Abstract
AIM: To characterize H+ and HCO3- transporters in polarized CFPAC-1 human pancreatic duct cells, which were derived from a cystic fibrosis patient with the ΔF508 CFTR mutation.
METHODS: CFPAC-1 cells were seeded at high density onto permeable supports and grown to confluence. The cells were loaded with the pH-sensitive fluorescent dye BCECF, and mounted into a perfusion chamber, which allowed the simultaneous perfusion of the basolateral and apical membranes. Transmembrane base flux was calculated from the changes in intracellular pH and the buffering capacity of the cells.
RESULTS: Our results showed differential permeability to HCO3-/CO2 at the apical and basolateral membranes of CFPAC-1 cells. Na+/HCO3- co-transporters (NBCs) and Cl-/HCO3- exchangers (AEs) were present on the basolateral membrane, and Na+/H+ exchangers (NHEs) on both the apical and basolateral membranes of the cells. Basolateral HCO3- uptake was sensitive to variations of extracellular K+ concentration, the membrane permeable carbonic anhydrase (CA) inhibitors acetazolamide (100 µmol/L) and ethoxyzolamide (100 µmol/L), and was partially inhibited by H2-DIDS (600 µmol/L). The membrane-impermeable CA inhibitor 1-N-(4-sulfamoylphenylethyl)-2,4,6-trimethylpyridine perchlorate did not have any effect on HCO3- uptake. The basolateral AE had a much higher activity than that in the apical membrane, whereas there was no such difference with the NHE under resting conditions. Also, 10 µmol/L forskolin did not significantly influence Cl-/HCO3- exchange on the apical and basolateral membranes. The administration of 250 µmol/L H2-DIDS significantly inhibited the basolateral AE. Amiloride (300 µmol/L) completely inhibited NHEs on both membranes of the cells. RT-PCR revealed the expression of pNBC1, AE2, and NHE1 mRNA.
CONCLUSION: These data suggest that apart from the lack of CFTR and apical Cl-/HCO3- exchanger activity, CFPAC-1 cells express similar H+ and HCO3- transporters to those observed in native animal tissue.
Keywords: CFPAC-1, Pancreatic duct cells, HCO3-, Intracellular pH
INTRODUCTION
There are two HCO3- transport processes in the pancreatic ductal epithelium. The first is the secretion, mainly by the smaller ducts, of HCO3- rich isotonic fluid that serves to flush digestive enzymes down the ductal tree and to neutralize gastric acid entering the duodenum[1,2]. The second HCO3- transport process is the flow-dependent exchange of luminal HCO3- for blood Cl- that is generally considered to occur in the larger ducts, including the main duct[1,2]. The physiological function of ductal HCO3- absorption (or salvage) may be protective as it will lower luminal HCO3- (and therefore luminal pH) during interdigestive periods when the flow is low or absent. A fall in luminal pH would reduce the activity of digestive enzymes within the static column of fluid in the ductal tree, thereby preventing damage to the ductal epithelium.
The initial step of HCO3- secretion is the accumulation of HCO3- within the duct cell[1,2]. This can occur by two mechanisms: (1) the forward transport of HCO3- by the Na+/HCO3- co-transporter (NBC); and (2) diffusion of CO2 into the duct cell which is then hydrated to carbonic acid by carbonic anhydrase (CA), followed by the backward transport of protons via the Na+/H+ exchangers and H+-pumps. The HCO3- ions are then secreted across the apical membrane via Cl-/HCO3- exchangers (SLC26A3, DRA and SLC26A6, PAT1)[3] and/or cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channels, which exhibit a finite permeability to HCO3-[4,5]. The exact mechanism how the SLC26 exchangers and apical Cl- channels produce a high HCO3- secretion is controversial[1,2]. Nevertheless, the key role of CFTR in HCO3- secretion is emphasized by the fact that the severity of the pancreatic phenotype in cystic fibrosis correlates best with the ability of mutant CFTRs to activate SLC26 exchangers, rather than their ability to conduct Cl- ions[6].
Rather less is known about the secondary, flow- and Cl--dependent absorption of HCO3- that occurs in the larger ducts. A luminal Na+/H+ exchanger (NHE3) and an electroneutral NBC (SLC4A7, NBCn1, NBC3) have been identified in the main duct of the mouse pancreas and probably explain HCO3- absorption across the apical membrane[7-9]. There is a clear evidence that activity of these luminal HCO3- salvage transporters is downregulated following stimulation of HCO3- secretion by a rise in intracellular cyclic AMP[7-9]. How the absorbed HCO3- exits the duct cell at the basolateral membrane is uncertain, but a candidate pathway is the basolateral Cl-/HCO3- exchanger (possibly AE2)[10]. The AE2 must be switched off during stimulation as the prevailing ion gradients would favor Cl- uptake and HCO3- efflux across the basolateral membrane.
Our current understanding of pancreatic ductal HCO3- secretion/salvage is mainly based on the experiments performed in animal tissues[1]. Studies in human tissue have been largely confined to adenocarcinoma duct cell lines; however, with the exception of CAPAN-1, human duct cell lines do not express CFTR to any significant degree[11]. An alternative approach is to use CFPAC-1 cells, which were derived from a 26-year-old cystic fibrosis (CF) patient with the ∆F508 mutation[12]. The utility of CFPAC-1 cells is that they allow us to examine HCO3- transport not only in the diseased state, but also in the corrected state by transfecting the cells with wild-type CFTR. A number of previous studies have shown that wild-type CFPAC-1 cells express some of the key transporters involved in HCO3- secretion; e.g. the basolateral NBC[13], anion exchanger-2 and the apical Cl-/HCO3- exchangers DRA and PAT-1[14]. Moreover, the expression of apical Cl-/HCO3- exchangers was significantly increased in CFPAC-1 cells expressing functional CFTR, as was the stimulatory effect of ATP on luminal Cl-/HCO3- exchange[15]. CFPAC-1 cells can also be reconstituted as polarized monolayers on permeable supports and the effects of various agents, such as angiotensin II[16,17] and PKC[18], have been studied using the short circuit current technique. Finally, in one study a stimulatory effect of ATP on HCO3- secretion from CFPAC cells was demonstrated by measuring the extracellular pH at the apical cell surface[19].
A number of issues regarding the mechanism of pancreatic ductal HCO3- secretion remain controversial. For instance, how much of the secreted HCO3- enters the lumen via CFTR and how much via SLC26 anion exchangers How is coordinate regulation of apical and basolateral acid base transporters achieved during secretion and salvage How do luminal signals regulate apical HCO3- and H+ transporters What is the molecular explanation for the strong outward rectification of HCO3- permeability exhibited by the apical membrane of the duct cell A human pancreatic duct cell line, which reconstituted as a polarized monolayer allowing easy access to the luminal membrane, would be a very useful resource for answering these questions. An added bonus would be a model system that facilitates investigation of how CFTR expression affects duct cell transporters. Therefore, in this study, we aimed to establish whether polarized CFPAC-1 cells grown on permeable supports could fulfill these requirements. To do this, we investigated the HCO3- permeability characteristics of the basolateral and apical membranes of CFPAC-1 cells, and the spatial distribution of acid/base transporters.
MATERIALS AND METHODS
Materials and solutions
All laboratory chemicals were purchased from Sigma (Poole, Dorset, UK). 2’,7’-Bis-(2-carboxyethyl)-5(6)-carboxyfluorescein-acetoxymethyl ester (BCECF-AM) and dihydro-4,4’-diisothiocyanostilbene-2,2’-disulfonic acid (H2-DIDS) were from Molecular Probes Inc. (Eugene, OR, USA); 1-N-(4-sulfamoylphenylethyl)-2,4,6-trimethylpyridine perchlorate (STP) was kindly provided by Prof. C Supuran. Stock solutions of BCECF (2 mmol/L), acetazolamide (0.4 mol/L), ethoxyzolamide (0.4 mol/L), amiloride (0.8 mol/L) and STP (10 mmol/L) were prepared in dimethyl sulfoxide (DMSO). Nigericin (10 mmol/L) and forskolin (50 mmol/L) were dissolved in ethanol and stored at -20 °C. Polyester Transwells were supplied by Corning-Costar (Buckinghamshire, UK). The standard HEPES-buffered solution contained 130 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 10 mmol/L Na-HEPES, and 10 mmol/L D-glucose (pH 7.4 with HCl). In the Na+-free HEPES solution, NaCl was replaced by 140 mmol/L N-methyl-D-glucamine (NMDG)-Cl and the Na-HEPES was replaced by equimolar HEPES-acid. The Cl--free HEPES solution contained 140 mmol/L Na-gluconate, 2.5 mmol/L K2SO4, 6 mmol/L Ca-gluconate, 1 mmol/L Mg-gluconate, 10 mmol/L HEPES-acid, 10 mmol/L D-glucose (pH 7.4 with NaOH). The Na+/Cl--free HEPES solution contained 130 mmol/L NMDG-gluconate, 2.5 mmol/L K2SO4, 1 mmol/L MgSO4, 1 mmol/L CaSO4, 10 mmol/L HEPES-acid, 10 mmol/L D-glucose (pH 7.4 with gluconate). The standard HCO3- solution contained 115 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 25 mmol/L Na-HCO3, 10 mmol/L D-glucose. In the K+-free HCO3- solution, KCl was replaced with equimolar NaCl. The high K+ HCO3- solution contained the same ingredients as the standard HCO3- solution but the KCl and NaCl concentrations were 115 and 5 mmol/L, respectively. The Na+-free HCO3- solution contained NMDG-Cl instead of NaCl and choline HCO3- in place of NaHCO3. Cl--free HCO3- solution contained 115 mmol/L sodium gluconate, 2.5 mmol/L K2SO4, 6 mmol/L Ca-gluconate, 1 mmol/L Mg-gluconate, 25 mmol/L Na-HCO3, 10 mmol/L D-glucose. The Na+/Cl--free HCO3- solution contained 115 mmol/L NMDG-gluconate, 2.5 mmol/L K2SO4, 1 mmol/L MgSO4, 1 mmol/L CaSO4, 25 mmol/L choline-HCO3-, 10 mmol/L D-glucose (pH 7.4 with gluconate). 10 µmol/L atropine was added to the solutions containing choline to prevent the possible activation of muscarinic receptors. In solutions containing NH4+, the concentration of Na+ or NMDG was replaced to maintain osmolarity. All the solutions containing HCO3- were continuously equilibrated with 5% CO2-95% O2 to maintain pH at 7.4.
Culture of CFPAC-1 cells
CFPAC-1 cells (passage number 27-60) were obtained from Prof. RA Frizzell (University of Pittsburgh, Pittsburgh, PA, USA) and were grown in Iscove’s modified Dulbecco’s medium supplemented with 100 mL/L fetal calf serum, 2 mmol/L glutamine, 100 units/mL penicillin and 0.1 mg/mL streptomycin and were cultured as described previously[12]. The media was changed every 1-2 d. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. Cell monolayers were prepared by seeding at high density (300-350 cells/cm2) onto polyester permeable supports (12 mm diameter, 0.4 µm pore size Transwells). Cell confluence was checked by microscopy and determination of transepithelial electrical resistance using an EVOM-G voltohmmeter (World Precision Instruments, Sarasota, FL, USA). Experiments were performed 4-6 d after seeding.
Measurement of intracellular pH
Intracellular pHi was estimated with the pH-sensitive dye BCECF[20,21]. CFPAC-1 cells were loaded with 2 µmol/L BCECF-AM in both the apical and basal chambers in standard HEPES for 30-45 min at 37 °C. After loading, the Transwells were transferred to a perfusion chamber mounted on an inverted Nikon Diaphot microscope (Nikon UK, Kingston upon Thames, UK). Apical and basal bath volumes were 0.5 and 1 mL and the perfusion rates were 3 and 6 mL/min, respectively. All the experiments started and ended with standard HEPES solution perfusing both sides of the cells. Experiments were performed at 37 °C. pHi was measured using a Life Sciences Microfluorimeter System (Life Sciences Resources, Cambridge, UK). About 10-15 cells were alternately excited with wavelengths of 440 and 490 nm, and the 490/440 fluorescence emission ratio was recorded at 535 nm over a sampling period of 256 ms. The resting pHi of CFPAC-1 cells in standard HEPES solution was determined using the method described by Hegyi et al.[21]. Calibration of 490/440 ratio to pHi was obtained using the high-K+/nigericin method. Extracellular pH was stepped between 5.94 and 8.46.
Determination of buffering capacity
The intrinsic buffering capacity (βi) of CFPAC-1 cells was estimated according to the NH4+ prepulse technique[22]. Briefly, the cells were exposed to varying concentrations of NH4Cl (0-30 mmol/L) while Na+ and HCO3- were omitted from the bath solution to block the Na+- and HCO3--dependent pH regulatory mechanisms. βi was estimated by the Henderson-Hasselbalch equation. The total buffering capacity (βtotal) was calculated as βtotal=βi+βHCO3-, where βHCO3- is the buffering capacity of the HCO3-/CO2 system. βHCO3-=2.3×[HCO3-]i. [HCO3-]i is the intracellular concentration of HCO3-.
Overall ∆pHi, base flux
The overall ∆pHi was measured by determining the pHi immediately before and at the peak level during the administration of solutions containing HCO3-/CO2 by averaging the values of 80 data points. The initial rates of ∆pHi (over 30 s) were used to calculate transmembrane base flux J(B) using the equation J(B)=∆pHi/∆t*βi. The βi value used in the calculation of J(B)s was obtained from Figure 1 by using the average pHi value over a 30-s period immediately before the administration of HCO3-/CO2 (virtually no HCO3- in the cells). Therefore, we were most likely underestimating J(B) because of the increase in buffering capacity as HCO3- fluxes into the cell during the administration of the basolateral HCO3-/CO2 solution. The only time we used βtotal to calculate J(B) was when we administered basolateral standard HCO3-/CO2 solution during perfusion with apical standard HCO3-/CO2. We denote base influx as J(B) and base efflux as -J(B).
Figure 1.
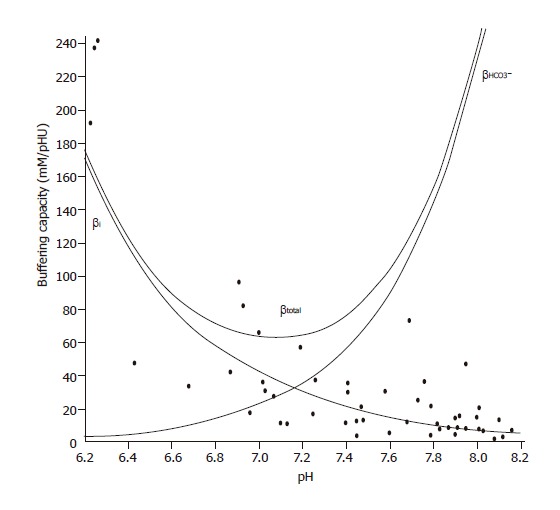
Buffering capacity of CFPAC-1 human pancreatic duct cells at different pHi values. Polarized CFPAC-1 cells were exposed to varying concentrations of NH4Cl (0–30 mmol/L), while Na+ and HCO3– were omitted from the bath solution to block the Na+ and HCO3–-dependent pH regulatory mechanisms. βi was estimated by the Henderson–Hasselbalch equation (n=50). The total buffering capacity (βtotal) was calculated as βtotal=βi+βHCO3–, where βHCO3– is the buffering capacity of the HCO3–/CO2 system. βHCO3–=2.3×[HCO3–]i. [HCO3–]i is the intracellular concentration of HCO3–.
RT-PCR
Total RNA was extracted from CFPAC-1 cells grown in tissue culture flasks using RNAzol solution (Gibco-BRL) according to the manufacturer’s instructions. For reverse transcriptase (RT)-PCR, 2 µg of RNA was reverse transcribed with 50 ng of random hexamer primers (GIBCO-BRL) in a final volume of 20 µL using 50 U of Moloney murine leukemia virus RT (Fermentas) at 37 °C for 1 h, following the manufacturer's instructions. The primers used for PCR amplification were previously described[23-25]. NHE1: 5’-CCAGCTCATTGCCTTCTACC-3’ (sense) and 5’-TGTGTCTGTTGTAGGACCGC-3’ (antisense) (length of amplified region 245 residues); AE2: 5’-GAAGATTCCTGAGAATGCCG-3’ (sense) and 5’-GTCCATGTTGGCACTACTCG-3’ (antisense) (length of amplified region 181 residues); pancreatic NBC1 (pNBC): 5’-ATGTGTGTGATGAAGAAGAAGTAGAAG-3’ (sense) and 5’-GACCGAAGGTTGGATTTCTTG-3’ (antisense) (length of amplified region 621 residues); glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5’-ATGGCACCGTCAAGGCTGAGA-3’ (sense) and 5’-GGCATGGACTGTGGTCATGAG-3’ (antisense) (length of amplified region 371 residues). The PCR reaction was started with a 3-min 94 °C step and was followed by standard step-cycling conditions with 30 cycles of amplification utilizing Taq DNA polymerase (Fermentas); the reaction was ended by a 10-min 72°C step. The cycling conditions for NHE1, AE2, and GAPDH were 94 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s; and for pNBC1 were 94 °C for 30 s, 58 °C for 30 s and 68 °C for 3 min. RT-PCR products were separated by electrophoresis on a 20 g/L agarose gel containing ethidium bromide (0.5 µg/mL) and were visualized under ultraviolet light.
Statistical analyses
In agreement with Zsembery et al[26], we noticed a variation in the rate and magnitude of HCO3- uptake between the different set of monolayers that we could not attribute to the passage number or time after seeding. To avoid errors arising from this fact, we (1) performed a particular experimental protocol on the same day using one set of cell cultures, (2) randomized the order in which monolayers in the same group were exposed to experimental maneuvers (i.e., exposure to inhibitors), and (3) always included internal controls where possible. Summary data in the figures are expressed as percent change from control and statistical analyses were performed using either Student’s paired or unpaired t test or the analysis of variance as appropriate. P values <0.05 were considered statistically significant.
RESULTS
Transepithelial electrical resistance, resting pHi and buffering capacity
The CFPAC-1 cells grown on polyester Transwells became confluent in 2-3 d as judged by visual observation. The net transepithelial resistance increased steadily over 4-5 d after seeding when it reached a plateau of 100±4 Ω/cm2 (n = 88). The resting pHi of the cells in standard HEPES solution was 7.11±0.08 (n = 6).
Apical administration of HCO3-/CO2
After perfusing both the apical and basolateral sides of the CFPAC-1 cells with standard HEPES solution, apical perfusion was switched to the standard HCO3-/CO2 solution (Figure 2). We then observed a rapid acidification (-17.5 ± 1.5 mmol/L B/min, n = 20) of pHi, most likely caused by the diffusion of CO2 into the cells. Following this, the pHi reached a new approximate steady state value (6.82 ± 0.02, n = 20) and remained at this level until the standard HEPES solution was restored to the apical membrane. Restoration of standard HEPES solution induced a rapid alkalinization of pHi towards the resting pHi value probably brought about by the diffusion of CO2 from the cells. These observations suggested that CO2 influx was very fast across the apical membrane of CFPAC-1 cells, and that significant HCO3- influx did not occur.
Figure 2.
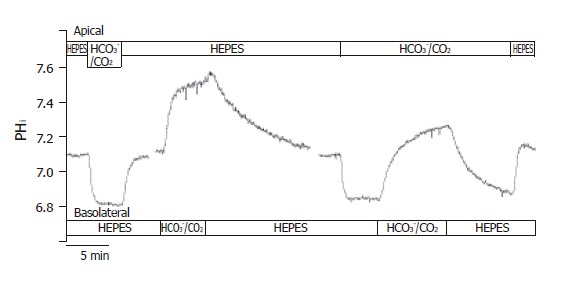
Functional polarities of CFPAC-1 cells. CFPAC-1 cells were grown to confluence on permeable supports, loaded with the pH-sensitive fluorescent dye BCECF-AM (2 µmol/L) and mounted into a perfusion chamber, which allowed the simultaneous perfusion of different solutions to the basolateral and apical membranes. The figure shows representative pHi traces demonstrating cell polarity. The administration of standard HCO3–/CO2 solution to the apical and the basolateral sides of the cells showed a marked difference in response (n = 12–20).
Basolateral administration of HCO3-/CO2
After perfusion of both the apical and basolateral membranes of CFPAC-1 cells with standard HEPES solution, the basolateral perfusion was switched to standard HCO3-/CO2 (Figure 2). Upon changing the basolateral perfusate, there was a rapid alkalinization of pHi (∆pHi = 0.48 ± 0.03, J(B) = 15.0±2.3 mmol/L B/min, n = 12), most likely induced by the transport of HCO3- into the cells. Removal of the standard HCO3-/CO2 solution from the basolateral side induced a further small transient increase in pHi consistent with efflux of CO2, followed by a slow acidification towards the resting pHi value brought about by the extrusion of HCO3- (Figure 2). These observations suggested that HCO3- might cross the basolateral membrane of CFPAC-1 cells much faster than CO2. To rule out the possibility that basolateral HCO3- entry was at least partly due to CO2 influx, followed by its rapid hydration to HCO3- ions, we perfused the apical side of CFPAC-1 cells with a standard HCO3-/CO2 solution for 4 min (intracellular pCO2 was increased) and then the same solution was added to the basolateral side (Figure 2). Under these conditions we still observed a rapid alkalinization of pHi, (∆pHi = 0.40 ± 0.05, J(B) = 14.1 ± 1.9 mmol/L B/min, n = 6) which was not significantly different compared to the absence of apical HCO3-/CO2.
Taken together with the effects of apical HCO3-/CO2 administration, these data showed that CFPAC-1 monolayers exhibited functional polarity with respect to HCO3- transport. Subsequently, we used the apical administration of HCO3-/CO2 to check the functional polarity of monolayers at the beginning of each experiment. The cells were considered to be polarized, if the pHi remained constant after the initial decrease in pHi caused by the diffusion of CO2 into the cells.
Investigation of basolateral HCO3- uptake
In theory, the alkalinization observed after the administration of basolateral standard HCO3-/CO2 could be explained by the action of the NBC and the reversal of Cl-/HCO3- anion exchanger. The initial rate of HCO3- uptake was significantly decreased in Cl--free vs standard conditions (J(B)=73.1±7.2% and 100±8.5%, respectively, n =5, Figures 3A and 4); however, no significant difference was noted for the overall ∆pHi for the two groups (85.5±6.0% and 100±6.3%, respectively). The administration of basolateral HCO3-/CO2 greatly decreased ∆pHi (25.6±5.1%) and J(B) (11.7±2.3%) in Na+-free conditions (n = 6) compared to those (100±7.5% and, 100±10.7%, respectively) in standard conditions, suggesting that a large proportion of the HCO3- uptake was due to a Na+-sensitive process, most likely via an NBC (Figures 3B and 4). Interestingly, the decrease in the rate of HCO3- uptake was somewhat attenuated in the absence of both Na+ and Cl- [n = 6, J(B) changed to 38.4±6.9%, Figures 3C and 4].
Figure 3.
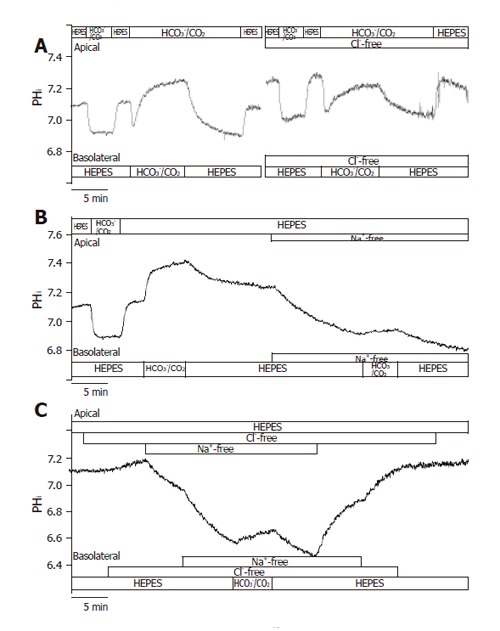
Basolateral HCO3– uptake – effects of Cl– and/or Na+ withdrawal. The figure shows representative pHi traces. A: The rate of HCO3– uptake was significantly decreased in Cl-free conditions compared to standard conditions (J(B) = 73.1 ± 7.2% and 100 ± 8.5%, respectively, n = 5); B: The administration of basolateral HCO3–/CO2 in Na+-free conditions (n = 6) greatly decreased ∆pHi and J(B) compared to standard conditions, suggesting that a large proportion of the HCO3– uptake was due to a Na+-sensitive process, most likely NBC; C: Interestingly, the decrease in the rate of HCO3– uptake was obviously ameliorated in the absence of both Na+ and Cl– (n = 6) compared to the Na+-free condition.
Figure 4.
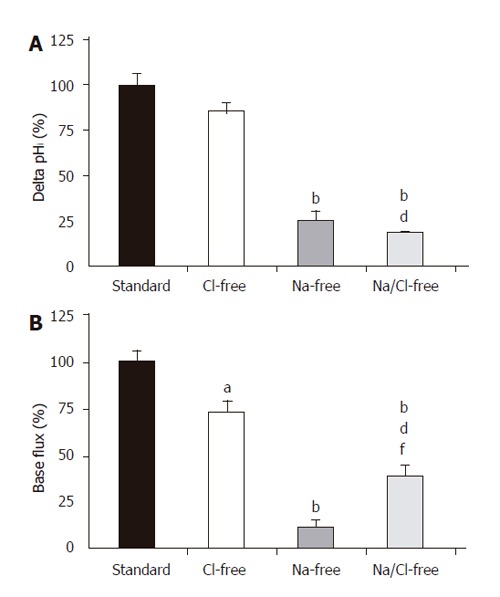
Basolateral HCO3– uptake – changes in (A) ∆pHi and (B) J(B) caused by the withdrawal of Cl– and/or Na+. SE of the control groups (standard, black bar) is somewhat different for each of the respective ion-withdrawal groups. The SEs depicted on the standard bars are the maximum values observed. aP<0.05 and bP<0.01 vs control group dP<0.01 vs Cl–-free group and/or fP<0.01 vs Na+-free group.
To further confirm the presence of NBC on the basolateral membrane of CFPAC-1 cells, we examined the recovery from a Na+-free acid load in the presence of HCO3−/CO2 by adding basolateral Na+ with/without 300 µmol/L amiloride (to block basolateral Na+/H+ exchange, n = 6). After acid loading of cells, the basolateral addition of Na+ stimulated a J(B) of 39.0±4.4 mmol/L B/min (please see below the much lower values in HEPES solution). In contrast, the basolateral addition of Na+ in the presence of amiloride decreased this value to 22.2±3.6 mmol/L B/min, suggesting that a third pathway of acid transport is due to a basolateral NHE. The anion transport inhibitor H2-DIDS (600 µmol/L, added to the basolateral membrane of cells for 2 min before and during the administration of basolateral HCO3-/CO2, n = 6), significantly decreased the extent of alkalinization (∆pHi) to 63.2±4.7% and rates of J(B) to 43.9±5.1% (n = 6, Figures 5A and 6). Varying the extracellular K+ concentrations (K+-free, high K+, n = 9-11) of the basolateral standard HCO3-/CO2 solution induced significant differences between the ∆pHi values vs the standard HCO3-/CO2 solution (41.5±24.5%, 139.6±15.0% and 100 ± 11.1%, respectively; Figures 5B and 6). The J(B) significantly decreased when K+-free HCO3-/CO2 (73.9±6.4%) was administered to the basolateral membrane, but was not affected by high K+, HCO3-/CO2 (96.6 ±11.0%) vs the standard HCO3-/CO2 solution (100.0 ±15.8%). Thus, our experiments showed that alteration of the membrane potential of CFPAC-1 cells, by varying basolateral extracellular K+ concentration, modified the extent of HCO3- uptake. The application of the membrane permeable CA inhibitors acetazolamide (100 µmol/L, n = 6, Figures 5C and 6) or ethoxyzolamide (100 µmol/L, n = 6) in the apical (standard HCO3-/CO2 solution) and basolateral (standard HEPES solution) perfusions, 10 min before and during the administration of basolateral standard HCO3-/CO2 solution, significantly decreased the overall ∆pHi to 56.8±4.2% or 59.0±9.8%, and J(B) to 50.5±4.6% or 63.7±8.3%, respectively. The basolateral addition of the membrane impermeable CA inhibitor STP (10 µmol/L, n = 6) in standard HEPES solution (the apical side of the cells was perfused with standard HCO3-/CO2 solution), 5 min before and during the administration of basolateral standard HCO3-/CO2 solution, did not significantly influence overall ∆pHi (114.3±14.3%) and J(B) (106.7±17.9%, Figure 6).
Figure 5.
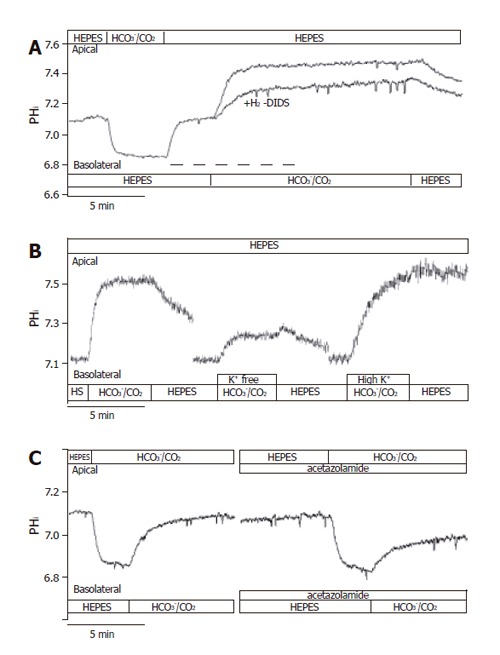
Basolateral HCO3– uptake – effects of H2-DIDS, acetazolamide and varying extracellular K+ concentration. The figure shows representative pHi traces. A: The anion transport inhibitor 600 µmol/L H2-DIDS (added to the basolateral membrane of cells for 2 min before and during the administration of basolateral HCO3–/CO2, dashed line, n=6) significantly decreased the extent of alkalinization (∆pHi) and rate of J(B) (n=6); B: Varying extracellular [K+] caused significant differences in ∆pHi and J(B) (n=9–11); C: The application of the membrane-permeable carbonic anhydrase inhibitor acetazolamide (100 µmol/L, n = 6) significantly decreased the overall ∆pHi and J(B). HS: HEPES.
Figure 6.
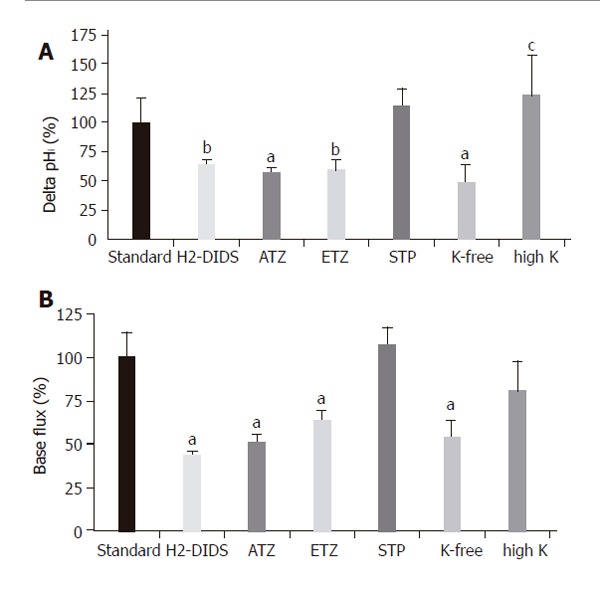
Basolateral HCO3– uptake – changes in (A) ∆pHi (B) J(B) caused by application of H2-DIDS, acetazolamide, ethoxyzolamide, STP and varying extracellular K+ concentration. Data are shown as mean ± SE (n = 6–11). SE of the control groups (standard, black bar) is somewhat different for each of the respective ion-withdrawal groups. The SE depicted on the control bar was the maximum values observed. aP<0.05 and bP<0.01 vs control group or cP<0.05 vs K+-free group. ATZ: acetazolamide; ETZ: ethoxyzolamide; STP: 1-N-(4-sulfamoylphenylethyl)-2,4,6-trimethylpyridine perchlorate. The SE depicted on the standard bars was the maximum values observed.
Localization of Cl-/HCO3- exchangers in CFPAC-1 cells
The localization of Cl-/HCO3- exchangers was performed by measuring the effect of changes in Cl- gradient across the luminal and/or basolateral membranes on pHi. In the presence of HCO3-, the removal of Cl- from the apical membrane did not result in changes of pHi. The removal of Cl- from the basolateral membrane caused a ∆pHi of 0.08±0.01 and a J(B) of 4.4±0.5 mmol/L B/min (n = 6, Figure 7). Furthermore, the basolateral administration of 250 µmol/L H2-DIDS (from 2 min before changing the solution to Cl--free HCO3-/CO2) completely blocked pHi changes caused by the withdrawal of Cl-. However, 10 µmol/L forskolin, 100 µmol/L acetazolamide or 100 µmol/L ethoxyzolamide (from 10 min before changing the solution to Cl--free HCO3-/CO2) did not significantly influence Cl-/HCO3- exchange on the apical and basolateral membranes (n = 6-8, data not shown).
Figure 7.
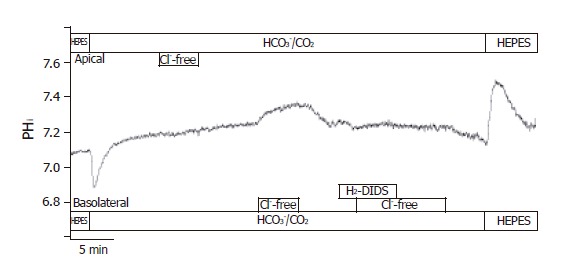
Localization of Cl–/HCO3– exchangers in CFPAC-1 cells. The figure shows representative pHi traces (n = 6). The localization of Cl–/HCO3– exchangers was performed by measuring the effect of changes in Cl– gradient across the luminal or basolateral membranes on pHi. In the presence of HCO3–, the removal of Cl– from the apical membrane did not result in changes of pHi. The removal of Cl– from the basolateral membrane caused a ∆pHi of 0.08 ± 0.01 and a J(B) of 4.37 ± 0.46 mmol/L B/min. Furthermore, the basolateral administration of 250 µmol/L H2-DIDS completely blocked pHi changes caused by the withdrawal of Cl–.
Localization of Na+/H+ exchangers in CFPAC-1 cells
In HEPES-buffered solutions, the removal of Na+ from the perfusing solutions had a small acidifying effect on pHi [J(B)=1.00±0.13 mmol/L B/min, n =12]. After exposure and then removal of the NH4+ in the absence of Na+ on both sides of the duct cells, pHi reduced to 6.73±0.03 (n = 12), and stabilized at this level (Figure 8A). We could not detect any active H+-pumps in CFPAC-1 cells, since pHi did not increase in the absence of extracellular Na+. Re-addition of Na+ to the apical or the basolateral side caused pHi recovery towards the resting values [J(B) = -6.6±2.6 mmol/L B/min and -7.5±1.3 mmol/L B/min, respectively, n = 6-10]. The administration of 300 µmol/L amiloride to the Na+-free (from 2 min before the addition of Na+) and Na+-containing HEPES solutions prevented the recovery of pHi in the presence of Na+ (n =6, Figure 8B). Removing amiloride resulted in the recovery of pHi to resting values. Interestingly, the removal of apical Na+ from the standard HCO3-/CO2 solution perfusing CFPAC-1 cells caused a sudden but small acidification of pHi (∆pHi = 0.14±0.01 and J(B) =-10.8±0.8 mmol/L B/min, n =6), whereas we did not see any change in pHi when we removed Na+ from the basolateral side of the cells (n =6). Taken together, our experiments showed that there are Na+/H+ exchangers on both the apical and the basolateral membranes. Finding a Na+/H+ exchanger on the apical side was somewhat unexpected. A possible explanation for this would be that the cells were leaky to Na+ ions. However, despite the great difference in Na+ concentration (0 mmol/L vs 140 mmol/L) of the apical and basolateral perfusates, analysis of the Na+ concentration of the solutions by mass spectrometry showed no contamination during perfusion.
Figure 8.
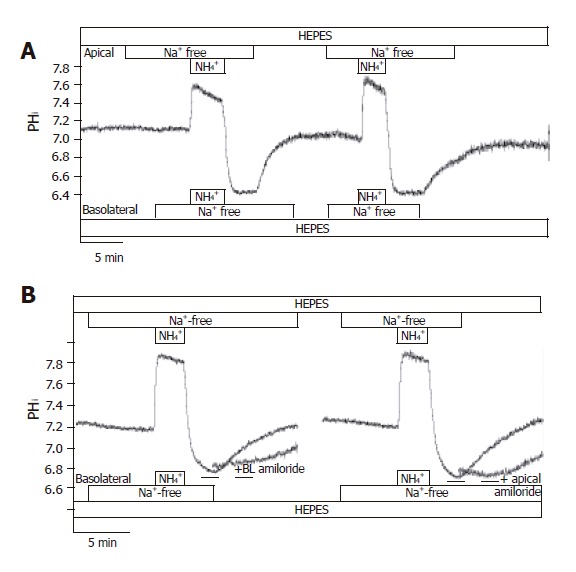
Localization of Na+/H+ exchangers in CFPAC-1 cells. The figure shows representative pHi traces. A: After exposure to NH4+ in the absence of Na+ on both sides of the duct cells, pHi reduced to 6.73 ± 0.03 (n = 12), and stabilized at this level. We could not detect any active H+-pumps in CFPAC-1 cells, since pHi did not increase in the absence of extracellular Na+. Upon addition of Na+ to the apical or the basolateral side prompted the pHi to recover towards the resting values (n = 6-10); B: The administration of 300 µmol/L amiloride (dashed line) to the Na+-free (from 2 min before the addition of Na+) and Na+-containing HEPES solutions prevented the recovery of pHi in the presence of Na+ (n = 6). Removal of amiloride resulted in the recovery of pHi to resting values.
Molecular identification of H+ and HCO3- transporters
To further investigate the presence of different H+ and HCO3- transporters in CFPAC-1 cells, we undertook RT-PCR analysis. We could detect mRNA expressions for pNBC1, AE2, and NHE1; the housekeeping gene GAPDH was used to normalize mRNA levels (Figure 9).
Figure 9.
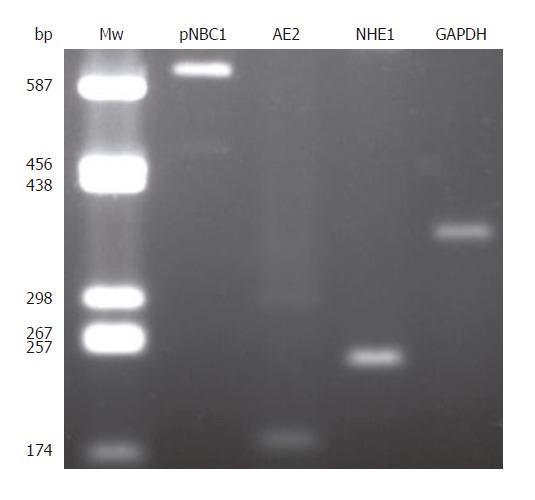
Expression of pNBC1, AE2, NHE1, and GAPDH mRNA in CFPAC-1 cells. Mw: molecular weight ladder (bp indicates number of base pairs).
DISCUSSION
Our results showed the functional presence of the NBC and Cl-/HCO3- exchanger on the basolateral membrane, together with Na+/H+ exchangers on both the apical and basolateral membranes of CFPAC-1 cells (Figure 10). That there was no detectable Cl-/HCO3- exchange activity on the apical membrane was not a surprise as CFTR is known to be required for its activation[3,27]. RT-PCR revealed the expression of pNBC1, AE2, and NHE1 mRNA.
Figure 10.
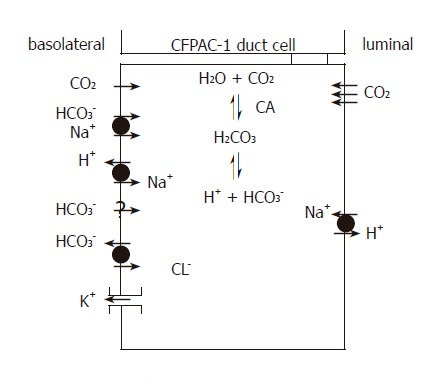
H+ and HCO3– transport mechanisms in CFPAC-1 human pancreatic duct cells. The basolateral membrane expresses Na+/HCO3– co-transporters, Na+/H+ exchangers and Cl–/HCO3– exchangers plus an unidentified Na+-independent HCO3– entry pathway. These transporters facilitate the rapid flux of HCO3– ions into the cell. K+ channels are also likely to be expressed on the basolateral membrane because altering extracellular K+ concentration affects HCO3– influx. The apical membrane expresses Na+/H+ exchangers, but not CFTR and Cl–/HCO3– exchangers found in normal pancreatic duct cells. The apical membrane is an effective barrier to HCO3– influx from the lumen, but is freely permeable to CO2. CA: Carbonic anhydrase.
CFPAC-1 cells clearly demonstrated a differential permeability to HCO3- at the apical and basolateral membranes. We found that the addition of 25 mmol/L HCO3-/CO2 to the basolateral side of the cells caused a marked alkalinization of pHi, whereas exposing the apical membrane to the same solution caused a sustained acidification. These data suggest that the basolateral membrane of CFPAC-1 cells is much more permeable to HCO3- than the apical membrane. Plasma membrane HCO3- and CO2 permeabilities have been reported to vary in different species and tissues. In perfused rabbit gastric glands, the luminal membranes of parietal and chief cells were impermeable to HCO3- and CO2, while the basolateral membranes were permeable to both[28]. Apical HCO3- permeability is relatively low in cultured bovine corneal endothelial cells[29]. In contrast, HCO3- permeability of the apical side of intact colonic mucosa of guinea pig is markedly higher[30]. Our results are in accordance with similar observations made by Ishiguro et al.[31] using guinea pig ducts. Administration of 25 mmol/L HCO3-/CO2 to the lumen of microperfused guinea pig pancreatic ducts decreased pHi rapidly. Following this, no subsequent increase in pHi was observed, indicating that little HCO3- was able to enter the cell from the lumen. HCO3- entry was detected only when the luminal HCO3- concentration was raised above 125 mmol/L. A low apical HCO3- permeability would make physiological sense in species, such as man and guinea pig, which can secrete near isotonic NaHCO3-. Our work is the first demonstration of HCO3-/CO2 asymmetry in polarized human pancreatic duct cells. Finally, it seems that CFPAC-1 cells, which express ∆F508 CFTR, exhibit the same resistance to HCO3- influx across the apical membrane as native guinea pig duct cells. This suggests that the presence of functional CFTR in the apical membrane is not required for this membrane to act as an effective barrier to HCO3- influx from the lumen.
A large proportion of the basolateral HCO3- uptake was Na+-dependent and was partially inhibited by H2-DIDS, therefore, it is most likely due to the action of pNBC1. However, we also detected a novel Na+-independent HCO3- influx mechanism that was significantly increased in Cl--free conditions, the identity of which remains unknown. NBCs have been detected in the guinea pig[32,33], rat[34,35] and mouse[36]. In contrast, Novak et al.[37,38] could not detect any HCO3- uptake by NBC in freshly prepared rat pancreatic ducts using electrophysiological and pHi measurements. Extracellular HCO3-/CO2 did not increase the rate of pHi recovery in experimental acidosis. The expressions of NBC mRNA and protein have been noted in the human pancreas[39]. Immunofluorescence localized the transporter to the basolateral but not the apical membrane of the pancreatic duct cells. Interestingly, immunohistological studies have shown the NBC in both the apical and basolateral membranes of rat pancreatic duct cells[35]. NBC1 mRNA was also detected in CFPAC-1 cells by Northern hybridization[13]. In accordance with our findings, expression of NBC1 was also found in non-polarized cells by recovery from acidosis in the presence of HCO3-, Na+ and amiloride and its sensitivity to the administration of 200 µmol/L DIDS[13]. cAMP had no effect on NBC activity in CFPAC-1 cells, whereas NBC activity was stimulated in CAPAN-1 cells expressing wild-type CFTR[13]. The basolateral localization of the NBC was shown by 22Na+ flux studies in CFPAC-1 cells stably transfected with wild-type CFTR grown on permeable supports (no data was reported for untransfected CFPAC-1 cells)[13].
The application of the membrane permeable CA inhibitor acetazolamide or ethoxyzolamide partially inhibited the rate of basolateral base flux in CFPAC-1 cells, whereas the membrane-impermeable CA inhibitor STP[40] had no such effect. Carbonic anhydrases are known to be coupled to HCO3- transporters, such as Cl-/HCO3- anion exchangers[41-44], DRA[45] and NBC[46]. There are at least 14 different isoenzymes in the α-CA gene family present in vertebrates[47]. CA I-III and VII are cytosolic, while CA IV, IX, XII and XIII are membrane-associated, V is mitochondrial and VI is secreted. Of the 14 different isoforms, CA II, IV, IX and XII were shown to be expressed in the human pancreas and cultured pancreatic tumor cells[48]. Interestingly, CA II and IV expressions were found to be associated with apical HCO3- channels in human pancreatic duct cells CAPAN-1[49], although the targeting of CA IV to the apical plasma membrane was impaired in the CFPAC-1 cells[50]. Kivela et al.[51] have clearly detected the transmembrane CA isoenzymes IX and XII on the basolateral membrane of the normal and pathological pancreas by immunohistochemistry. However, it is unlikely that the latter isoenzymes are associated with NBC as STP had no inhibitory effect on HCO3- uptake. Furthermore, in accordance with our results, Cheng et al.[52] have shown in CAPAN-1 cells that HCO3--dependent forskolin-induced Isc could be significantly reduced by acetazolamide. Moreover, the CA inhibitor also had an inhibitory effect on dome formation, which was thought to be due to exchange of water and electrolytes[49]. Taken together, intracellular CAs may be involved in the rapid basolateral transport of HCO3- by NBC. The enzyme converts HCO3- to carbonic acid and possibly maintains a concentration gradient for HCO3- across the basolateral membrane of CFPAC-1 cells.
Alteration of extracellular K+ concentration significantly influenced HCO3- uptake in our experiments. The presence of K+ channels in the basolateral membranes of CFPAC-1 cells is highly likely, and one report has identified ATP-sensitive K+ channels[53] in these cells. A basolateral K+ conductance will influence the rate of HCO3- secretion by altering the cells membrane potential. In our experiments, high extracellular K+ concentrations increased HCO3- entry, which was likely due to basolateral membrane depolarization, and a consequent increase in the electrochemical driving force for HCO3- entry on the electrogenic NBC. In contrast, low extracellular K+ concentration inhibited HCO3- uptake, probably due to membrane hyperpolarization.
Novak and Greger[37] demonstrated that the basolateral membrane of rat intra- and inter-lobular pancreatic ducts had a relatively large K+ conductance, which could be decreased by the K+ channel blocker Ba2+. The luminal membrane had no significant K+ conductance. Interestingly, Fong et al.[54] detected K+ conductances not only on the basolateral but also on the apical sides of HPAF human pancreatic duct cells.
CFPAC-1 cells exhibited Na+/H+ exchange activity on both the apical and basolateral membranes (the latter is most likely to be due to NHE1). Na+/H+ exchangers were identified in guinea pig[10,28-30], rat[55,56] and mouse[8] pancreatic ducts. These exchangers were localized to the basolateral membrane of intra/interlobular duct cells. However, the main and common segments of the rat pancreatic ducts also showed Na+/H+ exchange activity in the apical membrane[10]. There is also evidence for an apical Na+/H+ exchanger in bovine main pancreatic duct segments[57]. Furthermore, the main pancreatic duct of mice expressed NHE1 in the basolateral membrane, and NHE2 and NHE3 in the luminal membrane[8]. Disruption of the NHE2 gene had no effect on luminal H+ transport, while disruption of the NHE3 gene reduced luminal Na+-dependent H+ efflux by 45%. The remaining 55% of the luminal Na+-dependent H+ efflux from NHE3-knockout mice was inhibited by 50 µmol/L HOE694. Na+-dependent H+ transport mechanisms were inhibited by cAMP, therefore, they are thought to be inhibited during stimulated secretion and are most likely to be active during the resting state[8]. CFTR in the pancreatic duct not only affected the activity, but also the expression of NHE3 protein[7]. NHE3 expression was reduced by about 50%, luminal Na+-dependent and HOE694-sensitive recovery from acid load was reduced by 60% in the ducts of ∆508 CFTR mice[7]. To our knowledge, we are the first to detect NHEs on both the apical and basolateral membranes of human pancreatic duct cells. The presence of NHE on the apical membrane was unexpected, since it acted against HCO3- secretion. We do not know which section of the pancreatic duct CFPAC-1 cells originate from, but they might be from the main region where the expression of the apical NHEs was noted in animal studies. In accordance with our results, failure of pHi to recover from acid loading in the absence of extracellular Na+ suggests that there is little H+-pump activity in the unstimulated guinea pig ducts[32]. This is in contrast with the observations made in rat[10] and pig[58] pancreatic ducts, which showed H+-pump activity.
The role of apical Cl-/HCO3- exchangers in pancreatic ductal HCO3- secretion is a controversial issue. One hypothesis is that all of the HCO3- is secreted on an anion exchanger and that CFTR’s role is to activate the exchangers and to provide a route for the recycling of Cl- across the apical membrane[59]. An alternative proposal is that HCO3- secretion occurs on the exchanger until the luminal concentration reaches about 70 mmol/L after which HCO3- exits the duct cell via CFTR[60]. Previously, it has been shown that CFPAC-1 cells express AE2 on their basolateral membrane (which was also confirmed in our study) and SLC26A3 (DRA) and SLC26A6 (PAT1) on their apical membrane, and that expression of the apical SLC26 exchangers is upregulated by CFTR[14]. In accordance with these data, we observed basolateral Cl-/HCO3- exchange activity (which was inhibited by H2-DIDS) in wild-type CFPAC-1 cells, but no apical activity, presumably due to the lack of CFTR. We also found that forskolin had no effect on either the basolateral or apical anion exchange in CFPAC-1 cells.
In conclusion, apart from the lack of CFTR and apical Cl-/HCO3- exchange activity, CFPAC-1 cells have similar H+ and HCO3- transporters to those observed in native animal tissue. Moreover, intracellular CAs appear to play a role in the basolateral uptake of HCO3-. Taken together, polarized CFPAC-1 human pancreatic duct cells are likely to be a useful model to study H+ and HCO3- transporters, and their dependence on CFTR, in human pancreatic duct cells.
ACKNOWLEDGMENTS
We thank Professor RA Frizzell for kindly providing us with the CFPAC-1 cells, and Professor C Supuran for providing the 1-N-(4-sulfamoylphenylethyl)-2,4,6-trimethylpyridine perchlorate.
Footnotes
Supported by a Wellcome Trust Travelling Fellowship to Z.R., No. 069470, and a Wellcome Trust IRDA Grant to P.H., No. 068096
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Liu WF
References
- 1.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 2.Argent BE, Gray MA, Steward MC. Case RM Cell physiology of pancreatic ducts. Johnson LR eds Physiology of the Gastrointestinal Tract, 4th edn. Elsevier, San Diego. 2005 (in press) [Google Scholar]
- 3.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, et al. A molecular mechanism for aberrant CFTR-dependent HCO(3)(-)transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990;259:C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- 5.Linsdell P, Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3- salvage mechanisms in model systems and the mouse pancreatic duct. J Biol Chem. 2001;276:17236–17243. doi: 10.1074/jbc.M011763200. [DOI] [PubMed] [Google Scholar]
- 8.Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S. Na(+)-dependent transporters mediate HCO(3) (-) salvage across the luminal membrane of the main pancreatic duct. J Clin Invest. 2000;105:1651–1658. doi: 10.1172/JCI9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, et al. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3- salvage transporter human Na+-HCO3- cotransport isoform 3. J Biol Chem. 2002;277:50503–50509. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3- transporters in the rat pancreatic duct. J Gen Physiol. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers JA, Harris A. Expression of the cystic fibrosis gene and the major pancreatic mucin gene, MUC1, in human ductal epithelial cells. J Cell Sci. 1993;105(Pt 2):417–422. doi: 10.1242/jcs.105.2.417. [DOI] [PubMed] [Google Scholar]
- 12.Schoumacher RA, Ram J, Iannuzzi MC, Bradbury NA, Wallace RW, Hon CT, Kelly DR, Schmid SM, Gelder FB, Rado TA. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl Acad Sci U S A. 1990;87:4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shumaker H, Amlal H, Frizzell R, Ulrich CD, Soleimani M. CFTR drives Na+-nHCO3- cotransport in pancreatic duct cells: a basis for defective HCO3- secretion in CF. Am J Physiol. 1999;276:C16–C25. doi: 10.1152/ajpcell.1999.276.1.C16. [DOI] [PubMed] [Google Scholar]
- 14.Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1301–G1308. doi: 10.1152/ajpgi.2001.281.5.G1301. [DOI] [PubMed] [Google Scholar]
- 15.Namkung W, Lee JA, Ahn W, Han W, Kwon SW, Ahn DS, Kim KH, Lee MG. Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl- -dependent HCO3 transport in pancreatic duct cells. J Biol Chem. 2003;278:200–207. doi: 10.1074/jbc.M207199200. [DOI] [PubMed] [Google Scholar]
- 16.Chan HC, Law SH, Leung PS, Fu LX, Wong PY. Angiotensin II receptor type I-regulated anion secretion in cystic fibrosis pancreatic duct cells. J Membr Biol. 1997;156:241–249. doi: 10.1007/s002329900204. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HS, So SC, Law SH, Chan HC. Angiotensin II-mediated signal transduction events in cystic fibrosis pancreatic duct cells. Biochim Biophys Acta. 1999;1449:254–260. doi: 10.1016/s0167-4889(99)00017-8. [DOI] [PubMed] [Google Scholar]
- 18.Cheng HS, Wong WS, Chan KT, Wang XF, Wang ZD, Chan HC. Modulation of Ca2+-dependent anion secretion by protein kinase C in normal and cystic fibrosis pancreatic duct cells. Biochim Biophys Acta. 1999;1418:31–38. doi: 10.1016/s0005-2736(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 19.Cheung CY, Wang XF, Chan HC. Stimulation of HCO3- secretion across cystic fibrosis pancreatic duct cells by extracellular ATP. Biol Signals Recept. 1998;7:321–327. doi: 10.1159/000014555. [DOI] [PubMed] [Google Scholar]
- 20.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 21.Hegyi P, Rakonczay Z Jr, Gray MA, Argent BE. Measurement of intracellular pH in pancreatic duct cells: a new method for calibrating the fluorescence data. Pancreas. 2004;28:427–434. doi: 10.1097/00006676-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub WH, Machen TE. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989;257:G317–G327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- 23.Inglis SK, Finlay L, Ramminger SJ, Richard K, Ward MR, Wilson SM, Olver RE. Regulation of intracellular pH in Calu-3 human airway cells. J Physiol. 2002;538:527–539. doi: 10.1113/jphysiol.2001.012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudeja PK, Hafez N, Tyagi S, Gailey CA, Toofanfard M, Alrefai WA, Nazir TM, Ramaswamy K, Al-Bazzaz FJ. Expression of the Na+/H+ and Cl-/HCO-3 exchanger isoforms in proximal and distal human airways. Am J Physiol. 1999;276:L971–L978. doi: 10.1152/ajplung.1999.276.6.L971. [DOI] [PubMed] [Google Scholar]
- 25.Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal RY, Gardner C, Risk MC, Schmidt L, Bavishi D, Kim KE, et al. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am J Physiol. 1996;271:G483–G493. doi: 10.1152/ajpgi.1996.271.3.G483. [DOI] [PubMed] [Google Scholar]
- 26.Zsembery A, Strazzabosco M, Graf J. Ca2+-activated Cl- channels can substitute for CFTR in stimulation of pancreatic duct bicarbonate secretion. FASEB J. 2000;14:2345–2356. doi: 10.1096/fj.99-0509com. [DOI] [PubMed] [Google Scholar]
- 27.Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl-/HCO3- exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 28.Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature. 1994;368:332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- 29.Bonanno JA, Guan Y, Jelamskii S, Kang XJ. Apical and basolateral CO2-HCO3- permeability in cultured bovine corneal endothelial cells. Am J Physiol. 1999;277:C545–C553. doi: 10.1152/ajpcell.1999.277.3.C545. [DOI] [PubMed] [Google Scholar]
- 30.Böllert P, Peters T, von Engelhardt W, Gros G. Mass spectrometric determination of HCO3- permeability and carbonic anhydrase activity in intact guinea-pig colon epithelium. J Physiol. 1997;502(Pt 3):679–691. doi: 10.1111/j.1469-7793.1997.679bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 2000;528 Pt 2:305–315. doi: 10.1111/j.1469-7793.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3- by Na(+)-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Ondarza J, Hootman SR. Confocal microscopic analysis of intracellular pH regulation in isolated guinea pig pancreatic ducts. Am J Physiol. 1997;272:G124–G134. doi: 10.1152/ajpgi.1997.272.1.G124. [DOI] [PubMed] [Google Scholar]
- 34.Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso-O N, Inatomi J, Kawakami H, et al. Localization of Na+-HCO-3 cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729–C737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- 35.Thévenod F, Roussa E, Schmitt BM, Romero MF. Cloning and immunolocalization of a rat pancreatic Na(+) bicarbonate cotransporter. Biochem Biophys Res Commun. 1999;264:291–298. doi: 10.1006/bbrc.1999.1484. [DOI] [PubMed] [Google Scholar]
- 36.Gross E, Abuladze N, Pushkin A, Kurtz I, Cotton CU. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO(3)(-): 1 Na(+) J Physiol. 2001;531:375–382. doi: 10.1111/j.1469-7793.2001.0375i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflugers Arch. 1988;411:58–68. doi: 10.1007/BF00581647. [DOI] [PubMed] [Google Scholar]
- 38.Novak I, Christoffersen BC. Secretin stimulates HCO(3)- and acetate efflux but not Na+/HCO(3)- uptake in rat pancreatic ducts. Pflugers Arch. 2001;441:761–771. doi: 10.1007/s004240000472. [DOI] [PubMed] [Google Scholar]
- 39.Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na(+)-HCO (-)(3) cotransporter in human pancreas. Am J Physiol. 1999;277:G487–G494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- 40.Scozzafava A, Briganti F, Ilies MA, Supuran CT. Carbonic anhydrase inhibitors: synthesis of membrane-impermeant low molecular weight sulfonamides possessing in vivo selectivity for the membrane-bound versus cytosolic isozymes. J Med Chem. 2000;43:292–300. doi: 10.1021/jm990479+. [DOI] [PubMed] [Google Scholar]
- 41.Dahl NK, Jiang L, Chernova MN, Stuart-Tilley AK, Shmukler BE, Alper SL. Deficient HCO3- transport in an AE1 mutant with normal Cl- transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. J Biol Chem. 2003;278:44949–44958. doi: 10.1074/jbc.M308660200. [DOI] [PubMed] [Google Scholar]
- 42.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 43.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV. J Biol Chem. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- 44.Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J Biol Chem. 1998;273:28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 45.Sterling D, Brown NJ, Supuran CT, Casey JR. The functional and physical relationship between the DRA bicarbonate transporter and carbonic anhydrase II. Am J Physiol Cell Physiol. 2002;283:C1522–C1529. doi: 10.1152/ajpcell.00115.2002. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry. 2003;42:12321–12329. doi: 10.1021/bi0353124. [DOI] [PubMed] [Google Scholar]
- 47.Hewett-Emmett D. Evolution and distribution of the carbonic anhydrase gene families. EXS. 2000:29–76. doi: 10.1007/978-3-0348-8446-4_3. [DOI] [PubMed] [Google Scholar]
- 48.Nishimori I, FujikawaAdachi K, Onishi S, Hollingsworth MA. Carbonic anhydrase in human pancreas: hypotheses for the pathophysiological roles of CA isozymes. Ann N Y Acad Sci. 1999;880:5–16. doi: 10.1111/j.1749-6632.1999.tb09505.x. [DOI] [PubMed] [Google Scholar]
- 49.Mahieu I, Becq F, Wolfensberger T, Gola M, Carter N, Hollande E. The expression of carbonic anhydrases II and IV in the human pancreatic cancer cell line (Capan 1) is associated with bicarbonate ion channels. Biol Cell. 1994;81:131–141. doi: 10.1016/s0248-4900(94)80004-9. [DOI] [PubMed] [Google Scholar]
- 50.Fanjul M, Salvador C, Alvarez L, Cantet S, Hollande E. Targeting of carbonic anhydrase IV to plasma membranes is altered in cultured human pancreatic duct cells expressing a mutated (deltaF508) CFTR. Eur J Cell Biol. 2002;81:437–447. doi: 10.1078/0171-9335-00264. [DOI] [PubMed] [Google Scholar]
- 51.Kivelä AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastoreková S, Pastorek J, Waheed A, Sly WS, et al. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol. 2000;114:197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]
- 52.Cheng HS, Leung PY, Cheng Chew SB, Leung PS, Lam SY, Wong WS, Wang ZD, Chan HC. Concurrent and independent HCO3- and Cl- secretion in a human pancreatic duct cell line (CAPAN-1) J Membr Biol. 1998;164:155–167. doi: 10.1007/s002329900401. [DOI] [PubMed] [Google Scholar]
- 53.Roch B, Baró I, Hongre AS, Escande D. ATP-sensitive K+ channels regulated by intracellular Ca2+ and phosphorylation in normal (T84) and cystic fibrosis (CFPAC-1) epithelial cells. Pflugers Arch. 1995;429:355–363. doi: 10.1007/BF00374150. [DOI] [PubMed] [Google Scholar]
- 54.Fong P, Argent BE, Guggino WB, Gray MA. Characterization of vectorial chloride transport pathways in the human pancreatic duct adenocarcinoma cell line HPAF. Am J Physiol Cell Physiol. 2003;285:C433–C445. doi: 10.1152/ajpcell.00509.2002. [DOI] [PubMed] [Google Scholar]
- 55.Stuenkel EL, Machen TE, Williams JA. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988;254:G925–G930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- 56.Novak I, Hug M, Greger R. Intracellular pH in rat pancreatic ducts. Comp Biochem Physiol A Physiol. 1997;118:409–411. doi: 10.1016/s0300-9629(96)00331-3. [DOI] [PubMed] [Google Scholar]
- 57.Marteau C, Silviani V, Ducroc R, Crotte C, Gerolami A. Evidence for apical Na+ /H+ exchanger in bovine main pancreatic duct. Dig Dis Sci. 1995;40:2336–2340. doi: 10.1007/BF02063234. [DOI] [PubMed] [Google Scholar]
- 58.Villanger O, Veel T, Raeder MG. Secretin causes H+/HCO3- secretion from pig pancreatic ductules by vacuolar-type H(+)-adenosine triphosphatase. Gastroenterology. 1995;108:850–859. doi: 10.1016/0016-5085(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 59.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishiguro H, Steward MC, Sohma Y, Kubota T, Kitagawa M, Kondo T, Case RM, Hayakawa T, Naruse S. Membrane potential and bicarbonate secretion in isolated interlobular ducts from guinea-pig pancreas. J Gen Physiol. 2002;120:617–628. doi: 10.1085/jgp.20028631. [DOI] [PMC free article] [PubMed] [Google Scholar]


