Abstract
AIM: Chronic organ-donor shortage has led to the acceptance of steatotic livers for transplantation, despite the higher risk of graft dysfunction or nonfunction associated with the ischemic preservation period of these organs. The present study evaluates the effects of trimetazidine (TMZ) on an isolated perfused liver model.
METHODS: Steatotic and non-steatotic livers were preserved for 24 h in the University of Wisconsin (UW) solution with or without TMZ. Hepatic injury and function (transaminases, bile production and sulfobromophthalein (BSP) clearance) and factors potentially involved in the susceptibility of steatotic livers to ischemia–reperfusion (I/R) injury, including oxidative stress, mitochondrial damage, microcirculatory diseases, and ATP depletion were evaluated.
RESULTS: Steatotic livers preserved in UW solution showed higher transaminase levels, lower bile production and BSP clearance compared with non-steatotic livers. Alterations in perfusion flow rate and vascular resistance, mitochondrial damage, and reduced ATP content were more evident in steatotic livers. TMZ addition to UW solution reduced hepatic injury and ameliorated hepatic functionality in both types of the liver and protected against the mechanisms potentially responsible for the poor tolerance of steatotic livers to I/R.
CONCLUSION: TMZ may constitute a useful approach in fatty liver surgery, limiting the inherent risk of steatotic liver failure following transplantation.
Keywords: Steatotic liver, Ischemia–reperfusion, UW preservation solution
INTRODUCTION
The mounting number of patients awaiting liver transplantation and the limited pool of donor organs have led to the acceptance of marginal livers such as steatotic livers, for transplantation, despite the higher risk of graft dysfunction or nonfunction which is associated with their ischemic preservation[1-3].
There is evidence indicating that the composition of preservation solutions is critical for the quality of livers kept for prolonged ischemic periods. University of Wisconsin (UW) preservation solution, considered as the gold standard of such solutions, has proved itself effective in preventing liver damage during cold ischemia and has extended storage time limits[4,5]. However, irreversible injury occurring after prolonged cold periods (between 16 and 24 h) has also been reported. The main aims of organ preservation, therefore, are striving to prolong organ tolerance[6,7].
Trimetazidine (TMZ), introduced as an anti-ischemic drug into the heart for over 35 years[8,9], has also been used to protect kidneys exposed to the prolonged cold ischemia (48 h)[10,11] and is reported to protect liver against the deleterious effects of warm ischemia[12,13]. In addition, recently, it has been demonstrated that TMZ pre-treatment reduces liver injury and improves liver regeneration and survival rate in an experimental model of partial hepatectomy under hepatic blood inflow occlusion[14]. Studies examining the underlying protective mechanisms of TMZ suggest that this drug protects mitochondria in cardiomyocytes and isolated perfused heart by releasing the calcium accumulated in the matrix and by restoring mitochondrial membrane impermeability[8,15]. TMZ improves energy recovery in vitro and ex vivo in models of myocardial ischemia[8,15] and reduces oxidative stress in the liver under warm ischemia[12]. Additionally, TMZ improves microcirculatory alterations in isolated perfused rat kidneys[10]. Taken together, these findings suggest that mitochondria, energy metabolism, oxidative stress, and microcirculation might be important targets through which TMZ exerts its cytoprotective effect. Interestingly, severe mitochondrial damage[16,17], decreased ATP level[16,18], increased reactive oxygen species (ROS) production[19,20], and impaired microcirculation[21,22] have also been proposed as factors that might leave steatotic livers vulnerable to ischemia–reperfusion (I/R) injury. Taking these previous observations into account, here we report the results of a study aimed at investigating whether the addition of TMZ to the standard preservation solution (UW) protects steatotic liver grafts against the deleterious effects of I/R injury.
MATERIALS AND METHODS
Homozygous (obese, Ob) and heterozygous (lean, Ln) Zucker rats (used as reference group), aged 16-18 wk, were purchased from Iffa-Credo (L’Arbresle, France)[19,20]. In this study we used isolated perfused rat liver. It is a useful experimental system for evaluating hepatic function, isolated from the influence of other organ systems, undefined plasma constituents, and neural-hormonal effects. Hepatic architectures, microcirculation, and bile production were preserved in this experimental model[23,24]. All procedures were performed under isofluorane inhalation anesthesia. This study respected the European Union regulations (Directive 86/609 EEC) for animal experiments.
Liver procurement and experimental groups
The surgical technique was performed as described elsewhere[25]. After cannulation of the common bile duct, the portal vein was isolated, and the splenic and gastroduodenal veins were ligated. The steatotic and non-steatotic livers were flushed and preserved in cold UW solution for 24 h with or without the addition of TMZ (10-6 mol/L). This dose of TMZ has been shown to be beneficial in various experimental models of I/R[10,26,27]. At higher concentration, TMZ exerted no protective effect[28]. TMZ was kindly supplied by the Institut de Recherches Internationales Servier (Courbevoie, France).
Protocol 1: Effect of TMZ on steatotic liver injury after cold ischemia
After 24 h of cold storage, livers from 16 Zucker rats (8 Ln and 8 Ob) preserved in UW solution (UW) and livers from 16 Zucker rats (8 Ln and 8 Ob) preserved in UW solution with TMZ (UW+TMZ) were flushed with Ringer’s lactate solution. The aliquots of the effluent flush were sampled for the measurements of cumulative ALT and AST after prolonged ischemia.
Protocol 2: Effect of TMZ on steatotic liver injury after normothermic reperfusion
After 24 h of cold preservation, and in order to account for the period of rewarming during surgical implantation in vivo[29,30], livers from 16 Zucker rats (8 Ln and 8 Ob) preserved in UW solution and livers from 16 Zucker rats (8 Ln and 8 Ob) preserved in UW+TMZ solution were exposed at 22 °C for 30 min prior to reperfusion. Livers were then connected via the portal vein to a recirculating perfusion system for 120 min at 37 °C[6,25]. Time 0 was the point at which the portal catheter was satisfactorily connected to the circuit. As previously reported[25,31], during the first 15 min of perfusion (initial equilibration period), the flow was progressively increased in order to stabilize the portal pressure at 12 mmHg (Pressure Monitor BP-1, Instruments, Inc., Sarasota, FL, USA). The flow was controlled using a peristaltic pump (Minipuls 3, Gilson, France)[6,25]. The reperfusion liquid consisted of a cell culture medium (William’s medium E, BioWhittaker, Spain) with a Krebs-Henseleit-like electrolyte composition enriched with 5% albumin, as oncotic supply. The buffer was continuously ventilated with 95% O2 and 50 mL/L CO2 gas mixture. The buffer was subsequently passed through a heat-exchanger (37 °C) and a bubble trap prior to entering the liver[25,30]. During 120 min of normothermic reperfusion, the effluent fluid was collected at 30 min intervals to measure transaminases. After the initial equilibration period of 15 min, flow rate and vascular resistance were assessed continuously throughout the reperfusion period. Bile output and hepatic clearance (expressed as percentage of BSP in bile samples) were determined at 120 min of reperfusion. ATP, adenine nucleotides, and lipid peroxidation were evaluated in the liver samples at 120 min of reperfusion. Light/electron microscopy analysis of the liver was also performed at 120 min of reperfusion.
Biochemical determinations
Transaminase assay Hepatic injury was evaluated according to transaminase levels using commercial kit from Boehringer Mannheim (Munich, Germany).
Nucleotide analysis Livers were homogenized in perchloric acid solution, and the adenine nucleotides pool was measured by high-performance liquid chromatography[32].
Bile output Liver function was assessed by measuring bile production[33,34]. Bile was collected through the cannulated bile duct and output reported as µL/g liver.
Hepatic clearance As with bile output, hepatic clearance was considered as another parameter of hepatic function[35,36]. Thirty minutes after the onset of the perfusion (t30), 1 mg of BSP (Sigma, Spain) was added to the perfusate. The concentration of BSP in bile samples (t120) was measured at 580 nm with an UV-visible spectrometer. Bile BSP excretion was expressed as a percentage of perfusate content (t120 bile/t30 perfusate×100)[35,36].
Flow rate and vascular resistance Liver circulation was assessed by measuring perfusion flow rate and vascular resistance[34,37]. Perfusion flow rate was assessed continuously throughout the reperfusion period and expressed as mL/min·g. Vascular resistance was defined as the ratio of portal venous pressure to flow rate and expressed in mmHg·min·g/mL[34,37].
Lipid peroxidation assay Lipid peroxidation was used as an indirect measure of the oxidative injury induced by ROS[19,38]. Lipid peroxidation was determined by measuring the formation of malondialdehyde (MDA) with the thiobarbiturate reaction[19].
Light/electron microscopy
For light microscopy examinations, liver samples were fixed in 10% neutral buffered formalin and embedded in Paraplast, and 5-µm sections were stained with hematoxylin and eosin according to standard procedures[19]. For electron microscopy, the fixation of hepatic tissue was performed using a 2.5% glutaraldehyde/2% paraformaldehyde. These samples were post-fixed with osmium tetroxide and potassium ferrocyanide, dehydrated in acetone, and embedded in Spurr’s medium. Ultrathin sections were prepared with an ultracut-E ultramicrotome and contrasted with uranyl acetate and lead citrate. Stained sections were reviewed under an H 600-AB Hitachi electron microscope[39].
Statistics
Data were expressed as mean±SE, and were compared statistically by variance analysis, followed by Student-Newman-Keuls test. P < 0.05 was considered significant.
RESULTS
Protocol 1: Effect of TMZ on steatotic liver injury after 24 h of cold ischemia
Flushing of livers before reperfusion allowed effluent fluid to collect for the determination of transaminases[6,40]. This measure proved to be a valuable tool for predicting organ damage after cold preservation[6,40]. As shown in Figure 1, the higher levels of transaminases released by steatotic livers after cold preservation in UW solution confirm the increased sensitivity of this type of liver to cold ischemia. The addition of TMZ to UW solution (UW+TMZ) reduced transaminase levels in the flushing effluent of non-steatotic livers and, in particular in that of steatotic livers.
Figure 1.
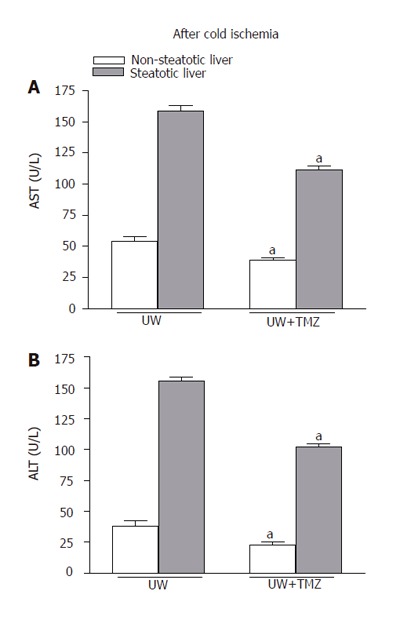
AST (A) and ALT (B) levels in flushing effluent after 24-h cold storage. UW: liver preserved in UW solution; UW+TMZ: liver preserved in UW with TMZ. aP < 0.05 vs UW.
Protocol 2: Effect of TMZ on steatotic liver injury after 24 h of cold ischemia followed by 2 h of normothermic reperfusion
Higher perfusate transaminase levels were observed in steatotic livers as the reperfusion period progressed compared with those found in non-steatotic livers (Figure 2). The addition of TMZ to UW solution reduced the perfusate transaminase release in both types of liver, but especially in steatotic livers. The histological light microscopic study showed a marked disintegration of hepatic architecture in both types of liver preserved in UW solution (Figures 3A and B), but especially in steatotic livers (Figure 3B), whereas in the UW+TMZ group hepatocyte integrity was maintained (Figures 3C and D).
Figure 2.
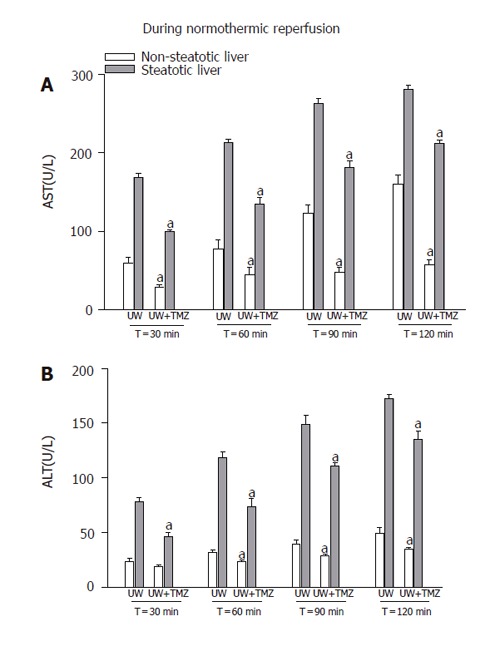
AST (A) and ALT (B) levels in perfusate after 30, 60, 90, and 120 min of normothermic reperfusion. UW: liver preserved in UW solution; UW+TMZ: liver preserved in UW with TMZ. aP < 0.05 vs UW.
Figure 3.
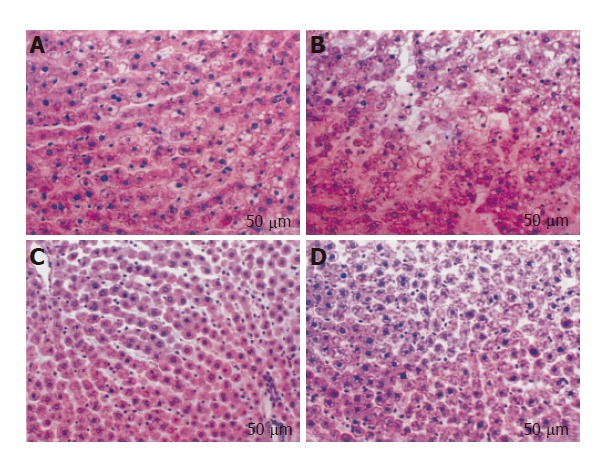
Histological change in steatotic and non-steatotic livers after 120 min of normothermic reperfusion. Non-steatotic (A) and steatotic (B) livers preserved in UW solution: Cell swelling and disintegration of hepatic architecture were more evident in steatotic livers. Non-steatotic (C) and steatotic (D) livers preserved in UW solution with TMZ: Hepatic morphology was better preserved in both types of the liver compared to that recorded in UW solution.
Liver function was assessed by measuring bile production and BSP clearance. Bile output and the percentage of BSP in bile were lower in steatotic livers preserved in UW solution than in non-steatotic livers (Figure 4). Both liver function parameters improved significantly in the two liver types following the addition of TMZ to UW solution (UW+TMZ).
Figure 4.
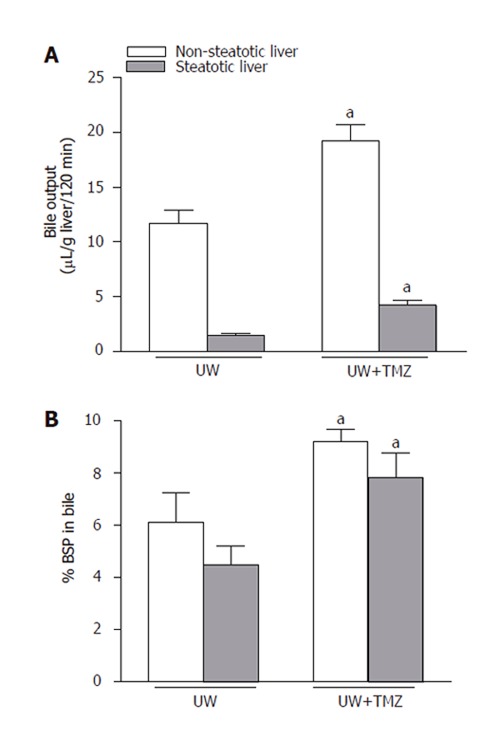
Bile output (A) and percentage of sulfobromophthalein (BSP) in bile (B) of steatotic and non-steatotic livers after 120 min of normothermic reperfusion. UW: livers were preserved in UW solution; UW+TMZ: liver preserved in UW with TMZ. aP < 0.05 vs UW.
The mechanisms by which TMZ was able to protect steatotic livers against the deleterious effects of I/R injury were also evaluated. As shown in Figure 5, MDA levels during reperfusion increased, particularly in the case of steatotic livers. The addition of TMZ to UW solution (UW+TMZ) brought about a reduction in these levels of increase. Marked increases in the vascular resistance and lower perfusion flow rate were observed in steatotic livers preserved in UW solution during the reperfusion period (t30,60,90,120) compared with the rates in non-steatotic livers (Figure 6). The liver weight was not statistically significant in both liver types. Vascular resistance was lower and perfusion flow rate was higher in both types of liver following the addition of TMZ to UW preservation solution (UW+TMZ). The beneficial effect of TMZ on the vascular resistance and perfusion flow rate were more evident in the steatotic livers (Figure 6). Lower ATP and adenine nucleotides levels during reperfusion were observed in steatotic livers preserved in UW solution than those recorded for non-steatotic livers (Figure 7). The results reported here show that TMZ led to the preservation of more ATP and adenine nucleotides content in both types of liver.
Figure 5.
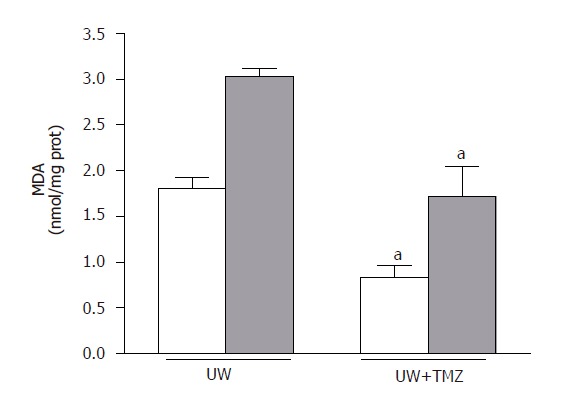
Hepatic malondialdehyde (MDA) levels after 120 min of normothermic reperfusion. UW: liver preserved in UW solution; UW+TMZ: liver preserved in UW with TMZ. aP < 0.05 vs UW.
Figure 6.
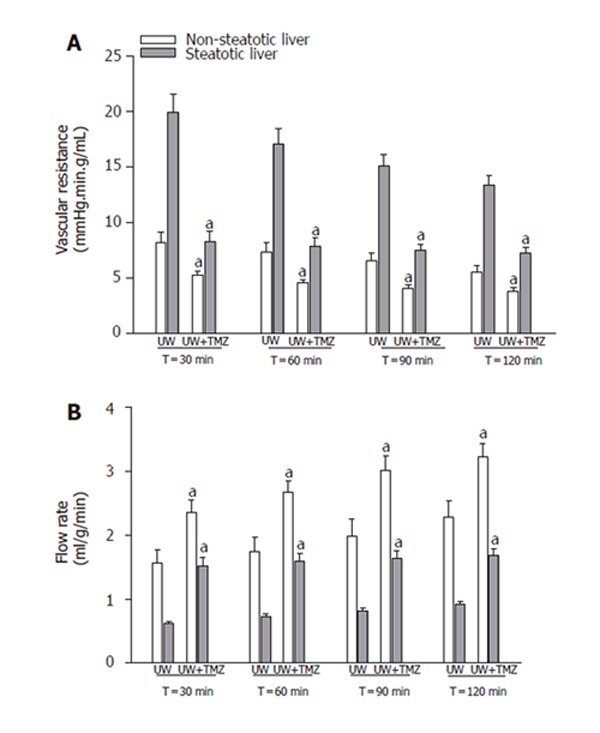
Vascular resistance (A) and perfusion flow rate (B) of steatotic and non-steatotic livers after 120 min of normothermic reperfusion. UW: livers were preserved in UW solution; UW+TMZ: liver preserved in UW with TMZ. aP < 0.05 vs UW.
Figure 7.
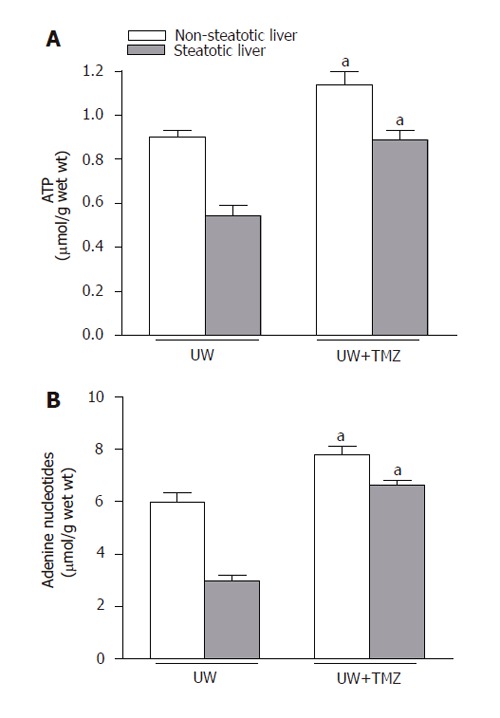
ATP (A) and adenine nucleotide (B) levels of steatotic and non-steatotic livers after 120 min of normothermic reperfusion. UW: livers were preserved in UW solution; UW+TMZ: liver preserved in UW with TMZ. aP < 0.05 vs UW.
Mitochondrial damage was evaluated by electron microscopy. Ultra-structural analysis after reperfusion in both types of liver preserved in UW solution revealed swelling of mitochondria, a significant decrease in the electron density of their matrices, while cristae were only seldom visible (Figures 8A and B). These morphological alterations were more evident in steatotic livers (Figure 8B). Following the addition of TMZ to UW preservation (UW+TMZ) a greater degree of mitochondrial preservation in both types of liver was recorded (Figures 8C and D) compared to the results obtained for the livers in UW solution.
Figure 8.
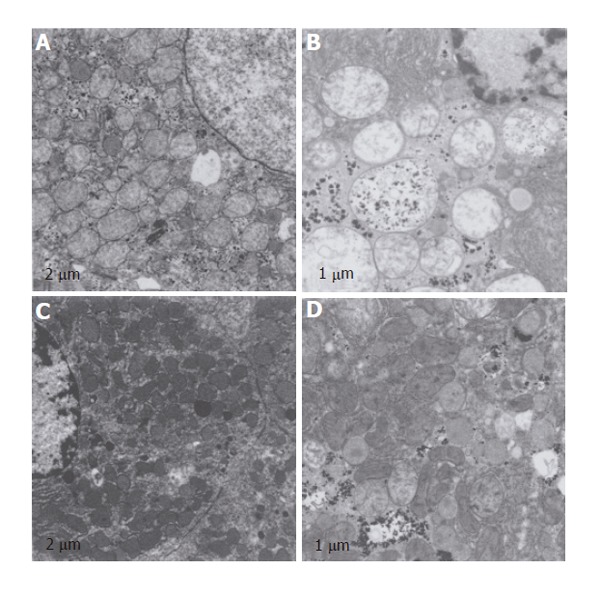
Electron microscopy analysis of structural integrity of the mitochondria. Non-steatotic (A) and steatotic (B) livers preserved in UW solution: mitochondria swelling and the alterations in the electron density of their matrices were more evident in steatotic livers. Cristae are only seldom visible in steatotic livers. Non-steatotic (C) and steatotic (D) livers preserved in UW solution with TMZ. Mitochondrial integrity was better preserved in both types of liver compared to that recorded in UW solution.
DISCUSSION
The results of the present study indicate that the addition of TMZ to UW solution improved the capacity of this standard preservation solution in both types of liver subjected to prolonged ischemic period especially in steatotic livers.
It is widely accepted that bile analysis is a useful means for assessing the integrity of biliary epithelial cells after cold ischemia. If cold storage time exceeds 10-12 h, complications in biliary structures occur in more than 25% of liver transplant recipients[41-43]. Several factors, including poor recovery after ATP depletion appear to contribute to bile duct cell damage after liver transplantation. Furthermore, isolated rat bile duct epithelial cells are noticeably sensitive to oxidative stress, possibly because their cellular stores of reduced glutathione are seven times lower than those of hepatocytes[44]. Taking these observations into account, bile production failure in steatotic livers could be explained, at least partially, by the lower ATP and increased oxidative stress presented by this type of liver compared with non-steatotic liver. Liver transplantation may benefit from strategies such as the addition of TMZ to the preservation solution as this seems to help maintain appropriate bile duct cell functions. Here, this strategy increased ATP recovery and reduced oxidative stress. This was also associated with better bile production.
The lower perfusate flow rate and the higher resistance to flow in steatotic, as opposed to non-steatotic livers indicate that steatotic livers offer greater impediments to perfusion. Fat accumulation in the cytoplasm of the hepatocytes is associated with an increase in cell volume, which may result in the partial or complete obstruction of the hepatic sinusoidal space[3,21]. Our results indicate the beneficial effects of TMZ on the flow rate and vascular resistance especially in the steatotic livers which were preserved during prolonged cold ischemia. Various hypotheses could be forwarded in an attempt at explaining the underlying protective mechanisms. In fact, according to the literature, TMZ reduces the leakage of intracellular potassium, which is a vasoconstrictor; it affects vasoactive mediators including prostaglandins; and it reduces tissue edema, which has been related to the disturbance of microvascular circulation[10,45,46].
Fatty degeneration, which induces a series of ultrastructural and biochemical alterations both in human and animal mitochondria[17,47] may render these organelles intrinsically more susceptible to I/R injury. Given that mitochondria are the main sites for ATP and ROS production in I/R[16], the lower ATP and adenine nucleotides content and the increased oxidative stress observed in steatotic livers preserved in UW solution could be attributed to mitochondrial damage. Thus, the identification of new strategies to prevent mitochondrial injury should represent an important research goal in attempts to optimize the use of donor steatotic organs for liver transplantation. Our result indicates that the addition of TMZ to UW preservation solution protects against mitochondrial damage caused by I/R, preserves more ATP and adenine nucleotide content during reperfusion and decreases oxidative stress, thus showing a protective effect against I/R injury. Taken together, these data suggest that mitochondria might be an important target through which TMZ exerts its cytoprotective effect. However, the underlying mechanisms are still far from being defined and other possibilities should not be ruled out. Thus, another possible reason for the higher ATP levels that are induced by TMZ might be improved microcirculation at the time of reperfusion. This could increase the availability of oxygen and, therefore, facilitate ATP production. It is well known that failure of liver perfusion can impair the ability of the steatotic liver to restore ATP levels[16,22,48]. Similarly, in addition to mitochondria, the beneficial effects of TMZ on oxidative stress could be exerted on other sources of ROS, including endothelial cells[10].
The addition of TMZ to UW solution ensured that steatotic grafts were less susceptible to the mechanisms involved in I/R injury including microcirculatory diseases, ATP and adenine content, oxidative stress and mitochondrial damage. In addition to allopurinol and glutathione, TMZ could be another antioxidant that should be added to UW solution, considering the benefits of TMZ in terms of oxidative stress and the difficulties for preventing oxidative stress damage in steatotic liver by other pharmacological treatments[19,33,49]. The ability of TMZ to protect both types of the liver is particularly attractive since some strategies that are effective in non-steatotic livers may not be useful in the presence of steatosis[19,20,50].
In conclusion, TMZ protected against hepatic injury and ameliorated liver function in steatotic and non-steatotic livers preserved in UW solution. Further studies will be required to elucidate whether TMZ is such a promising drug for the field of liver transplantation, and if this pharmacological substance can reduce the inherent risk of steatotic liver grafts for transplantation.
ACKNOWLEDGMENTS
I. Ben Mosbah holds a fellowship from the AECI and A. Casillas-Ramírez holds one from José E. González Hospital-UANL. We would like to thank Robin Rycroft at the Language Advisory Service of the University of Barcelona for revising the English text.
Footnotes
supported by the Ministerio de Ciencia y Tecnología (project grants HP 2003-0051, BFI 2002-00704 and BFI 2003-00912) and the Agencia Española de Cooperación Internacional (AECI, project grant 25/03/P) (Madrid, Spain
S- Editor Guo SY L- Editor Elsevier HK E- Editor Bai SH
References
- 1.Todo S, Demetris AJ, Makowka L, Teperman L, Podesta L, Shaver T, Tzakis A, Starzl TE. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47:903–905. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415–423. doi: 10.1053/jlts.2002.32275. [DOI] [PubMed] [Google Scholar]
- 3.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 4.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 5.Stratta RJ, Wood RP, Langnas AN, Duckworth RM, Markin RS, Marujo W, Grazi GL, Saito S, Dawidson I, Rikkers LF. The impact of extended preservation on clinical liver transplantation. Transplantation. 1990;50:438–443. doi: 10.1097/00007890-199009000-00015. [DOI] [PubMed] [Google Scholar]
- 6.El-Gibaly AM, Scheuer C, Menger MD, Vollmar B. Improvement of rat liver graft quality by pifithrin-alpha-mediated inhibition of hepatocyte necrapoptosis. Hepatology. 2004;39:1553–1562. doi: 10.1002/hep.20243. [DOI] [PubMed] [Google Scholar]
- 7.Klar E, Angelescu M, Zapletal C, Kraus T, Bredt M, Herfarth C. Definition of maximum cold ischemia time without reduction of graft quality in clinical liver transplantation. Transplant Proc. 1998;30:3683–3685. doi: 10.1016/s0041-1345(98)01193-2. [DOI] [PubMed] [Google Scholar]
- 8.Ikizler M, Dernek S, Sevin B, Kural T. Trimetazidine improves recovery during reperfusion in isolated rat hearts after prolonged ischemia. Anadolu Kardiyol Derg. 2003;3:303–308. [PubMed] [Google Scholar]
- 9.Veitch K, Maisin L, Hue L. Trimetazidine effects on the damage to mitochondrial functions caused by ischemia and reperfusion. Am J Cardiol. 1995;76:25B–30B. [PubMed] [Google Scholar]
- 10.Hauet T, Bauza G, Goujon JM, Caritez JC, Carretier M, Eugene M, Tillement JP. Effects of trimetazidine on lipid peroxidation and phosphorus metabolites during cold storage and reperfusion of isolated perfused rat kidneys. J Pharmacol Exp Ther. 1998;285:1061–1067. [PubMed] [Google Scholar]
- 11.Hauet T, Baumert H, Amor IB, Gibelin H, Tallineau C, Eugene M, Tillement JP, Carretier M. Pharmacological limitation of damage to renal medulla after cold storage and transplantation by trimetazidine. J Pharmacol Exp Ther. 2000;292:254–260. [PubMed] [Google Scholar]
- 12.Elimadi A, Sapena R, Settaf A, Le Louet H, Tillement J, Morin D. Attenuation of liver normothermic ischemia--reperfusion injury by preservation of mitochondrial functions with S-15176, a potent trimetazidine derivative. Biochem Pharmacol. 2001;62:509–516. doi: 10.1016/s0006-2952(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 13.Settaf A, Morin D, Lamchouri F, Elimadi A, Cherrah Y, Tillement JP. Trimetazidine ameliorates the hepatic injury associated with ischaemia-reperfusion in rats. Pharmacol Res. 1999;39:211–216. doi: 10.1006/phrs.1998.0427. [DOI] [PubMed] [Google Scholar]
- 14.Kaya Y, Coskun T, Aral E, Erkasap N, Var A. The effect of trimetazidine on liver regeneration after partial hepatectomy under hepatic blood inflow occlusion. Hepatogastroenterology. 2003;50:651–655. [PubMed] [Google Scholar]
- 15.Guarnieri C, Muscari C. Effect of trimetazidine on mitochondrial function and oxidative damage during reperfusion of ischemic hypertrophied rat myocardium. Pharmacology. 1993;46:324–331. doi: 10.1159/000139070. [DOI] [PubMed] [Google Scholar]
- 16.Caraceni P, Bianchi C, Domenicali M, Maria Pertosa A, Maiolini E, Parenti Castelli G, Nardo B, Trevisani F, Lenaz G, Bernardi M. Impairment of mitochondrial oxidative phosphorylation in rat fatty liver exposed to preservation-reperfusion injury. J Hepatol. 2004;41:82–88. doi: 10.1016/j.jhep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Rashid A, Wu TC, Huang CC, Chen CH, Lin HZ, Yang SQ, Lee FY, Diehl AM. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131–1138. doi: 10.1002/hep.510290428. [DOI] [PubMed] [Google Scholar]
- 18.Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55–61. doi: 10.1016/s0168-8278(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 19.Serafin A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 21.Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076–1082. doi: 10.1097/00007890-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hakamada K, Sasaki M, Takahashi K, Umehara Y, Konn M. Sinusoidal flow block after warm ischemia in rats with diet-induced fatty liver. J Surg Res. 1997;70:12–20. doi: 10.1006/jsre.1997.5077. [DOI] [PubMed] [Google Scholar]
- 23.Gores GJ, Kost LJ, LaRusso NF. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 1986;6:511–517. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed I, Attia MS, Ahmad N, Lodge JP, Potts DJ. Use of isolated perfused rat liver model for testing liver preservation solutions. Transplant Proc. 2001;33:3709–3711. doi: 10.1016/s0041-1345(01)02513-1. [DOI] [PubMed] [Google Scholar]
- 25.Ben Abdennebi H, Steghens JP, Margonari J, Ramella-Virieux S, Barbieux A, Boillot O. High-Na+ low-K+ UW cold storage solution reduces reperfusion injuries of the rat liver graft. Transpl Int. 1998;11:223–230. doi: 10.1007/s001470050132. [DOI] [PubMed] [Google Scholar]
- 26.Hauet T, Goujon JM, Vandewalle A, Baumert H, Lacoste L, Tillement JP, Eugene M, Carretier M. Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys. J Am Soc Nephrol. 2000;11:138–148. doi: 10.1681/ASN.V111138. [DOI] [PubMed] [Google Scholar]
- 27.Faure JP, Petit I, Zhang K, Dutheil D, Doucet C, Favreau F, Eugène M, Goujon JM, Tillement JP, Mauco G, et al. Protective roles of polyethylene glycol and trimetazidine against cold ischemia and reperfusion injuries of pig kidney graft. Am J Transplant. 2004;4:495–504. doi: 10.1111/j.1600-6143.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 28.Boucher FR, Hearse DJ, Opie LH. Effects of trimetazidine on ischemic contracture in isolated perfused rat hearts. J Cardiovasc Pharmacol. 1994;24:45–49. doi: 10.1097/00005344-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Minor T, Yamaguchi T, Isselhard W. Effects of taurine on liver preservation in UW solution with consecutive ischemic rewarming in the isolated perfused rat liver. Transpl Int. 1995;8:174–179. doi: 10.1007/BF00336533. [DOI] [PubMed] [Google Scholar]
- 30.Minor T, Akbar S, Tolba R, Dombrowski F. Cold preservation of fatty liver grafts: prevention of functional and ultrastructural impairments by venous oxygen persufflation. J Hepatol. 2000;32:105–111. doi: 10.1016/s0168-8278(00)80196-8. [DOI] [PubMed] [Google Scholar]
- 31.Ben Abdennebi H, Steghens JP, Hadj-Aïssa A, Barbieux A, Ramella-Virieux S, Gharib C, Boillot O. A preservation solution with polyethylene glycol and calcium: a possible multiorgan liquid. Transpl Int. 2002;15:348–354. doi: 10.1007/s00147-002-0427-8. [DOI] [PubMed] [Google Scholar]
- 32.Peralta C, Bartrons R, Serafin A, Blázquez C, Guzmán M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- 33.Nakano H, Nagasaki H, Barama A, Boudjema K, Jaeck D, Kumada K, Tatsuno M, Baek Y, Kitamura N, Suzuki T, et al. The effects of N-acetylcysteine and anti-intercellular adhesion molecule-1 monoclonal antibody against ischemia-reperfusion injury of the rat steatotic liver produced by a choline-methionine-deficient diet. Hepatology. 1997;26:670–678. doi: 10.1053/jhep.1997.v26.pm0009303498. [DOI] [PubMed] [Google Scholar]
- 34.Taneja C, Prescott L, Koneru B. Critical preservation injury in rat fatty liver is to hepatocytes, not sinusoidal lining cells. Transplantation. 1998;65:167–172. doi: 10.1097/00007890-199801270-00004. [DOI] [PubMed] [Google Scholar]
- 35.Soto A, Foy BD, Frazier JM. Effect of cadmium on bromosulfophthalein kinetics in the isolated perfused rat liver system. Toxicol Sci. 2002;69:460–469. doi: 10.1093/toxsci/69.2.460. [DOI] [PubMed] [Google Scholar]
- 36.Céspedes JM, Rodríguez Garay EA. Sulfobromophthalein metabolism in isolated perfused rat liver. Factors determining the applicability of the model. Acta Physiol Lat Am. 1979;29:207–215. [PubMed] [Google Scholar]
- 37.Arnault I, Bao YM, Sebagh M, Anjo A, Dimicoli JL, Lemoine A, Delvart V, Adam R. Beneficial effect of pentoxifylline on microvesicular steatotic livers submitted to a prolonged cold ischemia. Transplantation. 2003;76:77–83. doi: 10.1097/01.TP.0000071846.35825.B1. [DOI] [PubMed] [Google Scholar]
- 38.Peralta C, Bulbena O, Xaus C, Prats N, Cutrin JC, Poli G, Gelpi E, Roselló-Catafau J. Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203–1211. doi: 10.1097/00007890-200204270-00004. [DOI] [PubMed] [Google Scholar]
- 39.Peralta C, Bulbena O, Bargalló R, Prats N, Gelpí E, Roselló-Catafau J. Strategies to modulate the deleterious effects of endothelin in hepatic ischemia-reperfusion. Transplantation. 2000;70:1761–1770. doi: 10.1097/00007890-200012270-00016. [DOI] [PubMed] [Google Scholar]
- 40.Chimalakonda AP, Mehvar R. Effects of duration of ischemia and donor pretreatment with methylprednisolone or its macromolecular prodrug on the disposition of indocyanine green in cold-preserved rat livers. Pharm Res. 2004;21:1000–1008. doi: 10.1023/b:pham.0000029290.54167.7c. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 42.Kukan M, Haddad PS. Role of hepatocytes and bile duct cells in preservation-reperfusion injury of liver grafts. Liver Transpl. 2001;7:381–400. doi: 10.1053/jlts.2001.23913. [DOI] [PubMed] [Google Scholar]
- 43.Popescu I, Sheiner P, Mor E, Forman W, Borcich A, Emre S, Kishikawa K, Schwartz M, Miller C. Biliary complications in 400 cases of liver transplantation. Mt Sinai J Med. 1994;61:57–62. [PubMed] [Google Scholar]
- 44.Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495–500. doi: 10.1097/00007890-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Maridonneau-Parini I, Harpey C. Effect of trimetazidine on membrane damage induced by oxygen free radicals in human red cells. Br J Clin Pharmacol. 1985;20:148–151. doi: 10.1111/j.1365-2125.1985.tb05047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsimoyiannis EC, Moutesidou KJ, Moschos CM, Karayianni M, Karkabounas S, Kotoulas OB. Trimetazidine for prevention of hepatic injury induced by ischaemia and reperfusion in rats. Eur J Surg. 1993;159:89–93. [PubMed] [Google Scholar]
- 47.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 48.Hui AM, Kawasaki S, Makuuchi M, Nakayama J, Ikegami T, Miyagawa S. Liver injury following normothermic ischemia in steatotic rat liver. Hepatology. 1994;20:1287–1293. doi: 10.1002/hep.1840200528. [DOI] [PubMed] [Google Scholar]
- 49.Gao W, Connor HD, Lemasters JJ, Mason RP, Thurman RG. Primary nonfunction of fatty livers produced by alcohol is associated with a new, antioxidant-insensitive free radical species. Transplantation. 1995;59:674–679. doi: 10.1097/00007890-199503150-00005. [DOI] [PubMed] [Google Scholar]
- 50.Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]


