Abstract
Aims
We reported the attenuation of diabetes-induced renal dysfunction by exposure to multiple low-dose radiation (LDR) at 25 mGy every other day via suppressing renal oxidative damage. We here explored the optimal conditions of LDR to protect the kidney from diabetes.
Main methods
Type 1 diabetic mice were induced with multiple injections of low-dose streptozotocin in male C57BL/6J mice. Diabetic mice received whole body X-irradiation at dose of 12.5, 25 or 50 mGy every other day for either 4 or 8 weeks. Age-matched normal mice were similarly irradiated at the dose of 25 mGy for 4 or 8 weeks. The renal function and histopathological changes were examined at the 4th and 8th week of the study.
Key findings
Diabetes induced renal dysfunction, shown by the decreased creatinine and increased microalbumin in urinary. Renal oxidative damage, detected by protein nitration and lipid oxidation, and remodeling, reflected by increased expression of connective tissue growth factor, collagen IV and fibronectin, were significantly increased in diabetic mice. All these renal pathological and function changes in diabetic mice were significantly attenuated by exposure to LDR at all regimens, among which, however, exposure to LDR at 12.5 mGy for 8 weeks provided the best preventive effect on the kidney of diabetic mice.
Significance
Our results suggest that whole-body LDR at 12.5 mGy every other day for 8 weeks is the optimal condition of LDR to protect the kidney from diabetes.
Keywords: Low-dose radiation, Diabetic nephropathy, Renal remodeling, Renal pathogenesis, Radiation hormesis, Radiation adaptive response
Introduction
Diabetic nephropathy (DN) is the most common disorder leading to renal failure worldwide, with a high prevalence, a poor prognosis and high economic burden for diabetic patients. Although many mechanisms have been proposed to be involved in the development and progression of DN, oxidative stress is believed to play a critically initiating role in the onset and development of DN (Ha et al., 2008; Kataya and Hamza, 2008) through the aforementioned possible mechanisms (Baricos et al., 1999; Pacher et al., 2005).
Current therapies for DN predominantly include hyperglycemic control, blood pressure reduction, inflammatory response reduction, carbonaceous adsorbent, and antioxidant therapy (Bloomgarden, 2008). To date, there is no single approach to efficiently preventing or treating DN; therefore, combined use of several drugs has been explored. However, it is appreciated that most, if not all, drugs need to be metabolized in the liver and excreted via the kidney. The combined usage of drugs significantly increases the renal working load so that it will not be used for the DN patients at the late stage. Hence, new and preferably non-invasive methods which can prevent or delay the progression of DN to avoid the toxic side-effects are urgently needed.
Low-dose radiation (LDR) is extensively found to elicit hormesis and adaptive response (Luckey, 2006). Unlike high-dose radiation which generates cytotoxic, cytogenetic and inheritable effects, LDR was documented for the immune-stimulatory, anti-oxidative, and anti-inflammatory functions (Cai, 1999; Luckey, 1982). Our previous studies have shown that multiple exposures to LDR at 25 mGy significantly suppress diabetes-induced systemic and renal inflammatory response and oxidative damage, resulting in a prevention of the renal dysfunction and fibrosis (Xing et al., 2012; Zhang et al., 2009). Nomura and his colleagues also reported that continuous LDR ameliorated DN and increased life span in db/db mice through the activation of renal antioxidants (Nomura et al., 2011). These findings imply that LDR might be a novel approach for clinical treatment of DN. However, as far as application of LDR for DN therapy is considered, there will be many questions remain to be answered. For instance, what would be the optimal condition of LDR to protect the kidney from diabetes?
In the present study we have explored two important issues: (1) In previous study we exposed diabetic mice to 25 mGy every other day for 16 weeks, but there was no significant difference for the renal improvement between 8-week and 12-week LDR exposures. Can we expose animals for only 8 weeks or even shorter to reduce LDR accumulation doses without significant reduction of the renal protection from diabetes? (2) In previous studies, we used a single dose of LDR (i.e. 25 mGy), can we either reduce or increase the dose levels of LDR to more efficiently protect the kidney from diabetes? To these ends, we systemically compared the renal protection by exposure of diabetic mice to different LDR regiments, by examining the renal function, pathology and biochemical changes, examined at the 4th and 8th of LDR exposure at doses of 12.5, 25, or 50 mGy every other day.
Material and Methods
Animals
Male C57BL/6J mice, 10-week old (18–22 g of body weight), from the Jilin University Animal Center were maintained in light- (12:12-h light-dark cycle) and temperature-controlled quarters (22 °C) with rodent chow and water. Animals were kept under these conditions for ≥2 weeks before being used for the experiments. All animal protocols were approved by the University Animal Care and Use Committee, which is certified by the Chinese Association of Accreditation of Laboratory Animal Care. The body weights of mice were measured every 3 days.
Type 1 diabetes
Type 1 diabetic mice were generated with injection of multiple low doses of streptozotocin (STZ, Sigma Chemical, St. Louis, MO) at 60 mg/kg daily for 5 days with male mice to keep the gender consistence with those used in our previous studies (Xing et al., 2012; Zhang et al., 2009). At one week after the last injection blood glucose levels of mice were determined using a freestyle glucometer. Mice with fasting blood glucose ≥12 mmol/l were considered hyperglycemic.
Whole-body LDR
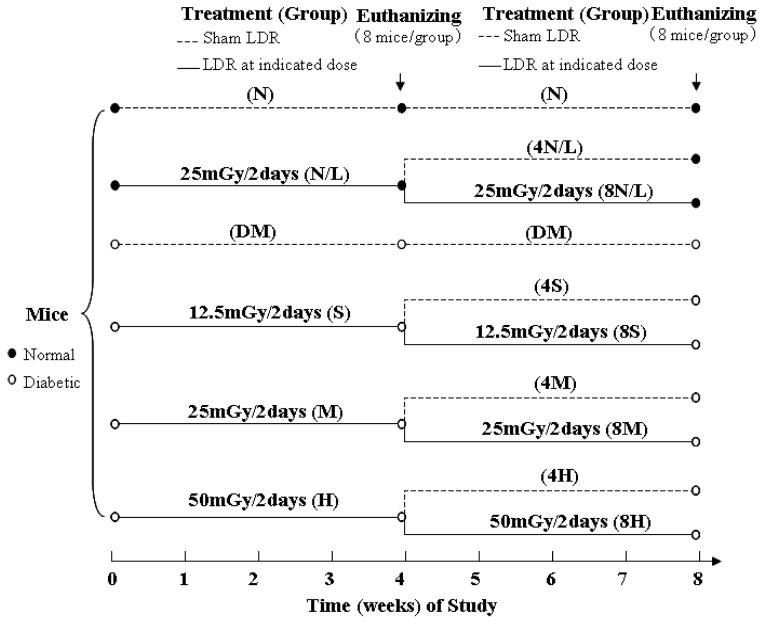
The animal groups and treatments were described in Figure 1. Briefly, mice from both diabetic and age-matched control groups were randomly divided into two groups with and without LDR: control, LDR, DM, and DM/LDR. For DM/LDR group, mice were further divided into 3 groups (n=24 mice) with multiple exposures to LDR at doses of 12.5, 25, or 50 mGy every other day. At the 4th week of exposure to LDR (or called study), 8 mice from each group were euthanized. The remaining 16 mice in each group were equally divided into 2 groups: one group was continuously given the same regimen of multiple exposures to LDR for additional 4 weeks and another group was given multiple sham exposures for 4 weeks. Then all mice were euthanized at the 8th week of the study. For mice in LDR group (labelled as N/L in the figure), the treatment was similar to that in DM/LDR group, except that the dose of LDR was 25 mGy, as shown in Figure 1.
Fig. 1. Experimental approach, and animal groups and treatments.
Mice from both diabetic and age-matched control groups were randomly divided into two groups with and without LDR. Diabetic mice in LDR group (DM/LDR) were further divided into 3 groups (n=24 mice) with multiple exposures to LDR either at 12.5, 25, or 50 mGy every other day. These 3 groups were named as S (small dose: 12.5 mGy), M (medium dose: 25 mGy) and H (high dose: 50 mGy) group, respectively. Unirradiated diabetic mice were given sham exposure, named as DM group. The control mice in LDR group received multiple exposures at dose of 25 mGy every other day, expressed as N/L (normal/LDR) group. Untreated normal mice were given sham LDR in parallel, named as N (normal) group. At the 4th week of exposure to LDR, 8 mice from each group were euthanized, and the remaining 16 mice in N/L, S, M and H groups were equally divided into 2 groups: one group was continuously given the same regimens of exposures for additional 4 weeks (named as 8N/L, 8S, 8M, and 8H, respectively), and the other group was given multiple sham exposures for 4 weeks (named as 4N/L, 4S, 4M, 4L, respectively). The mice in N and DM groups were continually given sham LDR in parallel, and still named as N and DM groups, respectively. Then all the mice were euthanized at the 8th week of the study.
Measurement for renal function
To collect urine samples, mice were individually placed in metabolic cages for 24 h prior to sacrifice under the condition of only access to tap water. The urinary microalbumin (Malb) and creatinine (Cre) contents as parameters of renal function were detected using Mouse MAU/ALB ELISA kit and Mouse UCR ELISA kit (Boster Biological Technology, Wuhan, China).
Histopathological examination
The fixed kidney tissues were cut into 3 mm thick blocks. The tissue blocks were embedded in paraffin and cut into 4 μm slices. After being deparaffinized using xylene and ethanol dilutions and rehydration, the sections were stained with hematoxylin and eosin (H & E). In addition, periodic acid Schiff (PAS) staining for the renal tissues was also performed.
Western blotting
Total protein was isolated from kidneys, and equal amounts (50 μg protein/lane) of proteins were loaded onto 12% SDS-PAGE, and proteins were transferred to PVDF membranes. The membrane was blocked with a 5% non-fat, dried milk for 1 h and then incubated with anti-mouse 3-nitrotyrosine (3-NT) antibody (1:1400, Abcam, Cambridge, MA, USA), anti-mouse 4-hydroxynonenal (4-HNE) antibody (1:50, Abcam), anti-mouse connective tissue growth factor (CTGF) antibody (1:200, Santa Cruz, CA, USA), anti-mouse collagen IV (Col IV) antibody (1:500, Abcam), and anti-mouse fibronectin (FN) antibody (1:5000, Abcam) overnight at 4 °C. Membranes were washed to remove the unbound antibodies with Tris-buffered saline (pH 7.2) containing 0.05% Tween 20 for three times, and then incubated with the secondary antibody. Antigen-antibody complexes were visualized with ECL system. The image was analyzed with Bio-Rad Quantity One.
Statistical analysis
Experimental data were collected from multiple animals under each experimental condition (n=8) and are presented as the means ± SD. Comparisons were performed by paired Student’s t-test, one-way ANOVA followed by SNK (Student-Newman-Keuls) q-test, and chi-square test for the different groups using statistical software SPSS 19.0. Differences were considered to be significant at P<0.05.
Results
Effects of LDR on blood glucose in diabetic mice
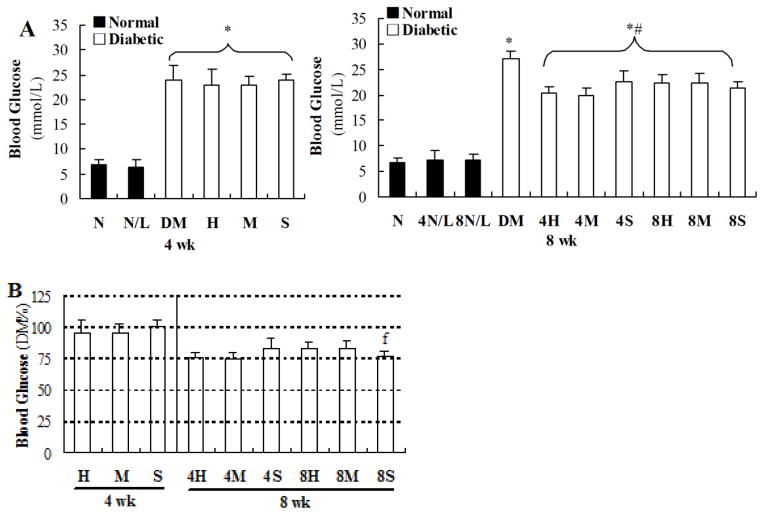
As shown in Figure 2, blood glucose levels of diabetic mice were significantly increased (≥20 mmol/l) compared to those of age-matched control mice, examined at the 4th week of the study (left panel of Figure 2A). LDR did not affect blood glucose either in normal or diabetic mice, examined at this time point. However, blood glucose levels, examined at the 8th week of the study, were significantly decreased in the mice of all DM/LDR groups, no matter exposed to LDR for only 4 weeks or 8 weeks, compared to DM mice with sham exposures for 8 weeks (P<0.05, right panel of Figure 2A). In order to easily compare effects of LDR on DM among different DM/LDR groups, we calculated the percentage of blood glucose levels of different DM/LDR groups relative to DM group’s glucose effect (Figure 2B). It clearly showed that in terms of the reduced blood glucose level, there was no effect of LDR on diabetic blood glucose level when examined at the 4th week of the study, while there was significant reduction of diabetic blood glucose levels among DM/LDR groups when examined at the 8th week of the study. It also clearly indicated that exposure to diabetic mice to 25 (4M) or 50 (4H) mGy for only 4 weeks showed a similar reductive effect to those exposed to 12.5 mGy (8S) for 8 weeks.
Fig. 2. Effects of different LDR regimens on blood glucose in normal and diabetic mice.
Blood samples of mice from each group were collected at the 4th and 8th week of the study, respectively. Blood glucose levels were measured using a freestyle glucometer (A). The blood glucose levels in different DM/LDR groups were further shown as the percentage of that in DM group to easily compare the therapeutic effect of different LDR regimens (B). “wk” indicates the time when the samples were collected and examined. *, P < 0.05 vs. N; #, P < 0.05 vs. DM; f, P < 0.05 vs. 8H.
Exposure to LDR at 12.5 mGy for 8 weeks more efficiently improved diabetes-induced renal dysfunction than other exposure regimens
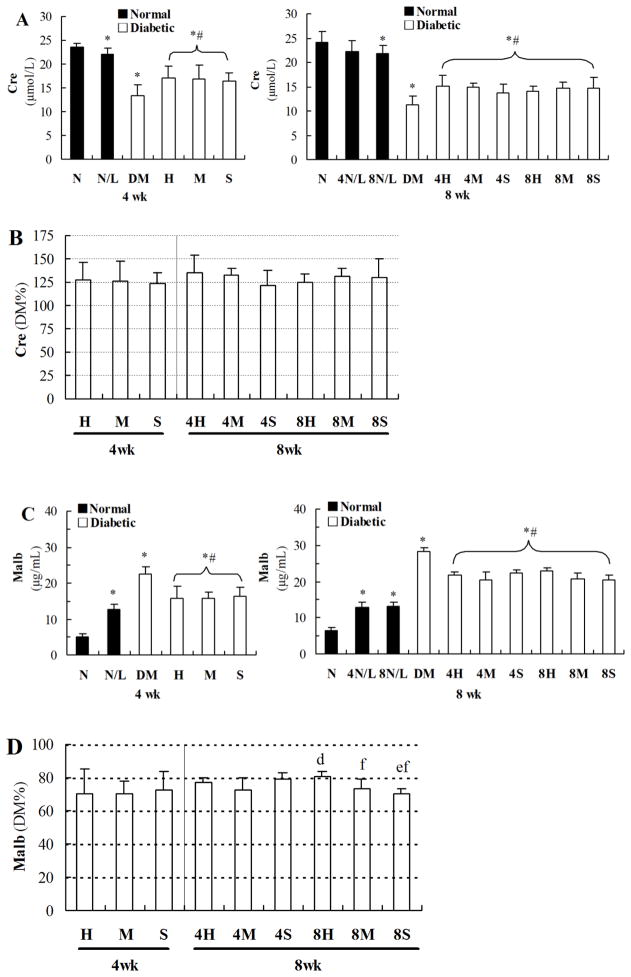
As shown in the left panel of Figure 3A, urinary Cre levels were decreased in diabetic mice, even examined at the 4th week of the study. LDR slightly decreased the level of urinary Cre in normal mice, but significantly improved diabetes-decreased Cre levels, examined at the 4th week of the study. It was noted that examined at the 8th of experiment, exposure to 25 mGy LDR for 8 weeks (8N/L), but not for 4 weeks (4N/L), slightly decreased Cre level compared to age-matched sham control (N, the right panel of Figure 3A), but significantly improved diabetes-decreased Cre levels without difference among different doses of LDR and between 4-week and 8-week exposures in DM/LDR groups (the right panel of Figure 3A). When the improvement effects of LDR on diabetes-decreased Cre levels were directly compared with their percentages relative to DM, there was no difference among the LDR groups (Figure 3B).
Fig. 3. Effects of different LDR regimens on biochemical markers in urine of normal and diabetic mice.
Urine samples of mice from each group were collected at the 4th and 8th week of the study, respectively. Cre (A) and Malb (C) levels in urine of mice were measured by ELISA method. The Cre and Malb levels in urine of different DM/LDR groups were further shown as the percentage of that in DM group (B and D, respectively). “wk” indicates the time when the samples were collected and examined. *, P < 0.05 vs. N; #, P < 0.05 vs. DM; d, P < 0.05 vs. 4M; e, P < 0.05 vs. 4S; f, P < 0.05 vs. 8H.
As shown in Figure 3C, urinary Malb was significantly and time-dependently increased in diabetic mice, examined at the 4th week (the left up-panel) and 8th week (the right up-panel) of the study, suggesting the progressed renal dysfunction. Urinary Malb was also increased in normal mice exposed to 25 mGy LDR for 4 and 8 weeks, examined at the 4th or 8th week of the study, without difference between two-time points. However, exposure to LDR for 4 or 8 weeks significantly reduced diabetes-increased urinary Malb levels, examined at the 4th or 8th week of the study. Furthermore, exposure to LDR at 12.5 mGy for 8 weeks was found to be more efficiently attenuated diabetes-increased urinary Malb than other exposure regimens (Figure 3D).
Exposure to LDR at 12.5 mGy for 8 weeks more efficiently suppressed diabetes-induced renal histopathological changes than other exposure regimens
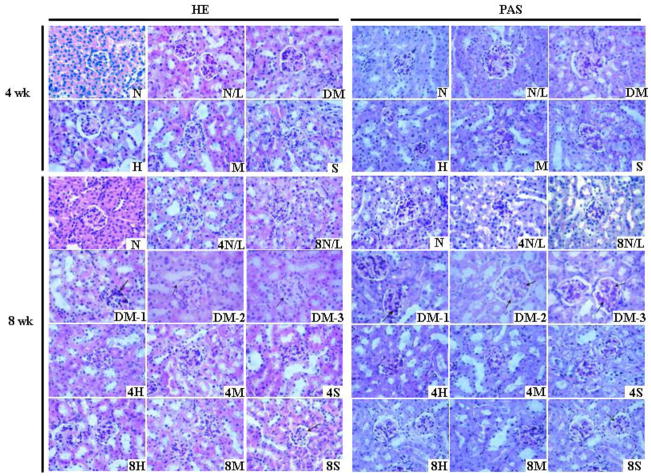
Histopathological assessment of renal sections with H&E staining showed (the left panel of Figure 4) that LDR had negligible impact on the histopathology of kidney of normal mice. These negligible impacts included rare inflammatory cell infiltration in renal interstitium, and inconspicuous mesangial cell proliferation and mesangial matrix expansion. In diabetic mice without LDR, the kidneys displayed typical pathological features of diabetic nephropathy, including increased size of glomerulus, obvious mesangial cell proliferation and mesangial matrix expansion, collapse of partial capillary, pink exudation in renal tubules, fibroplasia in interstitium and vacuolar degeneration of partial tubular epithelia. However, after treatment with any of the six LDR regimens, the diabetes-induced renal pathological changes were significantly attenuated, shown by the decreases in renal glomerulus structure damage, glomerulus size enlargement, mesangial cell proliferation, and mesangial matrix expansion. The most attenuated effect was seen in group exposed to 12.5 mGy LDR for 8 weeks.
Fig. 4. Effects of different LDR regimens on diabetes-induced renal histopathological changes.
At the 4th and 8th week of the study, renal pathology of mice from each group was examined with light microscope by H&E (left panel) and PAS (right panel) staining. In the images of H&E staining, arrows in DM-1,-2 and -3 indicate increased mesangial cells, vacuolar degeneration of renal tubular epithelial cells, and glomerular and renal capsule adhesion, respectively. Arrows in 8S indicate the glomerulus with reduced pathological change. In the images of PAS staining, arrows in DM-1,-2 and -3 indicate increased PAS-positive materials, and arrows in 8S indicated reduced deposition of PAS-positive materials. In the expression of DM-1, -2, and -3, numbers of -1, -2, and -3 indicate different images from same group to exhibit the various pathological changes. “wk” indicates the time when the samples were collected and examined.
To further observe the change of glomerular basement membrane, mesangial cell proliferation and mesangial matrix expansion, renal sections of these mice were stained by PAS. Results showed that LDR had little impact on glomerular basement membrane and mesangial matrix of normal mice, except for a slight increase of PAS-positive materials in the renal glomeruli region (the right panel of Figure 4). Diabetes significantly increased the thickness of glomerular capillary basement membrane, increased mesangial cell proliferation, and enhanced deposition of PAS-positive materials in glomeruli. These diabetes-induced changes were significantly suppressed by exposure to LDR regimens, especially exposure to LDR at 12.5 mGy for 8 weeks. This result was consistent with those observed with H&E staining, and both of these pathological changes were in a line with the renal function change.
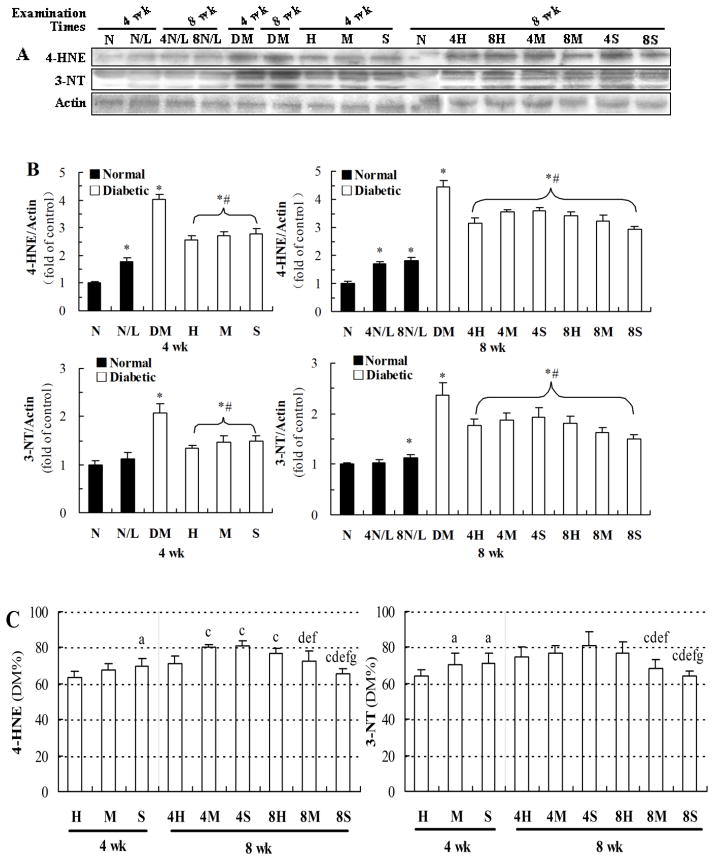
Exposure to LDR at 12.5 mGy for 8 weeks more efficiently reduced diabetes-induced renal oxidative and nitrosative damage than other exposure regimens
Our previous study had proved that LDR could suppress diabetes-induced oxidative damage (Zhang et al., 2009); therefore, renal oxidative damage was examined by western blot for oxidative and nitrosative damage markers: lipid peroxidation-related 4-HNE and protein-nitration-related 3-NT (Figure 5). Examination at the 4th week of the study showed that LDR induced increases in renal accumulation of 4-HNE, but not 3-NT, in normal mice (see gel profiles in Figure 5A, and quantitative data in the left panel of Figure 5B); however, exposure of diabetic mice to LDR for 4 weeks significantly attenuated the increases of renal accumulation of 4-HNE and 3-NT in DM/LDR compared to DM groups.
Fig. 5. Effects of different LDR regimens on the renal 3-NT and 4-HNE accumulation of normal and diabetic mice.
Renal tissues from mice exposed to different LDR regimens were collected at the 4th and 8th week of the study. The renal accumulation of 3-NT and 4-HNE was detected by western blotting (A) followed by quantitative analysis (B). The results of quantitative analysis were further shown as the percentage of DM group (C). “wk” indicates the time when the samples were collected and examined. *, P < 0.05 vs. N; #, P < 0.05 vs. DM; a, P < 0.05 vs. H; c, P < 0.05 vs. 4H; d, P < 0.05 vs. 4M; e, P < 0.05 vs. 4S; f, P < 0.05 vs. 8H; g, P < 0.05 vs. 8M.
Examination at the 8th week of exposure to LDR either for 4 weeks or 8 weeks showed that exposure to LDR for either 4 weeks or 8 weeks significantly increased renal accumulation of 4-HNE, and slightly increased 3-NT renal accumulation (only 8 week exposure) in normal mice (right panel of Figure 5B), but significantly attenuated diabetes-increased renal accumulation of 4-HNE and 3-NT.
When the effects of LDR on diabetes-increased renal accumulation of 4-HNE and 3-NT were directly compared by their preventive percentages, the most efficient attenuation was found in the group of diabetic mice exposed to 12.5 mGy for 8 weeks although exposure of diabetic mice to LDR 50 mGy for 4 weeks also provided the best prevention when examined at 4th week, but not at the 8th week (Figure 5C).
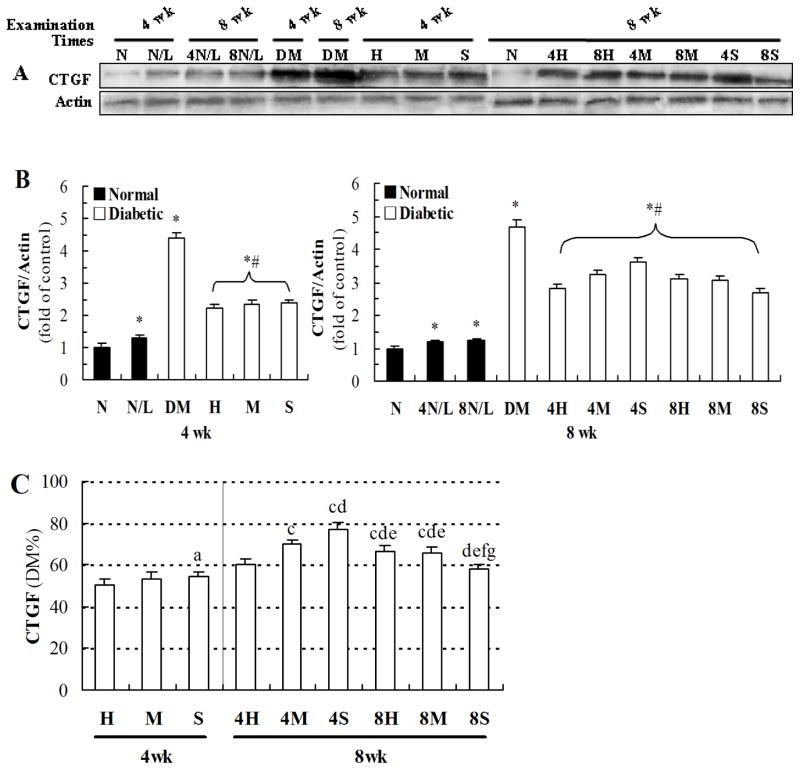
Exposure to LDR at 12.5 mGy for 8 weeks more efficiently attenuated diabetes-induced renal fibrosis than other exposure regimens
In the next study, renal fibrotic changes were examined by western blotting for CTGF, Col IV and FN. As shown in Figure 6A and B, exposure to 25 mGy for either 4 weeks or 8 weeks slightly increased renal expression of CTGF in normal mice, but significantly attenuated diabetes-increased renal expression of CTGF, not matter examined at the 4th week or 8th week. Figure 6C showed the best preventive effect should be in the groups of mice exposed to 50 mGy for 4 weeks, examined at the 4th week, but not at the 8th week. However, examination at the 8th week of the study showed that exposure to 12.5 mGy for 8 weeks and 50 mGy for 4 weeks provided the same preventive effect.
Fig. 6. Effects of different LDR regimens on diabetes-induced expression of CTGF.
Renal tissues from mice exposed to different LDR regimens were collected at the 4th and 8th week of the study. The expression level of CTGF was detected by western blotting (A) followed by quantitative analysis (B). The result of quantitative analysis was further shown as the percentage of DM group (C). “wk” indicates the time when the samples were collected and examined. *, P < 0.05 vs. N; #, P < 0.05 vs. DM; a, P < 0.05 vs. H; c, P < 0.05 vs. 4H; d, P < 0.05 vs. 4M; e, P < 0.05 vs. 4S; f, P < 0.05 vs. 8H; g, P < 0.05 vs. 8M.
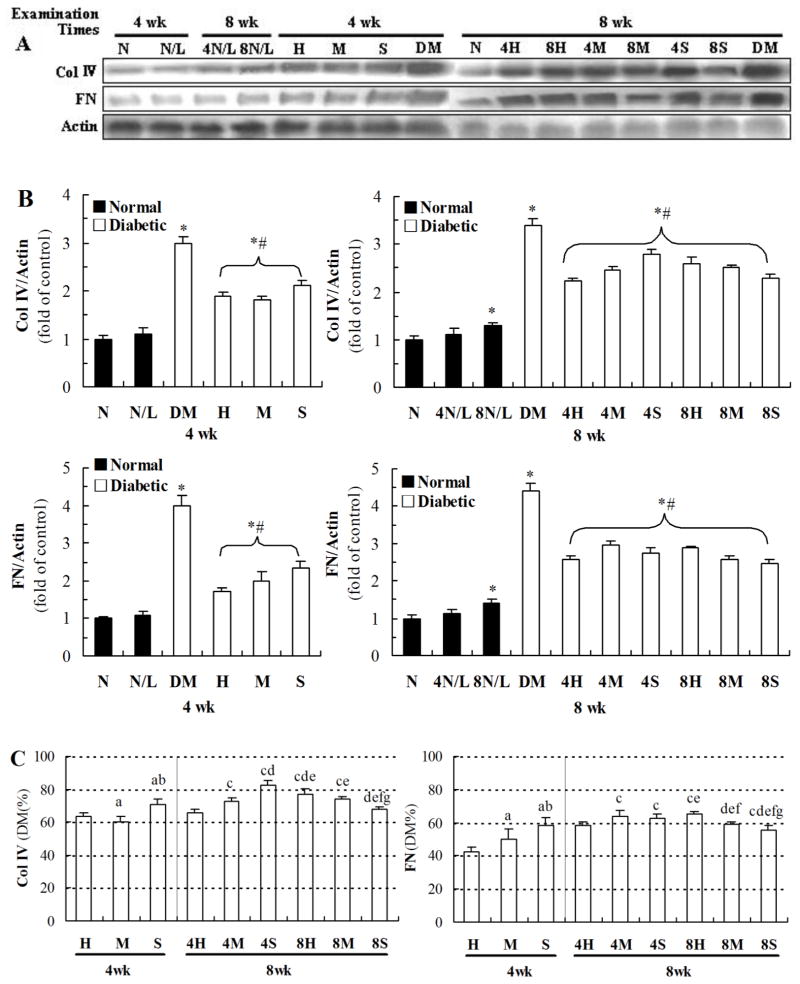
Figure 7A and B showed that exposure of normal mice to 25 mGy for 4 weeks did not affect the renal expression of Col IV and FN no matter examined at the 4th or 8th week of the study; while exposure of normal mice to 25 mGy for 8 weeks slightly increased renal expression of these two fibrotic factors. However, exposure of diabetic mice to LDR at all dose levels for either 4 weeks or 8 weeks significantly attenuated diabetes-increased renal accumulation of Col IV and FN no matter examined at the 4th or 8th week of the study. Figure 7C showed that exposure of diabetic mice to 50 mG for 4 weeks provided the best preventive effect among DM/LDR group, examined at the 4th week of the study, but provide the similar effect to that of mice to 12.5 mGy for 8 weeks, examined at the 8th week of the study.
Fig. 7. Effects of different LDR regimens on diabetes-induced expression of Col IV and FN.
Renal tissues from mice exposed to different LDR regimens were collected at the 4th and 8th week of the study. The expression levels of Col IV and FN were detected by western blotting (A) followed by quantitative analysis (B). The results of quantitative analysis were further shown as the percentage of DM group (C). “wk” indicates the time when the samples were collected and examined. *, P < 0.05 vs. N; #, P < 0.05 vs. DM; a, P < 0.05 vs. H; b, P < 0.05 vs. M; c, P < 0.05 vs. 4H; d, P < 0.05 vs. 4M; e, P < 0.05 vs. 4S; f, P < 0.05 vs. 8H; g, P < 0.05 vs. 8M.
Discussion
We have demonstrated that LDR significantly attenuates DN via suppression of renal oxidative stress and inflammation in our previous study (Zhang et al., 2009). Due to the lack of significant difference in renal improvement between 8-week and 12-week LDR exposures, and a significantly increased deleterious damage on normal mice with prolonged exposure duration, here we have systemically evaluated the preventive effects of LDR at three dose levels (12.5, 25 and 50 mGy) for two exposure times (4 weeks and 8 weeks). We found that: (1) In terms of renal function, there was no remarkable difference for the preventive effects among three doses of LDR, except exposure to 12.5 mGy for 8 weeks that showed the best effect when it was examined by Malb at the 8th week of study; (2) In terms of renal oxidative (4-HNE) and natrosative (3-NT) damage, exposure to 50 mGy for 4 weeks, examined at the 4th week of study, and exposure to 12.5 mGy for 8 weeks, examined at the 8th week of study showed a similar attenuated effect; (3) In terms of renal remodeling (fibrotic response), exposure to LDR at 50 or 25 mGy for 4 weeks showed a better preventive effect when it was examined at the 4th week, but not the 8th week of study. When compared to the attenuated effects examined at the 8th week of study, exposure to 12.5 mGy for 8 weeks showed the best prevention compared to those exposed to 25 or 50 mGy for 8 weeks and also to those exposed to LDR for 4 weeks.
DN is defined as any deleterious effect on kidney structure and/or function caused by diabetes mellitus (Musabayane, 2012); Renal fibrosis is the pathological basis of renal dysfunction in the patients with DN (Efstratiadis et al., 2009; Thomas et al., 2005), while increased oxidative stress plays an important role in fibrosis of diabetic kidney (Lee et al., 2003). Our previous studies have indicated that LDR suppressed the renal fibrosis of diabetic mice through inhibiting diabetes-induced oxidative damage (Zhang et al., 2009). In the present study, therefore, we have focused on the comparisons for oxidative and nitrosative damage and remodeling (fibrotic response and pathological examination) with renal function among groups with different exposure conditions. We demonstrated that the change patterns of oxidative damage markers (3-NT and 4-HNE) were similar to the changes of urinary Malb among groups, with the most effective inhibition of 3-NT and 4-HNE and urinary Malb levels by exposure of diabetic mice to 12.5 mGy for 8 weeks.
The protective effect of LDR on DN in the current study is related to the LDR-induced radio-adaptive response. It has been reported that there was a certain dose windows for the adaptive response in mammalian cells and mammals (Mitchel, 2010). In a couple studies, Ina and Sakai found that exposure to immune compromised mice at 0.35 mGy/h was less effective in prolongation of lifespan than exposure at 1.2 mGy/h, and lifelong low-dose-rate irradiation was more effective than exposure for 5 weeks (Ina and Sakai, 2004; 2005), which indicate that there should be a low-dose threshold below which the protective effect/ radio-adaptive response is not inducible; however, when the dose, no matter at a single dose or accumulated dose of multiple exposures, exceeds the lower dose threshold, the protective effect will be induced in a dose-dependent manner until the protective effect reaches to the maximal level when the dose is optimal. Then the protective effect will decrease with the further increased radiation dose. After the radiation dose reaches to the upper dose threshold, the protective effect will disappear. The upper single dose threshold for adaptive response that protected the mice from radiation-induced myeloid leukemia was about 100 mGy (Mitchel et al., 1999). In the current study, our results indicate that exposure to 50 mGy was more effective than other dose groups at 4th week; while at 8th week the most protective effect was observed in the group received 8-week exposure to 12.5 mGy. This might be because the accumulated doses of all of three dose groups were lower than optimal dose at 4th, when the protective effect was proportionate to the accumulated doses. However, with the increasing duration of exposure, the accumulated dose of high dose group (50 mGy), even the middle dose group (25 mGy), exceeded the optimal dose; while the accumulated dose of small dose group (12.5 mGy) was much close to the optimal dose, which lead to the result that the most protection was observed in the accumulated doses at 8th week. In addition, in order to investigate whether the protective effect could be kept after the LDR treatments was stopped, we designed a set of groups that were exposed for 4 weeks but sacrificed at 8th week. Our result showed that the protective effect induced by LDR could be observed 4 weeks after radiation cease. Actually, previous study had shown that the radio-adaptive response even could affect humans with respect to lifetime cancer incidence (Leonard, 2007).
Development of DN in people with diabetes is associated with higher blood glucose levels that may cause renal oxidative damage (Cade, 2008). Treatment of hyperglycemia is a basic approach for the prevention of DN (Gross et al., 2005). LDR may induce production of pancreatic antioxidants including superoxide dismutase, glutathione, and catalase to preserve pancreatic β-cell function (Wang et al., 2008; Yamaoka et al., 2004). In the present study, we demonstrated that exposure to LDR at 12.5, 25 or 50 mGy for 4 or 8 weeks significantly reduced the blood glucose of diabetic mice, examined at the 8th week but not the 4th week of the study. Similarly, several other studies also exhibited that LDR could reduce glucose levels in diabetic mouse or rat models. Takehara et al indicated that a single WB-LDR at dose of 500 mGy prevented alloxan-induced hyperglycemia in rats (Takehara et al., 1995) and NOD mice (Takahashi et al., 2000); however LDR at 1000 mGy had no effect on blood glucose (Takehara et al., 1995). Treatment with 500 mGy of irradiation did not change the blood glucose level in normal mice. Contrary to these studies, Tsuruga et al showed that blood glucose level was affected only marginally by LDR in diabetic mice (Tsuruga et al., 2007). These inconsistent results might be because the mice were continuously irradiated in an irradiation room (0.94 mGy/h), resulting in a relative higher accumulated doses. In this sense, it was consistent with Takehara’s finding that LDR at 1000 mGy had no effect on blood glucose. In addition, mice that were exposed to low-dose-rate γ radiation at 0.63 mGy/h from the age of 10 weeks until the end of their lives did not show significant decrease of blood glucose either (Nomura et al., 2011). Therefore, these studies indicated the different degree decreases in blood glucose; however, the decreased glucose levels may be not the major contributor to the renal protection from diabetes since significant renal protection by chronic exposure to life time was not accompanied a significant reduction of blood glucose (Nomura et al., 2011). In support of this finding, we demonstrated here that the renal protection by LDR from diabetes in the mice exposed to 4-week LDR could be found in terms of renal function, oxidative damage and remodeling, examined the 4th week of study, a time point when there was no significant decrease in blood glucose.
Our previous study showed that LDR at 25 mGy every other day significantly improved diabetes-caused urinary Malb and Cre levels (Zhang et al., 2009), but it also slightly produced deleterious effect in normal mice. Consistent with our previous study, here we remain demonstrated the mild toxic effect of exposure to 25 mGy either for 4 weeks or 8 weeks, shown by very slight decrease in Cre and increase in Malb. Furthermore, the increased Malb was irreversible since the increased level of Malb was similar when it was examined at the 4th and 8th week of study in normal mice exposed to 25 mGy for 4 weeks, suggesting that the damage in normal mice caused by 4-week exposure to 25 mGy, observed immediately after exposure, could last another 4 weeks without significant recovery. In parallel with functional changes, renal oxidative damage showed a similar pattern to functional changes. Both functional and biochemical measurements indicate the potential side toxic effect of 25 mGy for either 4 weeks or 8 weeks. However, whether these side effects really contribute certain carcinogenesis remains unclear. It is reported that when db/db mice were continuously exposed to low-dose-rate γ radiation at 0.63 mGy/h from the age of 10 weeks until the end of their lives, the life span of irradiated mice was significantly prolonged. No tumor incidence was noted in the organs of dead mice from any of the irradiated or control group, suggesting that continuous low-dose-rate irradiation at 0.63 mGy/h even for the entire life has no oncogenic effect (Nomura et al., 2011). Our results showed that, although all LDR regimens improved diabetes-induced renal dysfunction, LDR at 12.5 mGy for 8 weeks exhibited the most effective in preventing the kidney from diabetes than other LDR regimens. However, we did not include the normal mice exposed to 12.5 mGy LDR for either 4 or 8 weeks in the current study, which is a limitation of the present study and will be warranted in the future study. Therefore we do not know whether exposure of normal mice to 12.5 mGy for 8 weeks generates side toxic effects or not, but it is expected to be significantly reduced compared to 25 mGy exposure. Whether we can further decrease the LDR dose that remains effectively in preventing of diabetic kidney remains unclear, but it cannot be reduced to 6.25 mGy since we did not find any impact of it on diabetic pathological and functional changes (Data not shown).
Conclusions
In summary, our present study continually supports the hypothesis that LDR can delay the progression of diabetic nephropathy, as proposed in our previous study (Zhang et al., 2009). We also clarified here that when compared to the renal protection by LDR from diabetes, examined at the 8th week of the study, exposure to 12.5 mGy for 8 weeks showed the best preventive effect among those exposed to 25 and 50 mGy for 8 weeks and also to those exposed to LDR for 4 weeks, in terms of biochemical, pathological and functional changes. Therefore, LDR as a non-invasive treatment modality is able to avoid the accumulation of drug-derived toxic metabolic intermediates in the body since these intermediates are a big disadvantage associated with the use of medications in the treatment of diabetic patients suffering from late stage renal dysfunction. As stated by Pathak and Khanduja, “There is a need to evaluate the application of noninvasive technology like whole body (WB)-LDR to be as realistic and parallel as is done for other therapeutic modalities. If WB-LDR really can play a critical role in the prevention or treatment of certain disorders such as diabetes, then we should accept the same without any radiation phobia in the mind.” (Pathak and Khanduja, 2010).
Acknowledgments
This study was supported in part by grants from the National Science Foundation of China (81071920, to WL; 81202151, to FL), Ministry of Education Key Project of Science and Technology (311015, to JC), and the National Institutes of Health (1R01DK 091338-01A1, to LC).
Footnotes
Declaration of Interest
The authors report no declarations of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J Am Soc Nephrol. 1999;10:790–5. doi: 10.1681/ASN.V104790. [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT. Diabetic nephropathy. Diabetes Care. 2008;31:823–7. doi: 10.2337/dc08-zb04. [DOI] [PubMed] [Google Scholar]
- Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–35. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L. Research of the adaptive response induced by low-dose radiation: where have we been and where should we go? Hum Exp Toxicol. 1999;18:419–25. doi: 10.1191/096032799678840291. [DOI] [PubMed] [Google Scholar]
- Efstratiadis G, Divani M, Katsioulis E, Vergoulas G. Renal fibrosis. Hippokratia. 2009;13:224–9. [PMC free article] [PubMed] [Google Scholar]
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 (Suppl 1):S42–5. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice. Radiat Res. 2004;161:168–73. doi: 10.1667/rr3120. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Further study of prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole-life irradiation. Radiat Res. 2005;163:418–23. doi: 10.1667/rr3316. [DOI] [PubMed] [Google Scholar]
- Kataya HA, Hamza AA. Red Cabbage (Brassica oleracea) Ameliorates Diabetic Nephropathy in Rats. Evid Based Complement. Alternat Med. 2008;5:281–7. doi: 10.1093/ecam/nem029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241–5. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Adaptive response: Part II. Further modeling for dose rate and time influences. Int J Radiat Biol. 2007;83:395–408. doi: 10.1080/09553000701326995. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43:771–89. doi: 10.1097/00004032-198212000-00001. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Radiation hormesis: the good, the bad, and the ugly. Dose Response. 2006;4:169–90. doi: 10.2203/dose-response.06-102.Luckey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel RE. The dose window for radiation-induced protective adaptive responses. Dose Response. 2010;8:192–208. doi: 10.2203/dose-response.09-039.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–9. [PubMed] [Google Scholar]
- Musabayane CT. The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc J Afr. 2012;23:462–8. doi: 10.5830/CVJA-2012-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Li XH, Ogata H, Sakai K, Kondo T, Takano Y, et al. Suppressive effects of continuous low-dose-rate gamma irradiation on diabetic nephropathy in type II diabetes mellitus model mice. Radiat Res. 2011;176:356–65. doi: 10.1667/rr2559.1. [DOI] [PubMed] [Google Scholar]
- Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–75. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak CM, Khanduja KL. Letter to the editor: Low-dose whole body irradiation: a potential therapeutic modality? Am J Physiol Endocrinol Metab. 2010;299:E137. doi: 10.1152/ajpendo.00247.2010. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kojima S, Yamaoka K, Niki E. Prevention of type I diabetes by low-dose gamma irradiation in NOD mice. Radiat Res. 2000;154:680–5. doi: 10.1667/0033-7587(2000)154[0680:potidb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Takehara Y, Yamaoka K, Hiraki Y, Yoshioka T, Utsumi K. Protection against alloxan diabetes by low-dose 60Co gamma irradiation before alloxan administration. Physiol Chem Phys Med NMR. 1995;27:149–59. [PubMed] [Google Scholar]
- Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:177–86. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Tsuruga M, Taki K, Ishii G, Sasaki Y, Furukawa C, Sugihara T, et al. Amelioration of type II diabetes in db/db mice by continuous low-dose-rate gamma irradiation. Radiat Res. 2007;167:592–9. doi: 10.1667/RR0786.1. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Li XK, Sakai K, Lu C. Low-dose radiation and its clinical implications: diabetes. Hum Exp Toxicol. 2008;27:135–42. doi: 10.1177/0960327108090752. [DOI] [PubMed] [Google Scholar]
- Xing X, Zhang C, Shao M, Tong Q, Zhang G, Li C, et al. Low-dose radiation activates Akt and Nrf2 in the kidney of diabetic mice: a potential mechanism to prevent diabetic nephropathy. Oxid Med Cell Longev. 2012;2012:291087. doi: 10.1155/2012/291087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K, Mitsunobu F, Hanamoto K, Shibuya K, Mori S, Tanizaki Y, et al. Biochemical comparison between radon effects and thermal effects on humans in radon hot spring therapy. J Radiat Res. 2004;45:83–8. doi: 10.1269/jrr.45.83. [DOI] [PubMed] [Google Scholar]
- Zhang C, Tan Y, Guo W, Li C, Ji S, Li X, et al. Attenuation of diabetes-induced renal dysfunction by multiple exposures to low-dose radiation is associated with the suppression of systemic and renal inflammation. Am J Physiol Endocrinol Metab. 2009;297:E1366–77. doi: 10.1152/ajpendo.00478.2009. [DOI] [PubMed] [Google Scholar]