Abstract
AIM: To study the changes of portal blood flow in congestive heart failure.
METHODS: We studied the congestion index (CI) and portal vein pulsatility index (PI) in patients with varied degrees of congestive heart failure using ultrasonic Doppler. Ten patients with mean right atrial pressure (RA) < 10 mmHg were classified as group 1 and the remaining 10 patients with RA ≥ 10 mmHg as group 2.
RESULTS: There were no difference on cardiac index (HI, P = 0.28), aortic pressure (AO, P = 0.78), left ventricular end-diastolic pressure (LVED, P = 0.06), maximum portal blood velocity (Vmax, P = 0.17), mean portal blood velocity (Vmean, P = 0.15) and portal blood flow volume (PBF, P = 0.95) between the two groups. Group 2 patients had higher pulmonary wedge pressure (PW, 29.9 ± 9.3 mmHg vs 14.6 ± 7.3 mmHg, P = 0.002), pulmonary arterial pressure (PA, 46.3 ± 13.2 mmHg vs 25.0±8.2 mmHg, P =0.004), RA (17.5±5.7 mmHg vs 4.7 ± 2.4 mmHg, P < 0.001), right ventricular end-diastolic pressure (RVED, 18.3 ± 5.6 mmHg vs 6.4 ± 2.7 mmHg, P < 0.001), CI (8.7 ± 2.4 vs 5.8 ± 1.2, P = 0.03), and PI (87.8 ± 32.3% vs 27.0 ± 7.4%, P < 0.001) than Group 1. CI was correlated with PI (P < 0.001), PW (P < 0.001), PA (P < 0.001), RA (P = 0.043), RVED (P = 0.005), HI (P < 0.001), AO (P < 0.001), CO (P < 0.001), LVED (P < 0.001), Vmax (P < 0.001), Vmean (P < 0.001), cross-sectional area of the main portal vein (P < 0.001) and PBF (P < 0.001). CI could be as high as 8.3 in patients with RA < 10 mmHg and as low as 5.9 in those with RA ≥ 10 mmHg.
CONCLUSION: Our data show that RI is a more significant indicator than CI in the clinical evaluation of high RA ≥ 10 mmHg, whereas CI is better than PI in the assessment of left heart function.
Keywords: Portal blood flow, Heart failure, Ultrasonic Doppler, Congestion index, Portal vein pulsatility index
INTRODUCTION
Congestive heart failure increases the pressure in the inferior vena cava and hepatic veins[1-3]. Ultrasonic Doppler is a safe and non-invasive method in the clinical evaluation of portal blood flow and portal hypertension[4-9]. Portal vein pulsatility index (PI) is calculated by the percentage of peak-to-peak maximum portal vein velocities[10,11]. In our earlier study[10], patients with right heart failure developed transient reduced, stagnant, or hepatofugal portal blood flow with increased PI. However, the change of portal flow pattern and PI did not correlate with left heart function.
The congestion index (CI) has been used to assess the pathophysiological hemodynamics of portal venous system in different forms of liver diseases[12-14]. The correlation between CI and PI and the role of CI on right heart function remain uncertain. Therefore, we have studied the changes of portal blood flow in patients with different degrees of heart failure using non-invasive ultrasonic Doppler[15,16].
MATERIALS AND METHODS
We studied the portal hemodynamic profiles in 20 patients (9 males, 11 females, mean age: 49 ± 13 years) who received cardiac and Swan-Ganz catheterizations for cardiovascular disorders (16 rheumatic heart disease, 4 atherosclerotic heart disease) to compare with 20 healthy volunteers. All patients had medications affecting the hemodynamics such as isosorbide dinitrate and furosemide, and their systemic blood pressure and body weight were measured to be constant for more than 48 h prior to the study. Patients with fever, infection, and shock were excluded. All patients had no history of liver disease, alcoholism or other metabolic disorders. None of the patients received transfusion, inotropic agents or dopamine. All patients had an abdominal sonography to exclude chronic liver disease or splenomegaly. Patients with severe orthopnea were excluded if they were not able to remain in the supine position for the study of ultrasonic Doppler.
Cardiac profiles including cardiac index (HI), left ventricular end-diastolic pressure (LVED), mean aortic pressure (AO), pulmonary wedge pressure (PW), mean pulmonary arterial pressure (PA), mean right atrial pressure (RA), right ventricular end-diastolic pressure (RVED) were recorded during the cardiac and Swan-Ganz catheterizations. Ten patients with RA < 10 mmHg (range: 1-7 mmHg) and without right heart failure were classified as Group 1. The remaining 10 patients with right heart failure and RA ≥ 10 mmHg (range: 10-28 mmHg) were classified as Group 2.
The portal profiles were assessed using an ultrasonic Doppler composed of a real-time mechanical sector scanner and a 3.5 mHz pulsed Doppler flowmetry (Aloka Echo Camera, Model SSD-1700, Tokyo) within 12 h of cardiac catheterization. After more than 8 h of fasting, portal profiles were measured in the supine position for more than 30 min. Portal blood flow was measured from the main portal vein at a site just entering or immediately after entering the liver with the patient in expiratory apnea. The flow angle formed by the directions of ultrasonic beam and the portal blood flow below 55 degree was corrected to minimize the variation caused by the angle of insonation. The Doppler signal could be viewed on the screen and heard through a build-in speaker. Portal blood flow was measured by the same physician (SY) to avoid interobserver variation[17].
For each measurement, at least three reproducible spectral patterns were recorded for calculating the mean maximum portal blood velocity (Vmax) over a period of 3-4 s to ensure accuracy. Mean portal blood velocity (Vmean) was calculated by the equation “Vmean=0.57×Vmax” as described by Moriyasu et al.[18]. Cross-sectional area (area, cm2) was also recorded at the site of main portal vein where portal blood velocity was measured. The direction of portal blood velocity, antegrade or retrograde, was also measured. Positive velocity indicates the blood flow towards the transducer and vice versa. Portal blood flow volume (PBF, mL/min) was obtained by the equation “PBF=area×Vmean×60”[17,18]. PI was calculated by the equation “PI=(maximum-minimum)/maximum frequency shift”[6,15,17]. The waveforms were classified as continuous (PI ≤ 40%), decreased (PI 41-99%), stagnant (PI = 100%), or retrograde (PI>100%)[10,19]. CI was calculated by the equation “CI = (area/Vmean)×100”[12].
The study protocol was reviewed and approved by the Institutional Review Committee under the guidelines of the 1 975 Declaration of Helsinki. Statistical analysis was performed using Student’s t-test and simple linear regression as appropriate.
RESULTS
The biochemical data of the 20 patients (Table 1) showed total protein 7.0 ± 0.8 g/dL, albumin 3.8 ± 0.5 g/dL, total bilirubin 1.3 ± 0.6 mg/dL, AST 49.5 ± 23.4 IU/L, ALT 28.7 ± 10.4 IU/L, and prolonged prothrombin time 1.2 ± 0.9 s (normal < 3 s). All controls had normal blood chemistries. Gender (P = 0.11), age (P = 0.61), total protein (P = 0.85), albumin (P = 0.62), total bilirubin (P = 0.83), ALT (P = 0.15) and prolonged prothrombin time (P = 0.19) were not different between those with RA < 10 mmHg and ≥ 10 mmHg. Patients with RA ≥ 10 mmHg had higher serum AST activities (P = 0.009), which were related to ischemic hepatitis.
Table 1.
Clinical and biochemical data in patients with heart failure (mean¡ÀSD)
| Controls | RA < 10 mmHg | RA ¡Ý 10 mmHg | |
| Gender (M/F) | 10/10 | 4/6 | 5/5 |
| Age (yr) | 46 ± 12 | 50 ± 13 | 47 ± 19 |
| Total protein (g/dL) | 7.5 ± 0.6 | 7.1 ± 0.8 | 6.9 ± 1.1 |
| Albumin (g/dL) | 4.3 ± 0.2 | 3.9 ± 0.7 | 3.7 ± 0.5 |
| Total serum bilirubin (mg/dL) | 0.9 ± 0.4 | 1.3 ± 0.8 | 1.4 ± 0.8 |
| AST (U/L) | 21 ± 6 | 31 ± 11 | 59 ± 291b |
| ALT (U/L) | 24 ± 6 | 23 ± 8 | 35 ± 20 |
| Prolonged prothrombin time (s) | - | 1.1 ± 0.9 | 1.4 ± 0.8 |
RA, right atrial pressure ;
P=0.009 vs patients with RA < 10 mmHg and
P<0.001 vs controls.
HI (3.0 ± 0.9 L/min/m2; range: 1.6-5.3 L/min/m2 vs 2.4 ± 0.4 L/min/m2; range: 1.7-2.9 L/min/m2; P = 0.28), AO (89.0 ± 9.6 mmHg; range: 85-100 mmHg vs 87.3 ± 12.8 mmHg; range: 65-115 mmHg; P = 0.78), and LVED (12.2 ± 6.7; range: 4-34 mmHg vs 22.1 ± 10.9 mmHg; range: 10-40 mmHg; P = 0.06) were not statistically different between Groups 1 and 2 (Table 2).
Table 2.
Cardiac profiles in patients with congestive heart failure(mean¡ÀSD)
| RA < 10 mmHg | RA ¡Ý 10 mmHg | P | |
| HI (L/min/m2) | 3.0 ± 0.9 | 2.4 ± 0.4 | 0.28 |
| AO (mmHg) | 89.0 ± 9.6 | 87.3 ± 12.8 | 0.78 |
| LVED (mmHg) | 12.2 ± 6.7 | 22.1 ± 10.9 | 0.06 |
| PW (mmHg) | 14.6 ± 5.6 | 29.1 ± 7.7 | 0.002 |
| PA (mmHg) | 25.0 ± 6.8 | 42.4 ± 12.0 | 0.004 |
| RA (mmHg) | 4.7 ± 2.1 | 16.8 ± 4.9 | < 0.001 |
| RVED (mmHg) | 6.4 ± 2.1 | 17.8 ± 4.4 | < 0.001 |
RA, right atrial pressure; HI, cardiac index; LVED, left ventricular end-diastolic pressure; AO, mean aortic pressure; PW, pulmonary wedge pressure; PA, mean pulmonary arterial pressure; RA, mean right atrial pressure; RVED, right ventricular end-diastolic pressure.
For all Group 1 patients, the values of PW (mean: 14.6 ± 5.6 mmHg; range: 5-28 mmHg), PA (mean: 25.0 ± 6.8 mmHg; range: 16-38 mmHg), RA (mean: 4.7 ± 2.1 mmHg; range: 1-7 mmHg), and RVED (mean: 6.4 ± 2.1 mmHg; range: 2-11 mmHg) were within the normal limits.
All Group 2 patients had higher PW (mean: 29.1 ± 7.7 mmHg; range: 13-40 mmHg; P = 0.002), PA (mean: 42.4 ± 12.0 mmHg; range: 25-65 mmHg; P = 0.004), RA (mean: 16.8 ± 4.9 mmHg; range: 10-28 mmHg; P < 0.001), and RVED (mean: 17.8 ± 4.4 mmHg; range: 9-26 mmHg; P < 0.001) than Group 1 patients.
The healthy controls had Vmax 20.1 ± 3.1 cm/s, Vmean 11.2 ± 1.9 cm/s, area 1.01 ± 0.20 cm2, PBF 685 ± 136 mL/min, PI 23.3 ± 6.3%, and CI 5.3 ± 1.2. The mean values of Vmax (24.5 ± 3.9 cm/s; range 17-33 cm/s vs 21.1 ± 4.8 cm/s; range 14-33 cm/s; P = 0.17), Vmean (14.0 ± 2.3 cm/s; range: 9.7-18.8 cm/s vs 12.0±2.7 cm/s; range: 8.6-18.8 mmHg; P = 0.15) and PBF (678 ± 172 mL/min; range: 373-1 120 mL/min vs 672 ± 162 mL/min; range: 432-922 mL/min; P = 0.95) between Groups 1 and 2 did not show any statistical difference (Table 3). Group 2 patients had a larger area of portal vein than that of Group 1 (0.80 ± 0.13 cm2; range: 0.64-1.13 cm2 vs 0.96 ± 0.13 cm2; range: 0.79-1.33 cm2; P = 0.04).
Table 3.
Portal profiles in patients with congestive heart failure( mean¡ÀSD)
| Controls (n = 20) | RA ¡Ü 10 mmHg (n= 10) | RA > 10 mmHg (n = 10) | |
| Vmax (cm/s) | 20.1 ± 3.1 | 24.5 ± 3.9 | 21.1 ± 4.8 |
| Vmean (cm/s) | 11.2 ± 1.9 | 14.0 ± 2.3 | 12.0 ± 2.7 |
| Area (cm2) | 1.01 ± 0.20 | 0.80 ± 0.13 | 0.96 ± 0.13 |
| PBF (mL/min) | 685 ± 136 | 678 ± 172 | 672 ± 162 |
| PI (%) | 23.3 ± 6.3 | 27.0 ± 7.4 | 87.8 ± 32.3b |
| Congestion index | 5.3 ± 1.2 | 5.8 ± 1.2 | 8.7 ± 2.41 |
RA, right atrial pressure; Vmax, maximum portal velocity; Vmean, mean portal velocity; PBF, portal blood flow; PI, portal vein pulsatility index.
P<0.001,
P=0.03 vs controls and RA ≤ 10 mmHg.
All the 10 patients in Group 1 had a continuous antegrade portal flow with a mean PI 27.0 ± 7.4% (range: 17-40%) (Figure 1). The mean PI of the 10 patients in Group 2 was 87.8 ± 32.2% (range: 43-194%). In Group 2, all the patients had a PI > 40%. Six of them had transient reduced portal blood flow, one had stagnant flow, and three had hepatofugal flow.
Figure 1.
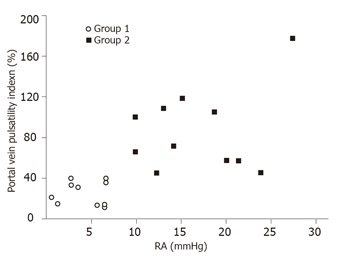
Portal vein pulsatility index of patients with right atrial pressure < 10 mmHg (Group 1) and ≥ 10 mmHg (Group 2).
Group 2 patients (mean: 8.7 ± 2.4, range: 5.9-16.7) had a higher CI than that of Group 1 patients (mean: 5.8 ± 1.2, range: 3.9-8.3; P = 0.03). Although Group 2 had a higher mean CI than Group 1, the CI could be as low as 5.9 in Group 2 and as high as 8.3 in Group 1 (Figure 2).
Figure 2.
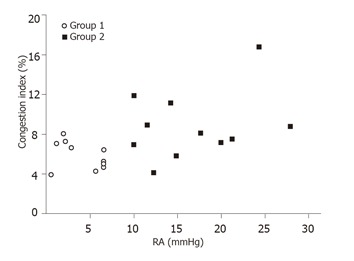
Congestion index of patients with right atrial pressure (RA) < 10 mmHg (Group 1) and ≥ 10 mmHg (Group 2).
Using linear regression, CI showed a good correlation with PI (P < 0.001), PW (P < 0.001), PA (P < 0.001), RA (P = 0.043), RVED (P = 0.005), HI (P < 0.001), AO (P < 0.001), CO (P < 0.001), LVED (P < 0.001), Vmax (P < 0.001), Vmean (P < 0.001), area (P < 0.001) and PBF (P < 0.001).
DISCUSSION
It is well known that the passive “backward” congested liver develops into hepatomegaly, synchronous pulsation, engorged and dilated terminal hepatic veins, atrophy of hepatocytes and eventually cardiac cirrhosis. The high hepatic vein pressure can transmit through the liver to cause post-sinusoidal portal hypertension, cardiac ascites and change of portal vein flow patterns[12,13]. Therefore, the changes of portal flow may help the assessment of heart function.
Prolonged right heart failure may result in atrophy of hepatocytes and eventually cardiac cirrhosis[3]. In the present study, we have strived to exclude those patients with chronic liver disease. The abdominal sonographies showed no splenomegaly or coarse liver echogenicity and the peripheral blood showed no abnormal reduction of leukocyte, hemoglobin or platelet account, which were common in cirrhosis. Furthermore, the portal flow pattern did not show reduced fluctuation, which was common in cirrhosis with portal hypertension[4]. Our patients were not likely to develop obvious cardiac cirrhosis.
In the present study, all patients with RA ≥ 10 mmHg had a PI > 40% and all patients with RA < 10 had a PI < 40% or less. The findings were consistent with our prior study[10] that PI showed a good correlation with PW, PA, RA, and RVED. The waveform changes of portal blood flow correlate well with right heart function, and the measurement of PI change is a simple and non-invasive method to identify right heart failure[10]. Our data also demonstrated that PI had no any correlation with HI, AO, CO, LVED, Vmax, Vmean and PBF. Furthermore, the waveform changes of portal blood flow correlated well with right heat function; and the PI is helpful for the diagnosis of stagnant or hepatofugal portal blood flow but not by the CI[10]. Therefore, CI is better than PI in the assessment of left heart function.
In addition to the assessment of left heart function, the CI correlated with all PBF, Vmax, Vmean, area, PI, HI, PW, PA, RA, AO, CO, LVED, and RVED. These results suggest that CI also correlates well with right heart profiles. Our findings were consistent with earlier studies[12,20,21]. However, the CI values could be as high as 8.3 in patients with RA < 10 mmHg and as low as 5.9 in those with RA ≥ 10 mmHg. If the CI value is between 5.9 and 8.3, it is difficult to predict whether or not the RA values ≥ 10 mmHg. Therefore, RI is a more significant indicator than CI in the clinical evaluation of high RA ≥ 10 mmHg.
The occurrence of congestive liver is not uncommon in patients with congestive heart failure. In addition to the occurrence of congestive hepatomegaly and dilatation of inferior vena cava and hepatic veins during abdominal sonography, the measurement of both CI and PI is helpful for the indirect non-invasive evaluation of cardiac function.
Footnotes
Supported by the grant from the Cathay Groups, Taipei, Taiwan, China
S- Editor Guo SY L- Editor Elsevier HK E- Editor Cao L
References
- 1.Pannen BH. New insights into the regulation of hepatic blood flow after ischemia and reperfusion. Anesth Analg. 2002;94:1448–1457. doi: 10.1097/00000539-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Shen B, Younossi ZM, Dolmatch B, Newman JS, Henderson JM, Ong JP, Gramlich T, Yamani M. Patent ductus venosus in an adult presenting as pulmonary hypertension, right-sided heart failure, and portosystemic encephalopathy. Am J Med. 2001;110:657–660. doi: 10.1016/s0002-9343(01)00701-x. [DOI] [PubMed] [Google Scholar]
- 3.Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis. 2002;6:947–67, viii-ix. doi: 10.1016/s1089-3261(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 4.Ohnishi K, Saito M, Sato S, Sugita S, Tanaka H, Okuda K. Clinical utility of pulsed Doppler flowmetry in patients with portal hypertension. Am J Gastroenterol. 1986;81:1–8. [PubMed] [Google Scholar]
- 5.Shapiro RS, Stancato-Pasik A, Glajchen N, Zalasin S. Color Doppler applications in hepatic imaging. Clin Imaging. 1998;22:272–279. doi: 10.1016/s0899-7071(98)00007-2. [DOI] [PubMed] [Google Scholar]
- 6.Yang SS, Wu CH, Huang CS, Ho MS, Lai MY, Kao JH, Chen DS. Early interferon therapy and abortion of posttransfusion hepatitis C viral infection. J Clin Gastroenterol. 1995;21:38–41. doi: 10.1097/00004836-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Yang SS, Ralls PW, Korula J. The effect of oral nitroglycerin on portal blood velocity as measured by ultrasonic Doppler. A double blind, placebo controlled study. J Clin Gastroenterol. 1991;13:173–177. doi: 10.1097/00004836-199104000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Gorka W, Gorka TS, Lewall DB. Doppler ultrasound evaluation of advanced portal vein pulsatility in patients with normal echocardiograms. Eur J Ultrasound. 1998;8:119–123. doi: 10.1016/s0929-8266(98)00060-3. [DOI] [PubMed] [Google Scholar]
- 9.Killi RM. Doppler sonography of the native liver. Eur J Radiol. 1999;32:21–35. doi: 10.1016/s0720-048x(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 10.Hu JT, Yang SS, Lai YC, Shih CY, Chang CW. Percentage of peak-to-peak pulsatility of portal blood flow can predict right-sided congestive heart failure. World J Gastroenterol. 2003;9:1828–1831. doi: 10.3748/wjg.v9.i8.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosoki T, Arisawa J, Marukawa T, Tokunaga K, Kuroda C, Kozuka T, Nakano S. Portal blood flow in congestive heart failure: pulsed duplex sonographic findings. Radiology. 1990;174:733–736. doi: 10.1148/radiology.174.3.2406781. [DOI] [PubMed] [Google Scholar]
- 12.Moriyasu F, Nishida O, Ban N, Nakamura T, Sakai M, Miyake T, Uchino H. "Congestion index" of the portal vein. AJR Am J Roentgenol. 1986;146:735–739. doi: 10.2214/ajr.146.4.735. [DOI] [PubMed] [Google Scholar]
- 13.Merkel C, Sacerdoti D, Bolognesi M, Bombonato G, Gatta A. Doppler sonography and hepatic vein catheterization in portal hypertension: assessment of agreement in evaluating severity and response to treatment. J Hepatol. 1998;28:622–630. doi: 10.1016/s0168-8278(98)80286-9. [DOI] [PubMed] [Google Scholar]
- 14.Moriyasu F, Nishida O, Ban N, Nakamura T, Miura K, Sakai M, Miyake T, Uchino H. Measurement of portal vascular resistance in patients with portal hypertension. Gastroenterology. 1986;90:710–717. doi: 10.1016/0016-5085(86)91127-3. [DOI] [PubMed] [Google Scholar]
- 15.Koslin DB, Mulligan SA, Berland LL. Duplex assessment of the portal venous system. Semin Ultrasound CT MR. 1992;13:22–33. [PubMed] [Google Scholar]
- 16.Bolondi L, Gaiani S, Barbara L. Accuracy and reproducibility of portal flow measurement by Doppler US. J Hepatol. 1991;13:269–273. doi: 10.1016/0168-8278(91)90067-l. [DOI] [PubMed] [Google Scholar]
- 17.Sabbá C, Weltin GG, Cicchetti DV, Ferraioli G, Taylor KJ, Nakamura T, Moriyasu F, Groszmann RJ. Observer variability in echo-Doppler measurements of portal flow in cirrhotic patients and normal volunteers. Gastroenterology. 1990;98:1603–1611. doi: 10.1016/0016-5085(90)91097-p. [DOI] [PubMed] [Google Scholar]
- 18.Moriyasu F, Ban N, Nishida O, Nakamura T, Miyake T, Uchino H, Kanematsu Y, Koizumi S. Clinical application of an ultrasonic duplex system in the quantitative measurement of portal blood flow. J Clin Ultrasound. 1986;14:579–588. doi: 10.1002/jcu.1870140802. [DOI] [PubMed] [Google Scholar]
- 19.Catalano D, Caruso G, DiFazzio S, Carpinteri G, Scalisi N, Trovato GM. Portal vein pulsatility ratio and heart failure. J Clin Ultrasound. 1998;26:27–31. doi: 10.1002/(sici)1097-0096(199801)26:1<27::aid-jcu6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.van Langen H, van Driel VJ, Skotnicki SH, Verheugt FW. Alterations in the peripheral circulation in patients with mild heart failure. Eur J Ultrasound. 2001;13:7–15. doi: 10.1016/s0929-8266(01)00115-x. [DOI] [PubMed] [Google Scholar]
- 21.Rengo C, Brevetti G, Sorrentino G, D'Amato T, Imparato M, Vitale DF, Acanfora D, Rengo F. Portal vein pulsatility ratio provides a measure of right heart function in chronic heart failure. Ultrasound Med Biol. 1998;24:327–332. doi: 10.1016/s0301-5629(97)00272-x. [DOI] [PubMed] [Google Scholar]


