Abstract
AIM: The beneficial effect of zinc supplementation on the efficacy of interferon as a treatment for chronic hepatitis C had been demonstrated in hepatitis virus genotype 1b of high viral load. This study focused on patients with genotype 2, which is more sensitive to interferon than genotype 1b, and used consensus interferon (CIFN) with or without zinc.
METHODS: We randomized 83 patients with chronic hepatitis C to CIFN at 18 MIU six times/wk for 4 wk, followed by CIFN at 18 MIU six times/wk for another 20 wk, in combination with polaprezinc 300 mg (regimen A, n = 41) or as monotherapy (regimen B, n = 42). Thirty-one patients in regimen A and 33 patients in regimen B completed the clinical trial; the remaining patients withdrew because of side effects or a transfer to another hospital.
RESULTS: Sustained biochemical response, defined as a normal aminotransferase level at the end of the 6-mo post-treatment observation, was 68% and 69%, and sustained virological response, defined as undetectable HCV-RNA at the end of the 6-mo post-treatment observation, was 54% and 67% for regimens A and B, respectively.
CONCLUSION: CIFN treatment combined with zinc did not enhance the effect of CIFN as shown by biochemical, virological criteria. No side effects related to polaprezinc were noted.
Keywords: Randomized controlled trial, Consensus interferon, Zinc, Chronic hepatitis C, Genotype 2
INTRODUCTION
Interferon (IFN) is an effective treatment for chronic hepatitis C and is the only method of treatment that eliminates hepatitis C virus (HCV)[1,2]. The incidence of hepatocellular carcinoma (HCC) decreases if liver function improves, even if the virus is not eradicated after IFN treatment[3-6], or if HCV RNA becomes undetectable during IFN treatment[7]. IFN treatment for chronic hepatitis C plays a pivotal role in the prevention of HCC.
HCV-RNA clearance rates are reported to be approximately 30-40% in patients treated with IFN alone[8-10]. However, better results have been reported when IFN-α is used in combination with ribavirin in both naïve patients[11,12] and in those who failed to respond to, or relapsed after, IFN-α monotherapy[13,14]. However, IFN plus ribavirin treatment for 24 wk causes more frequent side effects leading to dose reduction or discontinuation of treatment in 28% of cases compared that in 21% of cases in IFN alone treatment[11]. Furthermore, in Japan, the use of ribavirin is restricted to naïve patients with chronic hepatitis C who have high viral loads greater than 100 KIU/mL.
Consensus IFN (CIFN) is a genetically engineered novel type I IFN molecule derived from commonly observed amino acids of several natural IFN subtypes [15]. CIFN has demonstrated higher in vitro antiviral effects on an equal mass basis, than IFN- 2α or 2β[16,17]. In Japan, 9-18 MIU CIFN treatment for 6 mo is reported to be well tolerated and 18 MIU CIFN is considered to be the maximum tolerable dose in patients with chronic hepatitis C and is superior in efficacy, without additional toxicity, to 9 MIU CIFN in high-titer chronic hepatitis C patients[18].
Serum zinc levels are low in patients with chronic liver disease because of a trace element metabolism disorder[19]. Previously, we have demonstrated that zinc supplementation in chronic hepatitis C patients with genotype 1b of high viral load enhanced the response to IFN-α monotherapy[20]. Although zinc has diverse biological properties including antiviral, anti-inflammatory, and antioxidant functions[21-24], the mechanism responsible for the beneficial effect of zinc during treatment with IFN remains to be elucidated. Treatment efficacy depends on the genotype, HCV load, and staging; HCV genotypes 2a and 2b are more sensitive to IFN than genotype 1a or 1b[1,25,26].
We planned a randomized controlled study for the treatment of genotypes 2a and 2b hepatitis C to determine whether zinc supplementation could also enhance the efficacy of IFN in chronic hepatitis C patients as well as genotype 1b. We used CIFN instead of IFN plus ribavirin for the following reasons. (1) Naïve chronic hepatitis C patients with low viral load could not be treated by the standard treatment, IFN-α 2b plus ribavirin or pegylated-IFN plus ribavirin, under the current health insurance regulations in Japan, due to high sensitivity of HCV with low viral load to IFN. (2) Even with high viral loads, HCV genotype 2a/2b can be expected to be relatively sensitive to IFN monotherapy without ribavirin. (3) CIFN has fewer restrictions or contraindications, and is less expensive than the combination of IFN-α 2b and ribavirin.
MATERIALS AND METHODS
Patient selection criteria
Patients with chronic HCV infection who had never received IFN therapy were eligible for this study. The patients were continuously positive for HCV-RNA genotype 2 using the amplicor HCV monitor assay and were diagnosed with chronic active hepatitis from a liver biopsy. The eligibility criteria included histological evidence of chronic hepatitis, as judged on a liver biopsy performed no longer than 6 mo prior to enrolment, and confirmation of HCV infection by polymerase chain reaction (PCR) analysis. Liver-biopsy specimens were assessed independently by two pathologists who were blinded to the patients’ clinical details. Pathological diagnosis was made using the classification established by Desmet et al[27]. The exclusion criteria included: age lower than 18 years or older than 65 years, pregnancy or lack of appropriate contraceptive measures in women of child bearing age, previous treatment with antiviral or immunosuppressive drugs, current or previous drug addiction, alcoholism, positive HbsAg or HIV test, histological evidence of liver cirrhosis, concomitant metabolic, autoimmune or neoplastic liver diseases, severe concomitant diseases other than liver disease, history of depression or psychiatric diseases.
Study design
This was a randomized, open-label, controlled study conducted between December 2001 and December 2003. The study was approved by the Ethical Committee of Gunma University. Eighty-three patients gave written informed consent and were randomly assigned to two groups: patients in regimen A received CIFN with polaprezinc and patients in regimen B received CIFN alone. All patients were given CIFN subcutaneously every day for 4 wk and then thrice a wk for 20 wk at a dose of 18 mega units (MU). Forty-one patients in regimen A were given 75 mg of oral polaprezinc twice daily (Plomac; Zeria Pharmaceutical Co., Tokyo, Japan) with CIFN for 24 wk, and zinc administration was discontinued simultaneously with discontinuation of CIFN, for a total of 24 wk. Forty-three patients in regimen B were given CIFN according to the protocol for 24 wk. Serum HCV-RNA was measured before treatment and every mo for one year during and after CIFN therapy. Patient adherence to the protocol and compliance with treatment were encouraged by regular hospital visits.
Endpoints and definitions
Two endpoints were selected for comparing the efficacy of regimen A with the standard regimen B. The first endpoint involved serum HCV-RNA levels. ‘Sustained virological response (SVR)’ was defined as undetectable HCV-RNA at the end of the 6-mo post-treatment observation. ‘End-of treatment virological response (EVR)’ was defined as undetectable HCV-RNA at the end of the treatment. ‘Non virological response (NVR)’ was defined as detectable HCV-RNA at the end of the 6-mo post-treatment observation. The second endpoint was serum alanine aminotransferase (ALT) levels. Normalization of serum ALT at the end of treatment and at the end of the 6-mo post-treatment observation was defined as ‘end-of treatment biochemical response (EBR)’ and ‘sustained biochemical response (SBR)’, respectively. ‘Non biochemical response (NBR)’ was defined as elevation of serum ALT at the end of the 6-mo post-treatment observation.
Assessment of safety
We evaluated all adverse events, subjective and objective symptoms, and laboratory test results for safety. All adverse events were checked by the patients using questionnaires, double-checked, and recorded by the investigators. All adverse events were graded for severity based on the World Health Organization (WHO) criteria and the guideline to grading severity of adverse events determined by the Japanese Ministry of Health and Welfare. Global safety assessments were made at the end of post-treatment observation using the following classifications: no abnormality, abnormality requiring no symptomatic treatments, abnormality requiring symptomatic treatments, abnormality requiring study withdrawal, and non-evaluable.
Statistical analysis
Results were expressed as mean±SD of the mean on an intention to treat or per-protocol basis. Statistically significant differences in outcomes between the two regimens were assessed using both parametric and non-parametric tests including the Student’s t-test for paired and unpaired observations, and Fisher’ s exact test as appropriate.
A logistic multiple regression model was used to examine the relationship between baseline clinical characteristics and the binary outcome of response to CIFN therapy. Each variable was transformed into categorical data consisting of two simple ordinal numbers for the logistic multiple regression model. Variable selection was an important step in characterizing prognostic relations and identifying variables most strongly related to the outcome. We selected, as candidate predictors of SVR, age, sex, weight, body mass index (BMI), pretreatment platelet counts, pretreatment ALT, pretreatment γGTP levels, liver histology (staging and grading), pretreatment HCV-RNA viral load, wk of HCV-RNA clearance (less than 1, 4, 8, and 12 wk) and zinc supplementation. Thus, variables (P < 0.2) on univariate analysis, that is, zinc supplementation, BMI, staging, pretreatment HCV-RNA viral load (less than 400 KIU/mL), wk of HCV-RNA clearance (less than 8 wk) and pretreatment ALT were included in a logistic multiple regression model. Differences with a P < 0.05 were considered significant.
RESULTS
Eighty-three patients were enrolled into the trial; 41 were randomized to regimen A and 42 to regimen B. The pretreatment clinical characteristics of the patients assigned to the two regimens were similar (Table 1).
Table 1.
Characteristics of participating patients
| Regimen A IFN+zinc) | Regimen B (CIFN) | P | |
| Number of entry | 41 | 42 | |
| Male/Female | 28/13 | 21/21 | NS |
| Age (yr) | 60±8.5 | 55±21 | NS |
| Body mass index | 23±2.4 | 24±3.7 | NS |
| Pre-treatment zinc concentration (μg/dl) | 77.8±13.6 | 82.5±16.4 | NS |
| Pre-treatment ALT (IU/l) | 113±75 | 101±96 | NS |
| Pre-treatment leukocyte counts (x103mm-3) | 5.1±1.3 | 5.0±1.4 | NS |
| Pre-treatment platelet counts (x103mm-3) | 19±5 | 17±5 | NS |
| Pre-treatment HCV-RNA genotype (2A/2B) | 31/10 | 31/11 | NS |
| Pre-treatment HCV-RNA titer (KIU/mL) | 484±1075 | 304±318 | NS |
| 0<pretreatment HCV-RNA<100 | 11 | 16 | NS |
| 100≤pretreatment HCV-RNA<400 | 15 | 14 | NS |
| 400≤pretreatment HCV-RNA<700 | 6 | 2 | NS |
| 700≤pretreatment HCV-RNA | 9 | 9 | NS |
| Histological findings (staging) | 1.8±1 | 1.3±0.8 | NS |
| Histological findings (grading) | 1.6±0.7 | 1.3±0.9 | NS |
Mean±SD; NS: not significant
Follow-up and protocol compliance
The progression of randomized patients through the trial is summarized in Figure 1. Seven patients in regimen A withdrew prematurely from the study before wk 24 due to intolerance to IFN in five patients, and personal problems or low compliance in the other two patients. Seven patients in regimen B also withdrew prematurely from the study before wk 24 due to intolerance to IFN in six patients, and low compliance in the other patient. Three more patients, all in regimen A, withdrew prematurely from the study before wk 48 because of job transfer or personal problems. Two patients in regimen B withdrew prematurely due to personal problems.
Figure 1.
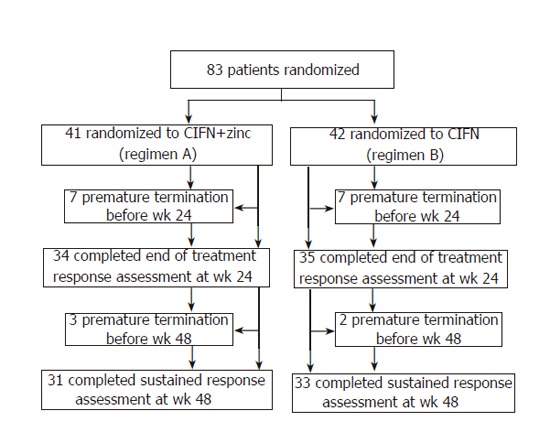
Trial profile.
Effect of treatment on viremia and serum transaminase
There were 28/41 (68%) end-of treatment virological responders for regimen A and 32/42 (76%) for regimen B when analyzed on an intention to treat basis; the corresponding values were 28/31 (90%) and 32/33 (97%), respectively, when analyzed on a per-protocol basis (Table 2). There were 22/41 (54%) sustained virological responders for regimen A and 28/42 (67%) responders for regimen B when analyzed on an intention to treat basis; the corresponding values were 22/31 (71%) and 28/33 (85%), respectively, when analyzed on a per-protocol basis. There were 19/41 (46%) end-of treatment biochemical responders for regimen A and 21/42 (50%) responders for regimen B when analyzed on an intention to treat basis; the corresponding values were 19/31 (61%) and 21/33 (64%), respectively, when analyzed on a per-protocol basis. There were 28/41 (68%) sustained biochemical responders for regimen A and 29/42 (69%) responders for regimen B when analyzed on an intention to treat basis; the corresponding values were 28/31 (90%) and 29/33 (88%), respectively, when analyzed on a per-protocol basis. There were no significant differences between the two regimens in these four responses.
Table 2.
Effect on viremia and serum transaminases
| Regimen A (CIFN+zinc) | Regimen B (CIFN) | P | |
| ALT (U/l): | |||
| Wk 0 | 113±75 | 101±96 | NS |
| Wk 24 | 46±51 | 40±30 | NS |
| Wk 48 | 26±17 | 22±12 | NS |
| Biochemical response (by intention to treat): | |||
| Wk 24 (EBR) | 46% | 50% | NS |
| Wk 48 (SBR) | 68% | 69% | NS |
| Virological response (by intention to treat): | |||
| Wk 24 (EVR) | 68% | 76% | NS |
| Wk 48 (SVR) | 54% | 67% | NS |
Mean±SD; EBR: End-of treatment biochemical response; SBR: Sustained biochemical response; EVR: End-of treatment virological response; SVR: Sustained virological response; NS: not significant
Factors contributing to sustained virological response
Variables that correlated with sustained virological response were analyzed by both univariate and multivariate analyses. In univariate analysis, the sustained virological response correlated significantly with liver histology (staging) and pretreatment HCV-RNA viral load. In multivariate analysis, only pretreatment HCV-RNA less than 400 KIU/mL was associated with a sustained virological response (Table 3). The other factors, including zinc supplementation, BMI, staging, wk of HCV-RNA clearance, and pretreatment ALT level, did not influence sustained virological response.
Table 3.
Logistic regression model of predictors of response to CIFN treatment
| Variables | Odds ratio (95% Confidence Interval) | |
| Zinc supplementation (regimen B vs. A) | 0.254 (0.033-1.948) | 0.1873 |
| BMI (<23.4 vs. ≥23.4) | 1.245 (0.162-9.579) | 0.8330 |
| Staging (0-1 vs. 2-3) | 0.213 (0.024-1.877) | 0.1635 |
| pretreatment HCV-RNA (<400 vs. ≥400) | 0.104 (0.011-0.951) | 0.0450 |
| Week of HCV-RNA clearance (<8 vs. ≥8) | 0.106(0.005-2.399) | 0.1584 |
| pretreatment ALT (<107 vs. ≥107) | 13.709 (0.960-195.749) | 0.1873 |
Serum zinc concentrations
Serum zinc concentrations at 4 wk after the commencement of polaprezinc treatment were elevated and significantly higher than pretreatment levels in regimen A patients (Figure 2). Serum zinc concentrations in patients of regimen A were significantly higher than those of regimen B patients at 4 wk after the start of administration of polaprezinc (Figure 2).
Figure 2.
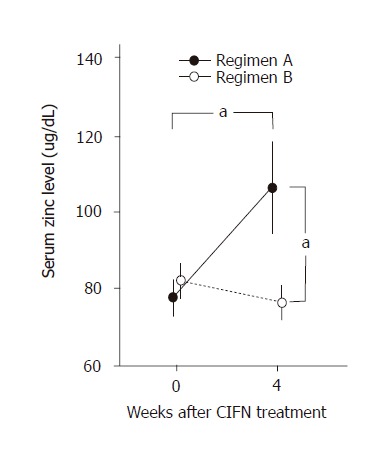
Serum zinc concentrations (mean±SD) at wk 0 (baseline) and after 4 wk of CIFN treatment in patients with regimen A (closed circles) and regimen B (open circles). Serum zinc levels at wk 4 were significantly higher in regimen A patients than those in regimen B patients (aP<0.05). Serum zinc levels at wk 4 were significantly higher than those recorded at wk 0 (baseline) in regimen A patients (aP < 0.05).
Adverse effects
Each of the serious adverse events including depression, thrombocytopenia, leukopenia, and severe elevations in γGTP was observed in one patient in regimen A. In addition, regimen B was also associated with serious adverse events such as depression in one patient, and severe leukopenia in two patients. Frequent adverse events and laboratory abnormalities recorded during the study are summarized in Tables 4 and 5. The type and frequency of the adverse events, and laboratory abnormalities were similar in both regimens. Five (12.2%) patients and six (14.3%) patients dropped out of the study due to the adverse events before wk 24 in regimen A and regimen B, respectively. There were no significant differences of grading severity of adverse effects as well as incidence of them in the global safety assessment of the two regimens (data not shown).
Table 4.
Incidence of selected adverse events (>10% frequency)
| Adverse event | RegimenA (CIFN+zinc) | Regimen B (CIFN) |
| n | 41 | 42 |
| Fever | 41 (100%) | 42 (100%) |
| Headache | 17 (41.5%) | 16 (38.1%) |
| Malaise | 15 (36.6%) | 12 (28.6%) |
| Anorexia | 14 (34.1%) | 19 (45.2%) |
| Arthralgia | 14 (34.1%) | 13 (31.0%) |
| Insomnia | 11 (26.8%) | 11 (26.2%) |
| Stomach discomfort | 7 (17.1%) | 5 (11.9%) |
| Alopecia | 6 (14.6%) | 6 (14.3%) |
| depression | 6 (14.6%) | 5 (11.9%) |
| Nausea | 6 (14.6%) | 5 (11.9%) |
| Myalgia | 5 (12.2%) | 6 ( 14.3%) |
| Rash | 5 (12.2%) | 4 (9.5%) |
Table 5.
Incidence of selected laboratory abnormalities (>10% frequency)
| Laboratory abnormality | Regimen A (CIFN+zinc) | Regimen B (CIFN) |
| n | 41 | 42 |
| Thrombocytopenia | 31 (78%) | 29 (69%) |
| Leucopenia | 24 (59%) | 30(71%) |
| Increase in AST | 20 (49%) | 18 (43%) |
| Increase in ALT | 18 (44%) | 16 (38%) |
| Increase in γ-GTP | 12 (29%) | 12 (29%) |
| Decrease in hemoglobin | 7 (17%) | 9 (21%) |
| Increase in ZTT | 7 (17%) | 7 (17%) |
DISCUSSION
The metabolism of trace elements is impaired in chronic liver disease[19]. Zinc deficiency in chronic hepatitis C is improved by IFN treatment[28]. Zinc supplementation enhances the response to IFN-α treatment in patients with genotype 1b chronic hepatitis C[20]. Conversely, zinc supplementation did not enhance the response to CIFN treatment in patients with chronic hepatitis C with genotype 2 in this trial. The reasons for the lack of synergy between zinc and IFN are unclear, however, there are several possible reasons that are worthy of consideration. The viral genotype 2 in both regimens may account for the virological response, differing from the previous study that involved patients with genotype Ib. It is also possible that the relatively higher response rate achieved with CIFN in both regimens in this trial compared with the previous study with IFN-α could have masked any differences in efficacy. In addition, individual differences in serum zinc concentration increases, as a result of zinc administration, may alter the virological response because the extent of the increases in serum zinc concentration varied in regimen A. Furthermore, differences in the type of IFN may affect the virological response, given that natural IFN-α was used in the previous study.
The overall therapeutic efficacy (60% SVR) of CIFN in our study was similar to that (50% SVR) of low-titer or genotype 2 group in a previous report from Japan[18]. Recent reports showed that pegylated-IFN plus ribavirin[29] had excellent treatment efficacy (100% SVR) in chronic hepatitis C patients with genotype 2[30]. SVR rates of IFN-α 2b plus ribavirin for chronic hepatitis C patients with genotypes except 1[11] or CIFN plus ribavirin for chronic hepatitis C patients with genotypes 2/3 were reported to be from 60% to 70%[31]. Given the overall 60% SVR in our study, CIFN without ribavirin can be considered as a good candidate for patients in whom ribavirin is contraindicated.
Interestingly, the cases of undetectable HCV-RNA titer at 1 wk, but not 4 or 12 wk after the start of treatment, showed 95% and 100% of sustained virological response rate in regimens A and B, respectively. Although only a pretreatment HCV-RNA less than 400 KIU/mL was considered to be a predictive factor for sustained virological response, based on a logistic multiple regression model in our trial, earlier responses at 1 wk after the start of treatment may also be a good predictor of sustained virological response.
The type and frequency of adverse events, and laboratory abnormalities were similar in both regimens. Overall, adverse events and laboratory abnormalities were similar to those in previous reports in Japan[18]. Although 18 MIU CIFN was relatively well tolerated in our trial, there were a few cases of serious adverse events. Twelve MIU and nine MIU CIFN, which are now available in Japan, may allow us to reduce the incidence of serious adverse events.
In conclusion, our study does not support the use of zinc as an adjunct to CIFN for the treatment of chronic hepatitis C with genotype 2, because combination treatment was not superior to CIFN monotherapy in achieving a sustained virological response. High viral loads of over 400 KIU/mL may indicate a low probability of sustained virological response for CIFN treatment, regardless of zinc supplementation.
Footnotes
Supported by the grant center of Excellence, Biomedical research using accelerator technology, Gunma, Japan
S- Editor Guo SY L- Editor Elsevier HK E- Editor Bai SH
References
- 1.Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC Jr, Perrillo RP, Carey W, Jacobson IM, Payne J, Dienstag JL. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, Goodman Z, Banks SM, Hoofnagle JH. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506–1510. doi: 10.1056/NEJM198911303212204. [DOI] [PubMed] [Google Scholar]
- 3.Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–1402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–1130. doi: 10.1002/hep.510290439. [DOI] [PubMed] [Google Scholar]
- 6.Okanoue T, Itoh Y, Minami M, Sakamoto S, Yasui K, Sakamoto M, Nishioji K, Murakami Y, Kashima K. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. Viral Hepatitis Therapy Study Group. J Hepatol. 1999;30:653–659. doi: 10.1016/s0168-8278(99)80196-2. [DOI] [PubMed] [Google Scholar]
- 7.Okanoue T, Itoh Y, Kirishima T, Daimon Y, Toyama T, Morita A, Nakajima T, Minami M. Transient biochemical response in interferon therapy decreases the development of hepatocellular carcinoma for five years and improves the long-term survival of chronic hepatitis C patients. Hepatol Res. 2002;23:62–77. doi: 10.1016/s1386-6346(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778–789. doi: 10.1002/hep.510240405. [DOI] [PubMed] [Google Scholar]
- 9.Carithers RL Jr, Emerson SS. Therapy of hepatitis C: meta-analysis of interferon alfa-2b trials. Hepatology. 1997;26:83S–88S. doi: 10.1002/hep.510260715. [DOI] [PubMed] [Google Scholar]
- 10.Orito E, Mizokami M, Nakano T, Terashima H, Nojiri O, Sakakibara K, Mizuno M, Ogino M, Nakamura M, Matsumoto Y. Serum hepatitis C virus RNA level as a predictor of subsequent response to interferon-alpha therapy in Japanese patients with chronic hepatitis C. J Med Virol. 1994;44:410–414. doi: 10.1002/jmv.1890440418. [DOI] [PubMed] [Google Scholar]
- 11.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 12.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 13.Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 14.Barbaro G, Di Lorenzo G, Belloni G, Ferrari L, Paiano A, Del Poggio P, Bacca D, Fruttaldo L, Mongio F, Francavilla R, et al. Interferon alpha-2B and ribavirin in combination for patients with chronic hepatitis C who failed to respond to, or relapsed after, interferon alpha therapy: a randomized trial. Am J Med. 1999;107:112–118. doi: 10.1016/s0002-9343(99)00160-6. [DOI] [PubMed] [Google Scholar]
- 15.Blatt LM, Davis JM, Klein SB, Taylor MW. The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J Interferon Cytokine Res. 1996;16:489–499. doi: 10.1089/jir.1996.16.489. [DOI] [PubMed] [Google Scholar]
- 16.Alton K, Stabinshy Y, Richards R, et al. Production, characterization and biological effects of recombinant DNA derived human interferon-α and interferon-γ analogs. In: The Biology of the Interferon System. Amsterdam: Elsevier Science. 1983:119–128. [Google Scholar]
- 17.Ozes ON, Reiter Z, Klein S, Blatt LM, Taylor MW. A comparison of interferon-Con1 with natural recombinant interferons-alpha: antiviral, antiproliferative, and natural killer-inducing activities. J Interferon Res. 1992;12:55–59. doi: 10.1089/jir.1992.12.55. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Tango T. A multicenter, randomized, controlled clinical trial of interferon alfacon-1 in comparison with lymphoblastoid interferon-alpha in patients with high-titer chronic hepatitis C virus infection. Hepatol Res. 2002;22:1–12. doi: 10.1016/s1386-6346(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 19.Loguercio C, De Girolamo V, Federico A, Feng SL, Cataldi V, Del Vecchio Blanco C, Gialanella G. Trace elements and chronic liver diseases. J Trace Elem Med Biol. 1997;11:158–161. doi: 10.1016/s0946-672x(97)80045-4. [DOI] [PubMed] [Google Scholar]
- 20.Takagi H, Nagamine T, Abe T, Takayama H, Sato K, Otsuka T, Kakizaki S, Hashimoto Y, Matsumoto T, Kojima A, et al. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J Viral Hepat. 2001;8:367–371. doi: 10.1046/j.1365-2893.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 21.Novick SG, Godfrey JC, Pollack RL, Wilder HR. Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Med Hypotheses. 1997;49:347–357. doi: 10.1016/s0306-9877(97)90201-2. [DOI] [PubMed] [Google Scholar]
- 22.Kümel G, Schrader S, Zentgraf H, Brendel M. [Therapy of banal HSV lesions: molecular mechanisms of the antiviral activity of zinc sulfate] Hautarzt. 1991;42:439–445. [PubMed] [Google Scholar]
- 23.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 24.Prasad AS. Zinc and immunity. Mol Cell Biochem. 1998;188:63–69. [PubMed] [Google Scholar]
- 25.Tsubota A, Chayama K, Ikeda K, Yasuji A, Koida I, Saitoh S, Hashimoto M, Iwasaki S, Kobayashi M, Hiromitsu K. Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology. 1994;19:1088–1094. [PubMed] [Google Scholar]
- 26.Martinot-Peignoux M, Marcellin P, Pouteau M, Castelnau C, Boyer N, Poliquin M, Degott C, Descombes I, Le Breton V, Milotova V. Pretreatment serum hepatitis C virus RNA levels and hepatitis C virus genotype are the main and independent prognostic factors of sustained response to interferon alfa therapy in chronic hepatitis C. Hepatology. 1995;22:1050–1056. [PubMed] [Google Scholar]
- 27.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 28.Nagamine T, Takagi H, Hashimoto Y, Takayama H, Shimoda R, Nomura N, Suzuki K, Mori M, Nakajima K. The possible role of zinc and metallothionein in the liver on the therapeutic effect of IFN-alpha to hepatitis C patients. Biol Trace Elem Res. 1997;58:65–76. doi: 10.1007/BF02910667. [DOI] [PubMed] [Google Scholar]
- 29.Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, Sarrazin C, Harvey J, Brass C, Albrecht J. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–999. doi: 10.1016/j.jhep.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36:S1–S2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 31.Fattovich G, Zagni I, Minola E, Felder M, Rovere P, Carlotto A, Suppressa S, Miracolo A, Paternoster C, Rizzo C, et al. A randomized trial of consensus interferon in combination with ribavirin as initial treatment for chronic hepatitis C. J Hepatol. 2003;39:843–849. doi: 10.1016/s0168-8278(03)00391-x. [DOI] [PubMed] [Google Scholar]


