastro-oesophageal reflux. Aliment Pharmacol Ther. 1998;12:159–165. doi: 10.1046/j.1365-2036.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Venables TL, Green JR, Armstrong D, O'Kane KP, Giaffer M, Bardhan KD, Carlsson RG, Chen S, Hasselgren GS. Esomeprazole 40 mg and 20 mg is efficacious in the long-term management of patients with endoscopy-negative gastro-oesophageal reflux disease: a placebo-controlled trial of on-demand therapy for 6 months. Eur J Gastroenterol Hepatol. 2002;14:857–863. doi: 10.1097/00042737-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Pace F, Bollani S, Molteni P, Bianchi Porro G. Natural history of gastro-oesophageal reflux disease without oesophagitis (NERD)--a reappraisal 10 years on. Dig Liver Dis. 2004;36:111–115. doi: 10.1016/j.dld.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Cange L, Johnsson E, Rydholm H, Lehmann A, Finizia C, Lundell L, Ruth M. Baclofen-mediated gastro-oesophageal acid reflux control in patients with established reflux disease. Aliment Pharmacol Ther. 2002;16:869–873. doi: 10.1046/j.1365-2036.2002.01250.x. [DOI] [PubMed] [Google Scholar]
- 13.Watson DI, Pike GK, Baigrie RJ, Mathew G, Devitt PG, Britten-Jones R, Jamieson GG. Prospective double-blind randomized trial of laparoscopic Nissen fundoplication with division and without division of short gastric vessels. Ann Surg. 1997;226:642–652. doi: 10.1097/00000658-199711000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JW, Seidl LG, Cluff LE. Studies on the epidemiology of adverse drug reactions. V. Clinical factors influencing susceptibility. Ann Intern Med. 1966;65:629–640. doi: 10.7326/0003-4819-65-4-629. [DOI] [PubMed] [Google Scholar]
- 15.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 16.Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment Pharmacol Ther. 2000;14:669–690. doi: 10.1046/j.1365-2036.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 17.Chevrel B. A comparative crossover study on the treatment of heartburn and epigastric pain: Liquid Gaviscon and a magnesium--aluminium antacid gel. J Int Med Res. 1980;8:300–302. doi: 10.1177/030006058000800411. [DOI] [PubMed] [Google Scholar]
- 18.Washington N, Greaves JL, Iftikhar SY. A comparison of gastro-oesophageal reflux in volunteers assessed by ambulatory pH and gamma monitoring after treatment with either Liquid Gaviscon or Algicon Suspension. Aliment Pharmacol Ther. 1992;6:579–588. doi: 10.1111/j.1365-2036.1992.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 19.Goves J, Oldring JK, Kerr D, Dallara RG, Roffe EJ, Powell JA, Taylor MD. First line treatment with omeprazole provides an effective and superior alternative strategy in the management of dyspepsia compared to antacid/alginate liquid: a multicentre study in general practice. Aliment Pharmacol Ther. 1998;12:147–157. doi: 10.1046/j.1365-2036.1998.0284f.x. [DOI] [PubMed] [Google Scholar]
- 20.Lindow SW, Regnéll P, Sykes J, Little S. An open-label, multicentre study to assess the safety and efficacy of a novel reflux suppressant (Gaviscon Advance) in the treatment of heartburn during pregnancy. Int J Clin Pract. 2003;57:175–179. [PubMed] [Google Scholar]
- 21.Buts JP, Barudi C, Otte JB. Double-blind controlled study on the efficacy of sodium alginate (Gaviscon) in reducing gastroesophageal reflux assessed by 24 h continuous pH monitoring in infants and children. Eur J Pediatr. 1987;146:156–158. doi: 10.1007/BF02343223. [DOI] [PubMed] [Google Scholar]


