TO THE EDITOR
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death in Japan, ranked 3rd in males and 5th in females. Thanks to recent progress, there are several definitive treatment modalities available for HCC, including surgery (liver resection and transplantation), ablation therapy, and transarterial chemoembolization (TACE). It is fortunate for both patients and doctors to have multiple treatment options, however, there have been very few evidence-based guidelines for decision-making[1]. Supported by the Japanese Ministry of Health, Labour and Welfare, we have compiled the “Clinical practice guidelines for hepatocellular carcinoma”[2]. This set of guidelines covers 6 fields for HCC, including prevention, diagnosis and surveillance, surgery, chemotherapy, TACE, and ablation therapy. We have surveyed 7 192 publications on HCC extracted mainly from MEDLINE (1966-2002), and selected 334 articles to form 58 pairs of research questions and recommendations. For convenience in practical use, we have also created algorithms for the surveillance and treatment of HCC.
The algorithm for the treatment of HCC was based on the evidence from the selected articles above and modified according to the current status in Japan, where liver resection for HCC is safe with less than 1% mortality, and cadaveric donors for liver transplantation are extremely difficult to obtain Figure 1. This algorithm was devised on the basis of three factors, namely, degree of liver damage[3], number of tumors, and diameter of the tumors. In patients with the severity of the liver damage categorized into class A or B: (1) If there is only one tumor, liver resection is recommended, irrespective of the diameter of the tumor. Ablation therapy may also be selected if the severity of liver damage is class B and the diameter of the tumor is not more than 2 cm; (2) From 2 to 3 tumors of no more than 3 cm in diameter, liver resection or ablation therapy is recommended; (3) From 2 to 3 tumors with a diameter of 3 cm or more, liver resection or TACE is recommended; (4) For 4 or more tumors, TACE or hepatic arterial infusion chemotherapy is recommended. For patients with class C liver damage: (1) If the tumor condition is within the so-called Milan criteria[4], liver transplantation is recommended; (2) If the number of tumors is 4 or more, palliative treatment is recommended. For patients with class A liver damage accompanied by vascular invasion, liver resection may be selected, and for patients with extra-hepatic metastasis, chemotherapy may be selected.
Figure 1.
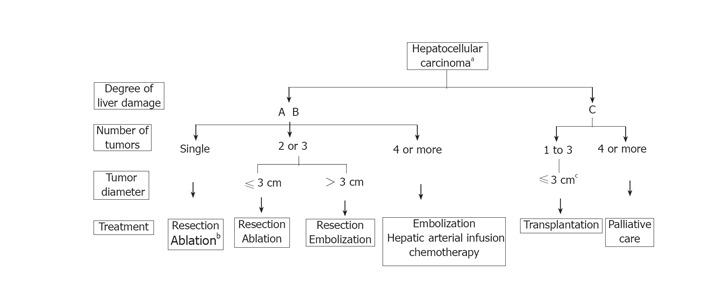
Treatment algorithm for hepatocellular carcinoma (cited from Ref.2 with permission). aPresence of vascular invasion or extrahepatic metastasis to be indicated separately. bSelected when the severity of liver damage is class B and tumor diameter is no greater than 2 cm. cTumor diameter should be no greater than 5 cm when there is only one tumor.
English translation for this guideline will be released on the website of The Japan Society of Hepatology (http://www.jsh.or.jp/) soon.
Co-investigators for “Clinical practice guidelines for hepatocellular carcinoma” (in alphabetical order): Shigeki Arii, MD, Shunji Futagawa, MD, Yuji Itai, MD, Shuichi Kaneko, MD, Seiji Kawasaki, MD, Ken-ichi Kobayashi, MD, Hiroshi Matsuyama, PhD, Masatoshi Okazaki, MD, Kiwamu Okita, MD, Masao Omata, MD, Yukihisa Saida, MD, Tadatoshi Takayama, MD, Yoshio Yamaoka, MD.
Footnotes
S- Editor Guo SY L- Editor Elsevier HK E- Editor Cao L
References
- 1.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52 Suppl 3:iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group formed to establish “Guidelines for evidence-based clinical practice for the treatment of liver cancer”. Clinical practice guidelines for hepatocellular carcinoma. Kanehara & Co., Ltd., Tokyo. 2005. p. (in Japanese). [Google Scholar]
- 3.Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. Second English edition. Kanehara & Co., Ltd., Tokyo. 2003. [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]


