Abstract
Liver transplantation is believed to reverse the clinical and metabolic abnormalities of cirrhosis. Reduced skeletal muscle mass or sarcopenia contributes to increased mortality and adverse consequences of cirrhosis. Failure of reversal of sarcopenia of cirrhosis after liver transplantation is not well recognized. Six temporally, geographically, and methodologically distinct follow-up studies in 304 cirrhotics reported conflicting data on changes in indirect measures of skeletal muscle mass after transplantation. Distinct measures of body composition but not skeletal muscle mass were used and did not focus on the clinical consequences of sarcopenia after transplantation. A number of studies reported an initial rapid postoperative loss of lean mass followed by incomplete recovery with a maximum follow-up of 2 years. Posttransplant sarcopenia may be responsible for metabolic syndrome and impaired quality of life after liver transplantation. Potential reasons for failure to reverse sarcopenia after liver transplantation include use of immunosuppressive agents [mammalian target of rapamycin (mTOR) and calcineurin inhibitors] that impair skeletal muscle growth and protein accretion. Repeated hospitalizations, posttransplant infections, and renal failure also contribute to posttransplant sarcopenia. Finally, recovery from muscle deconditioning is limited by lack of systematic nutritional and physical-activity-based interventions to improve muscle mass. Despite the compelling data on sarcopenia before liver transplantation, the impact of posttransplant sarcopenia on clinical outcomes is not known. There is a compelling need for studies to examine the mechanisms and consequences of sarcopenia post liver transplantation to permit development of therapies to prevent and reverse this disorder.
Keywords: Posttransplant sarcopenia, Cirrhosis, Liver transplantation, Immunosuppressants
Introduction
Liver transplantation is believed to be curative therapy for cirrhosis, and complications such as ascites, encephalopathy, variceal bleeding, hepatorenal syndrome, and pulmonary defects reverse after transplantation [1–4]. Malnutrition and its component, sarcopenia or loss of skeletal muscle mass, is the most common adverse consequence of cirrhosis [5–8]. Reversal of malnutrition after transplantation has not been examined critically, but there are sufficient data to suggest that, unlike other complications of cirrhosis that reverse after transplantation, sarcopenia may not [9]. Health-related quality of life (HRQOL) after liver transplantation continues to be impaired and contributes to the economic burden of cirrhosis and liver transplantation [10]. Malnutrition-related reduced HRQOL has been suggested to be due to inability to return to work, which itself is related to poor physical functioning and overall poor health [1, 10–14]. Even though they have not been specifically examined in patients with cirrhosis, reduced skeletal muscle mass and strength have been consistently associated with reduced quality of life in a number of disorders [15–17]. Therefore, reduction in or failure to regain skeletal muscle mass after liver transplantation is likely to have health-related and economic consequences. A recent comprehensive review showed limitation in exercise capacity following transplantation, and one of the potential reasons suggested was failure to reverse muscle loss after transplantation [18]. Posttrans-plant obesity and metabolic syndrome are common after transplantation [19]. Sarcopenia is also more prevalent in patients with different components of the metabolic syndrome [20, 21]. Hence, changes in skeletal muscle mass following liver transplantation may contribute to the reduced quality of life as well as the development of posttransplant metabolic syndrome. Since most studies to date have not specifically addressed post liver transplantation sarcopenia, a comprehensive review was performed to examine the current state of knowledge in this area. The primary focus of the present review is to determine whether the skeletal muscle loss that has been reported to affect over 60 % of cirrhotic patients [22, 23] was reversed after transplantation. Since there are no data on direct measures of skeletal muscle mass, indirect measures using lean body mass, and anthropometric measures of arm muscle area were examined.
Methods
Publications that referred to nutritional status after liver transplantation were evaluated. The search terms included: post liver transplant nutrition, sarcopenia after liver transplantation, malnutrition after liver transplantation, body composition after liver transplantation, nutritional status after liver transplantation, and skeletal muscle after liver transplantation. Medline, Ovid, and Google scholar search engines were used with no time limits. The last date of search was May 2013. A total of seven prospective studies [24–30], one retrospective study each in adults [31] and children [32], and two cross-sectional studies [33, 34] that evaluated the specific issue of nutrition and body composition changes after liver transplantation had been published. In six prospective studies that were included in this review, the data were of high quality as defined by prospective follow-up human studies, sequential comparisons of body composition before and after liver transplantation, and high retention with low dropout rates [24–29]. In one of the prospective studies, follow-up of body composition was not the primary aim [25]. In the other prospective study, protein calorie malnutrition was defined by an index using a percentage adequacy from reference values for both fat and muscle mass determined by anthropometry. Sequential data for muscle mass or muscle area were not provided, and data were presented as percentages of subjects who were eutrophic or had mild and moderate/severe protein calorie malnutrition [30]. Since it was not possible to determine whether muscle loss did indeed occur at the cost of gaining fat mass, this study [30] was not used for analyses or interpretation. The retrospective study used nutritional assessment data of a nonrandom subgroup of patients who had nutritional evaluations done for at least 180 days after transplantation, and the data were presented mostly as a ratio compared with expected values, so this study was also not used in this review [31]. The study in children used only anthropometric measures, the etiology was fulminant hepatitis in the majority, and the outcome measures included gain in height and body composition [32]. Despite these limitations, this study was included because other studies in children after liver transplant examined growth and height patterns only and did not consider body composition. Heterogeneous tools were employed to measure body composition, including anthropometry, bioelectrical impedance analysis, dual-energy X-ray absorptiometry (DEXA), in vitro neutron activation analysis, and total body potassium. There were two cross-sectional studies that were not of high quality because they did not examine the impact of liver transplantation on body composition or muscle loss on serial evaluation [33, 34]. Another cross-sectional study of body composition using DEXA in 17 children did not have a control group and used reference standards [35]. Other studies that reported posttransplant obesity were not included since their focus was not on skeletal muscle mass or sarcopenia.
Results
A summary of the data from the studies included in this review is shown in Table 1. Data from four longitudinal studies using paired DEXA, total body potassium, bio-electrical impedance, and skinfold anthropometric measures showed that the increase in body weight after liver transplantation was primarily due to an increase in body fat [24–26, 29]. Furthermore, there was a significant loss of lean body mass that continued for 9 months after transplantation, and there was no significant increase in lean weight for up to 24 months [24]. Low serum albumin pre transplantation, use of high-dose steroid immunosuppression regimens, and longer hospitalization after transplantation were risk factors for a reduction in lean body mass after liver transplantation in one study [24]. No comments were made regarding posttransplant recurrent disease or renal failure despite all patients being on triple immunosuppressive regimen of corticosteroids, azathioprine, and a calcineurin inhibitor. This was followed by a study in 41 subjects from Australia that evaluated body composition as part of quantifying bone density before and after transplantation. There was a lack of appreciable loss of lean body mass before transplantation in cirrhosis and a gain in total and fat mass after transplantation. This was accompanied by a 3.3 ± 0.8 kg reduction in lean body mass in the legs and trunk with a greater loss of lean mass in men than women [25]. Once again, the impact of etiology of liver disease, type of immunosuppression, and renal dys-function post transplant on loss of lean body mass was not reported. A subsequent detailed study in 14 patients undergoing liver transplantation who underwent sequential body composition analysis showed that total body protein content was significantly lower than the pre-illness protein content and decreased further in the immediate posttrans-plantation period [26]. Maximum loss of whole body protein was observed during the first 10 days after transplantation and was estimated to be 0.99 ± 0.12 kg (~1 % total body protein/day). After this period, the total body protein content gradually increased with a total gain of 0.53 ± 0.24 kg (~54 % of total lost after transplantation) by 12 months after transplantation [26]. Loss of total body protein due to cirrhosis was estimated to be ~18.5 % of the pre-illness total body protein. Further loss of skeletal muscle protein occurred in the first 10 days after transplantation, followed by a slow but incomplete recovery pattern between 10 and 90 days after transplantation with no further gain in skeletal muscle protein content. Changes in skeletal muscle protein content were accompanied by a similar pattern of increase in skeletal muscle mass that started after day 10 and continued only to day 90. These changes were accompanied by improvement in muscle strength measured by grip strength that improved after transplantation and recovered by 3 months, reaching predicted normal values by 12 months. Respiratory muscle strength also improved significantly by 12 months but remained below that predicted for these patients [26].
Table 1.
Published data on changes in body composition after liver transplantation
| Author (ref.) | Hussaini [24] | Keogh [25] | Platnk [26] | Krasnoff [27] | Krasnoff [28] | Merli [29] | Wagner [33] | Schutz [34] |
|---|---|---|---|---|---|---|---|---|
| Number of subjects | 55 | 41 | 14 | 50 | 119 (of 151 included initially) | 25 | 71 | 42 |
| Study type | Longitudinal | Longitudinal | Longitudinal | Longitudinal | Longitudinal | Longitudinal | Cross-sectional | Cross-sectional |
| Duration of follow-up after transplant (months) | 24 | 19.3 ± 2 (3–44) | 12 | 24 | 12 | 12 | Up to 120 | 17.7–100.6 |
| Body composition measures | DEXA, TBK | DEXA | IVNAA, DEXA, BIA, TBK, grip strength, respiratory muscle function | DEXA, quadriceps muscle strength, physical activity scale, step counter | DEXA, quadriceps muscle strength | Anthropometry | BIA | BIA, BMI |
| Outcome | Reduction in muscle mass, gain in fat mass | Loss of lean body mass with gain in fat mass | Reduction in muscle mass by 10 days but did not return to pretransplant values, fat mass returned to pretransplant values by 3 months | Increase in muscle mass in men for 24 months, but in women no change between 2 and 24 months after transplantation | Increase in lean body mass over time in exercise group but not in usual care group, increase in fat mass and muscle strength in all groups | No change in muscle mass, increase in fat mass by 12 months | No significant difference in phase angle | Higher fat mass and fat-free mass after transplantation |
BIA bioelectrical impedance analysis, BMI body mass index, DEXA dual-energy X-ray absorptiometry, IVNAA in vitro neutron activation absorptiometry, TBK total body potassium
In another study, body composition was determined by anthropometric measurements in 25 patients evaluated sequentially after liver transplantation [29]. This study reported a significant improvement in dietary intake by 3 months that persisted to 1 year after surgery. Three months post liver transplantation, whole body weight and triceps skinfold thickness were lower while mid-arm muscle circumference showed a nonsignificant reduction compared with pretransplant measurements. In the subsequent 9 months, body weight and triceps skinfold thickness improved to pretransplant values. Muscle circumference, however, did not show significant improvement. It was interesting that these authors did not observe hypermetabolism in these patients, but the estimated contribution of carbohydrates and fat to total energy changed after transplantation (carbohydrate from 54 to 47 % and fat from 31 to 35 %, pretransplant and at 12 months after transplantation) [29]. A study that examined the nutritional status by anthropometry in 31 consecutive cirrhotic patients at two time points, one 6 months before and another after liver transplantation, by anthropometry reported that nutritional parameters assessed by anthropometry did not show significant differences [36]. A French study that prospectively evaluated nutritional status as the percentage of patients with different degrees of malnutrition without separating changes in fat and fat-free mass showed that noncirrhotic patients had more severe malnutrition than those with cirrhosis at 1 year after transplantation [30].
However, two reports from a single center reported conflicting results in recipients of liver transplantation [27, 28]. In a large cohort of subjects (n = 169) an increase in lean body mass by DEXA was reported over periods ranging from 12 to 24 months. However, it was interesting that, in the initial report, an increase was reported in males while in female liver transplant recipients no change was observed between 2 and 24 months [27]. In a follow-up study to examine the impact of dietary counseling and exercise compared with usual care, the same group reported no significant change in lean body mass over time in patients who were not in the exercise–nutritional counseling group [28].
Two cross-sectional studies have been reported [33, 34]. In 71 liver transplant recipients studied at discrete time points beyond 10 years after transplantation, the prevalence of malnutrition identified by phase angle on bio-electrical impedance analysis (BIA) was significantly greater in the first 5 years after transplantation than thereafter [33]. A subsequent cross-sectional study from Germany reported an increase in sarcopenic obesity (loss of skeletal muscle mass with an increase in fat mass) after liver transplantation (n = 42) compared with a cohort of patients with cirrhosis who had not undergone transplantation [34]. Whole body weight, fat mass, and body cell mass measured by bioelectrical impedance analysis were higher at 50 (17.7–100.6) months. These authors also reported that nontransplanted cirrhotics were hypermetabolic while posttransplant resting energy expenditure was similar to predicted.
The study in children showed that anthropometric measures of muscle and fat mass increased after liver transplantation [32]. A careful analysis of the data however showed that about a third of children had failed to maintain growth. The authors did not identify differences between children with and without changes in body composition.
Discussion
Recovery of the metabolic and clinical consequences of cirrhosis is believed to be universal after liver transplantation [1, 11]. Following liver transplantation, even though the metabolic complications including changes in amino acid profile and hormonal changes of cirrhosis [37, 38] reverse, body composition did not improve in three well-conducted follow-up studies [24, 26, 29] and in one study where body composition was a secondary analysis [25]. In the retrospective study in adults, body weight and serum albumin improved [31]. Posttransplant weight gain has been consistently shown to be related to increased fat mass and obesity [39–44]. Since skeletal muscle contributes about 45–50 % of lean body mass in humans, a reduction in lean body mass is reflective of loss of muscle mass but may not be a precise measure of skeletal muscle loss [45]. In contrast, in two studies from a single center, lean body mass and muscle strength increased after transplantation primarily in males [27, 28]. These effects may be related to systematic differences in the patient population studied, indications for liver transplantation, and use of the Model for End-Stage Liver Disease (MELD) scoring system that altered the definition of severity of liver disease. It is interesting that all four studies reporting worsening loss of muscle mass or lean body mass were from Europe [24, 26, 29, 46] or Australia [25] while those reporting an increase in lean body mass were from a single center in the USA [27, 28]. The data in children that showed improvement in muscle area could not be compared with those in adults because the underlying liver disease and the responses in children are different from in adults.
Critical analyses of these data suggest that, in the majority of cirrhotic patients, the immediate postoperative period is associated with a further reduction in lean body mass. This period can range from 10 to 90 days, following which there is a compensatory recovery phase. Recovery is more robust for fat mass than for skeletal muscle and lean body mass. The posttransplant recovery of lean body mass reported by the majority of authors has been suboptimal and does not reach the premorbid levels [24–26, 29, 46]. Thus, loss of lean body mass is consistent prior to liver transplantation and worsens in the early posttransplant period; the recovery of protein synthesis is impaired, resulting in long-term persistence of the low muscle mass. These are supported by the retrospective study where patients were evaluated at two different time points after transplantation. In the two studies that reported an increase in lean body mass post transplantation from a single center, it is interesting that the focus was on exercise-mediated gain in lean body mass [27, 28]. In contrast, the other centers that published data on a loss of lean body mass did not report on posttransplant exercise or specific nutritional recommendations. A number of studies have examined the impact of nutritional interventions on the immediate postoperative period [47]. The Cochrane review did not find any benefit of nutritional interventions in the immediate postoperative period. Their role in the management of the posttransplant period has not been evaluated carefully. There may be a beneficial role for perioperative branched-chain amino acid supplementation in improving immediate post liver transplant prealbumin and substrate utilization [48]. The role of amino acid supplementation in the long-term management of the posttransplant patient needs careful assessment.
Data from these studies are of direct clinical significance for a number of reasons. The clinical compartmentalization of the body into fat, skeletal muscle, and bone mass is relevant since the adverse metabolic and clinical consequences of obesity are related to increased fat mass. Weight gain after transplantation has been now considered to be primarily in the adipose tissue compartment with loss in the skeletal muscle and bone compartment [49–60]. Reduction in muscle mass and muscle strength are associated with reduced survival especially in cirrhotics [6, 61, 62]. Few studies have examined the mechanisms of sarcopenia in cirrhosis [63–66], but no studies have evaluated the possible reasons for failure to regain skeletal muscle mass after transplantation. Since HRQOL has been shown to be persistently impaired after transplantation in a number of studies [10–13], the contribution of sarcopenia to this major patient-centric outcome measure is not known. Finally, there are ongoing studies to understand the mechanisms of sarcopenia in cirrhosis and portosystemic shunting [63, 67, 68], but there is a complete absence of any data on the mechanisms of posttransplant sarcopenia. There are recent data that a skeletal muscle secreted protein, irisin, drives brown-fat-like transformation of white adipose tissue, thereby increasing thermogenesis and potentially improving cardiometabolic risk factors in obesity [69]. This increases interest in studying muscle metabolism and biology with the goal of reducing post-transplant metabolic syndrome. It must also be reiterated that, despite median patient survival post transplant exceeding 10 years, the longest reported follow-up for nutritional assessment is only for a maximum of 2 years and the delayed responses are currently not clear.
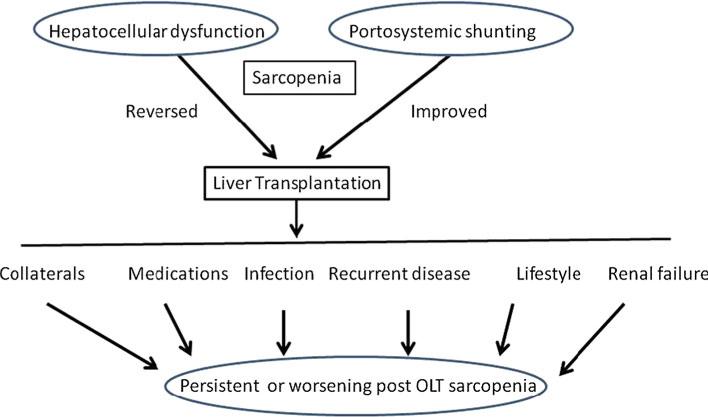
Despite a number of descriptive studies, mechanisms that contribute to posttransplant sarcopenia have not been evaluated carefully. One suggestion is that the elevated resting energy expenditure in cirrhotics persists after liver transplant [70, 71]. Persistence of hypermetabolism after transplantation is one potential contributor to posttrans-plant sarcopenia. Use of immunosuppressive agents that alter skeletal muscle protein metabolism [72–75] prevents recovery of skeletal muscle mass after transplantation. Corticosteroids increase proteolysis and impair protein synthesis. Myostatin, a potent inhibitor of muscle protein synthesis and regeneration, may be one mediator of the effects of steroids [76, 77]. Calcineurin inhibitors including tacrolimus and cyclosporine may also contribute to impaired muscle growth in response to anabolic stimuli [75, 78]. Cyclosporine has been reported to decrease skeletal muscle mitochondrial function in rats [79]. Tacrolimus has been reported to increase energy expenditure [80] and hypermetabolism, resulting in an impaired recovery pattern of skeletal muscle mass after transplantation. Immunosuppressants contribute to the risk of post-transplant infections and sepsis, which result in increased proteolysis and impaired protein synthesis [81]. Prevalence of posttransplant renal failure and renal impairment are increasing due to the use of calcineurin inhibitors [82]. Renal-sparing approaches, however, necessitate use of mTOR inhibitors that induce anabolic resistance and contribute to reduced muscle mass [83]. Renal failure, calcineurin inhibitors, and mTOR inhibitors all contribute to reduced muscle mass. Finally, increased physical activity after liver transplantation has been shown to be effective in improving quality of life and decreasing metabolic and orthopedic complications [84]. Even though increased physical activity and exercise have been shown to be beneficial in improving body composition and lean body mass after renal and heart transplantation, there are limited data on the impact of regular exercise on outcomes after liver transplantation [18, 28, 85–87]. Cross-sectional studies suggest persistently impaired exercise capacity after transplant [18]. These data suggest that, despite the large number of liver transplants performed in the USA and globally, little has been done to reverse the adverse skeletal muscle consequences. Reversal of posttransplant sarcopenia is likely to reduce the risk of posttransplant metabolic syndrome and consequent adverse outcomes in these patients [19]. Other contributors to post transplant sarcopenia include development of posttransplant infections, hospitalization, posttransplant renal failure, and insulin resistance (Fig. 1). Even though many of these are a direct consequence of potent immunosuppressive agents, disease recurrence in the graft can also contribute to persistence or worsening sarcopenia. However, rejection has not been reported to contribute to loss of lean body mass [46]. Of the available interventions to reverse reduction in muscle mass, exercise and nutraceutical amino acid supplementation using leucine-enriched mixtures hold the most potential. Increasing recognition that exercise and leucine can have beneficial effects on body composition by their regulation of mTOR signaling [88] suggests that these need to be evaluated carefully in this population with the most need for increasing muscle mass and reduction in fat mass. Despite recognition of the negative impact of liver transplantation on body composition, one randomized trial that evaluated preoperative nutritional supplementation showed posttransplant benefits [89]. The effect of ongoing preoperative immunonutrient intervention on postoperative outcomes including muscle mass is likely to be interesting but focuses on a specific protocol to modulate immune function by dietary interventions [90].
Fig. 1.
Hepatocellular dysfunction and portosystemic shunting in cirrhosis contribute to sarcopenia before transplantation and are reversed at least partially by liver transplantation. However, a number of factors contribute to the development of posttransplant sarcopenia, including persistence of collaterals even after transplantation, recurrent infections that induce a catabolic state, immunosuppressant medications that inhibit mTOR and stimulate myostatin, posttrans-plant renal failure, and lifestyle changes with little or no exercise. Recurrent disease also contributes to the worsening muscle mass after transplantation
In conclusion, the primary focus of this review is to recognize post liver transplant sarcopenia as a distinct disorder and identify its potential impact on the clinical management of these patients. Identifying the critical link between sarcopenia and increase in fat mass post transplant will reduce the consequences of posttransplant metabolic syndrome. It must also be reiterated that these alterations in body composition may not be specific to liver transplant alone, since sarcopenia is common in patients awaiting transplant of other organs including kidney, lung, and heart [91–94]. In all these patients, similar mechanisms including recurrent disease, impact of immunosuppressive regimen, and complications after transplantation may contribute to posttransplant sarcopenia. Long-term follow-up studies are necessary to determine whether these early alterations in body composition persist and affect the long-term outcome. Translation of data from studies on sarcopenia [5], identifying the molecular mechanisms, and evaluation of interventions including amino acid supplementation, exercise, and therapies targeted at improving skeletal muscle mass and function are likely to result in development of strategies to prevent and reverse post-transplant sarcopenia and improve outcomes in these patients.
Acknowledgments
I was supported in part by NIH DK 83414.
Footnotes
Conflict of interest None.
References
- 1.Aberg F, Isoniemi H, Hockerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14–21. doi: 10.1177/145749691110000104. [DOI] [PubMed] [Google Scholar]
- 2.Gouw AS, Haagsma EB, Manns M, Klompmaker IJ, Slooff MJ, Gerber MA. Is there recurrence of primary biliary cirrhosis after liver transplantation? A clinicopathologic study in long-term survivors. J Hepatol. 1994;20:500–507. doi: 10.1016/s0168-8278(05)80497-0. [DOI] [PubMed] [Google Scholar]
- 3.Schott R, Chaouat A, Launoy A, Pottecher T, Weitzenblum E. Improvement of pulmonary hypertension after liver transplantation. Chest. 1999;115:1748–1749. doi: 10.1378/chest.115.6.1748. [DOI] [PubMed] [Google Scholar]
- 4.Sussman AN, Boyer TD. Management of refractory ascites and hepatorenal syndrome. Curr Gastroenterol Rep. 2011;13:17–25. doi: 10.1007/s11894-010-0156-6. [DOI] [PubMed] [Google Scholar]
- 5.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merli M, Giusto M, Gentili F, et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208–214. doi: 10.1111/j.1478-3231.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 8.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Merli M, Giusto M, Giannelli V, Lucidi C, Riggio O. Nutritional Status and Liver Transplantation. J Clin Exp Hepatol. 2011;1:190–198. doi: 10.1016/S0973-6883(11)60237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y) 2011;7(10):661–671. [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652–661. doi: 10.1097/SLA.0b013e3181f5f23a. [DOI] [PubMed] [Google Scholar]
- 12.Santos JR, Miyazaki MC, Domingos NA, Valerio NI, Silva RF, Silva RC. Patients undergoing liver transplantation: psychosocial characteristics, depressive symptoms, and quality of life. Transpl Proc. 2008;40:802–804. doi: 10.1016/j.transproceed.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 13.Saab S, Wiese C, Ibrahim AB, et al. Employment and quality of life in liver transplant recipients. Liver Transpl. 2007;13:1330–1338. doi: 10.1002/lt.21247. [DOI] [PubMed] [Google Scholar]
- 14.Norman K, Kirchner H, Lochs H, Pirlich M. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol. 2006;12:3380–3385. doi: 10.3748/wjg.v12.i21.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoresen L, Frykholm G, Lydersen S, et al. The association of nutritional assessment criteria with health-related quality of life in patients with advanced colorectal carcinoma. Eur J Cancer Care (Engl) 2012;21:505–516. doi: 10.1111/j.1365-2354.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 16.Verhage TL, Heijdra Y, Molema J, Vercoulen J, Dekhuijzen R. Associations of muscle depletion with health status. Another gender difference in COPD? Clin Nutr. 2011;30:332–338. doi: 10.1016/j.clnu.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Vetta F, Ronzoni S, Taglieri G, Bollea MR. The impact of malnutrition on the quality of life in the elderly. Clin Nutr. 1999;18:259–267. doi: 10.1016/s0261-5614(98)80022-8. [DOI] [PubMed] [Google Scholar]
- 18.Williams TJ, McKenna MJ. Exercise limitation following transplantation. Compr Physiol. 2012;2:1937–1979. doi: 10.1002/cphy.c110021. [DOI] [PubMed] [Google Scholar]
- 19.Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Post-transplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 20.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 21.Kim TN, Park MS, Lim KI, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract. 2011;93:285–291. doi: 10.1016/j.diabres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(166–73):173. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 24.Hussaini SH, Oldroyd B, Stewart SP, et al. Effects of orthotopic liver transplantation on body composition. Liver. 1998;18:173–179. doi: 10.1111/j.1600-0676.1998.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 25.Keogh JB, Tsalamandris C, Sewell RB, et al. Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition. 1999;15:661–664. doi: 10.1016/s0899-9007(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 26.Plank LD, Metzger DJ, McCall JL, et al. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann Surg. 2001;234:245–255. doi: 10.1097/00000658-200108000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Dodd MJ, Painter PL. Objective measures of health-related quality of life over 24 months post-liver transplantation. Clin Transpl. 2005;19:1–9. doi: 10.1111/j.1399-0012.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- 28.Krasnoff JB, Vintro AQ, Ascher NL, et al. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transpl. 2006;6:1896–1905. doi: 10.1111/j.1600-6143.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 29.Merli M, Guisto M, Riggio O, Gentili F, Molinaro A, Attili A, et al. Improvement of nutritional status in malnourished cirrhotic patients one year after liver transplantation. Eur E J Clin Nutr Metab. 2011;6:e142–e147. [Google Scholar]
- 30.de Carvalho L, Parise ER, Samuel D. Factors associated with nutritional status in liver transplant patients who survived the first year after transplantation. J Gastroenterol Hepatol. 2010;25:391–396. doi: 10.1111/j.1440-1746.2009.06033.x. [DOI] [PubMed] [Google Scholar]
- 31.Zaina FE, Lopes RW, Souza MR. A comparison of nutritional status in three time points of liver transplant. Transpl Proc. 2004;36:949–950. doi: 10.1016/j.transproceed.2004.03.125. [DOI] [PubMed] [Google Scholar]
- 32.Holt RI, Broide E, Buchanan CR, et al. Orthotopic liver transplantation reverses the adverse nutritional changes of end-stage liver disease in children. Am J Clin Nutr. 1997;65:534–542. doi: 10.1093/ajcn/65.2.534. [DOI] [PubMed] [Google Scholar]
- 33.Wagner D, Adunka C, Kniepeiss D, et al. Serum albumin, subjective global assessment, body mass index and the bioimpedance analysis in the assessment of malnutrition in patients up to 15 years after liver transplantation. Clin Transpl. 2011;25:E396–E400. doi: 10.1111/j.1399-0012.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- 34.Schutz T, Hudjetz H, Roske AE, et al. Weight gain in long-term survivors of kidney or liver transplantation–another paradigm of sarcopenic obesity? Nutrition. 2012;28:378–383. doi: 10.1016/j.nut.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Krasnoff JB, Mathias R, Rosenthal P, Painter PL. The comprehensive assessment of physical fitness in children following kidney and liver transplantation. Transplantation. 2006;82:211–217. doi: 10.1097/01.tp.0000226160.40527.5f. [DOI] [PubMed] [Google Scholar]
- 36.de Luis DA, Izaola O, Velicia MC, et al. Impact of dietary intake and nutritional status on outcomes after liver transplantation. Rev Esp Enferm Dig. 2006;98:6–13. doi: 10.4321/s1130-01082006000100002. [DOI] [PubMed] [Google Scholar]
- 37.Madersbacher S, Ludvik G, Stulnig T, Grunberger T, Maier U. The impact of liver transplantation on endocrine status in men. Clin Endocrinol (Oxf) 1996;44:461–466. doi: 10.1046/j.1365-2265.1996.698519.x. [DOI] [PubMed] [Google Scholar]
- 38.Seehofer D, Steinmueller T, Graef KJ, et al. Pituitary function test and endocrine status in patient with cirrhosis of the liver before and after hepatic transplantation. Ann Transpl. 2002;7:32–37. [PubMed] [Google Scholar]
- 39.Wawrzynowicz-Syczewska M, Karpinska E, Jurczyk K, Laurans L, Boron-Kaczmarska A. Risk factors and dynamics of weight gain in patients after liver transplantation. Ann Transpl. 2009;14:45–50. [PubMed] [Google Scholar]
- 40.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461–466. doi: 10.1111/j.1432-2277.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 41.Toniutto P, Fabris C, Minisini R, et al. Weight gain after liver transplantation and the insertion/deletion polymorphism of the angiotensin-converting enzyme gene. Transplantation. 2005;79:1338–1343. doi: 10.1097/01.tp.0000158712.42875.51. [DOI] [PubMed] [Google Scholar]
- 42.Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl. 2001;7:S13–S21. doi: 10.1053/jlts.2001.29167. [DOI] [PubMed] [Google Scholar]
- 43.Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4:285–296. doi: 10.1002/lt.500040402. [DOI] [PubMed] [Google Scholar]
- 44.Palmer M, Schaffner F, Thung SN. Excessive weight gain after liver transplantation. Transplantation. 1991;51:797–800. doi: 10.1097/00007890-199104000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol. 1999;277:E489–E495. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- 46.Hussaini SH, Soo S, Stewart SP, et al. Risk factors for loss of lean body mass after liver transplantation. Appl Radiat Isot. 1998;49:663–664. doi: 10.1016/s0969-8043(97)00088-2. [DOI] [PubMed] [Google Scholar]
- 47.Langer G, Grossmann K, Fleischer S, et al. Nutritional interventions for liver-transplanted patients. Cochrane Database Syst Rev. 2012:8:CD007605. doi: 10.1002/14651858.CD007605.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida R, Yagi T, Sadamori H, et al. Branched-chain amino acid-enriched nutrients improve nutritional and metabolic abnormalities in the early post-transplant period after living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2012;19:438–448. doi: 10.1007/s00534-011-0459-5. [DOI] [PubMed] [Google Scholar]
- 49.Ebeling PR. Approach to the patient with transplantation-related bone loss. J Clin Endocrinol Metab. 2009;94:1483–1490. doi: 10.1210/jc.2009-0205. [DOI] [PubMed] [Google Scholar]
- 50.Guadalix S, Martinez-Diaz-Guerra G, Lora D, et al. Effect of early risedronate treatment on bone mineral density and bone turnover markers after liver transplantation: a prospective single-center study. Transpl Int. 2011;24:657–665. doi: 10.1111/j.1432-2277.2011.01253.x. [DOI] [PubMed] [Google Scholar]
- 51.Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Immunosuppressive and postoperative effects of orthotopic liver transplantation on bone metabolism. Liver Transpl. 2004;10:638–647. doi: 10.1002/lt.20160. [DOI] [PubMed] [Google Scholar]
- 52.Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12:1390–1402. doi: 10.1002/lt.20874. [DOI] [PubMed] [Google Scholar]
- 53.Kasturi KS, Chennareddygari S, Mummadi RR. Effect of bisphosphonates on bone mineral density in liver transplant patients: a meta-analysis and systematic review of randomized controlled trials. Transpl Int. 2010;23:200–207. doi: 10.1111/j.1432-2277.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 54.Kulak CA, Borba VZ, Kulak JJ, Shane E. Transplantation osteoporosis. Arq Bras Endocrinol Metabol. 2006;50:783–792. doi: 10.1590/s0004-27302006000400023. [DOI] [PubMed] [Google Scholar]
- 55.Kulak CA, Borba VZ, Kulak J, Jr, Custodio MR. Osteoporosis after transplantation. Curr Osteoporos Rep. 2012;10:48–55. doi: 10.1007/s11914-011-0083-y. [DOI] [PubMed] [Google Scholar]
- 56.Maalouf NM, Sakhaee K. Treatment of osteoporosis in patients with chronic liver disease and in liver transplant recipients. Curr Treat Options Gastroenterol. 2006;9:456–463. doi: 10.1007/s11938-006-0002-y. [DOI] [PubMed] [Google Scholar]
- 57.Millonig G, Graziadei IW, Eichler D, et al. Alendronate in combination with calcium and vitamin D prevents bone loss after orthotopic liver transplantation: a prospective single-center study. Liver Transpl. 2005;11:960–966. doi: 10.1002/lt.20466. [DOI] [PubMed] [Google Scholar]
- 58.Perkins JD. Bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Liver Transpl. 2006;12:1168–1169. [PubMed] [Google Scholar]
- 59.Scolapio JS, DeArment J, Hurley DL, Romano M, Harnois D, Weigand SD. Influence of tacrolimus and short-duration prednisone on bone mineral density following liver transplantation. JPEN J Parenter Enteral Nutr. 2003;27:427–432. doi: 10.1177/0148607103027006427. [DOI] [PubMed] [Google Scholar]
- 60.Stein E, Ebeling P, Shane E. Post-transplantation osteoporosis. Endocrinol Metab Clin North Am. 2007;36:937–963. doi: 10.1016/j.ecl.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 62.Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 63.Dasarathy S, McCullough AJ, Muc S, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707–709. doi: 10.1213/ANE.0b013e3181eac1c9. [DOI] [PubMed] [Google Scholar]
- 65.Morrison WL, Bouchier IA, Gibson JN, Rennie MJ. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin Sci (Lond) 1990;78:613–619. doi: 10.1042/cs0780613. [DOI] [PubMed] [Google Scholar]
- 66.Tessari P. Protein metabolism in liver cirrhosis: from albumin to muscle myofibrils. Curr Opin Clin Nutr Metab Care. 2003;6:79–85. doi: 10.1097/00075197-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Gayan-Ramirez G, van de Casteele M, Rollier H, et al. Biliary cirrhosis induces type IIx/b fiber atrophy in rat diaphragm and skeletal muscle, and decreases IGF-I mRNA in the liver but not in muscle. J Hepatol. 1998;29:241–249. doi: 10.1016/s0168-8278(98)80009-3. [DOI] [PubMed] [Google Scholar]
- 68.Lin SY, Chen WY, Lee FY, Huang CJ, Sheu WH. Activation of ubiquitin-proteasome pathway is involved in skeletal muscle wasting in a rat model with biliary cirrhosis: potential role of TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E493–E501. doi: 10.1152/ajpendo.00186.2004. [DOI] [PubMed] [Google Scholar]
- 69.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller MJ, Loyal S, Schwarze M, et al. Resting energy expenditure and nutritional state in patients with liver cirrhosis before and after liver transplantation. Clin Nutr. 1994;13:145–152. doi: 10.1016/0261-5614(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 71.Hasse JM, Blue LS, Liepa GU, et al. Early enteral nutrition support in patients undergoing liver transplantation. JPEN J Parenter Enteral Nutr. 1995;19:437–443. doi: 10.1177/0148607195019006437. [DOI] [PubMed] [Google Scholar]
- 72.Dickinson JM, Fry CS, Drummond MJ, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drummond MJ, Fry CS, Glynn EL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 75.Sakuma K, Nakao R, Aoi W, et al. Cyclosporin A treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration. Acta Neuropathol. 2005;110:269–280. doi: 10.1007/s00401-005-1049-x. [DOI] [PubMed] [Google Scholar]
- 76.Ma K, Mallidis C, Bhasin S, et al. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 77.Taylor WE, Bhasin S, Artaza J, et al. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E221–E228. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- 78.Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. 2010;2010:721219. doi: 10.1155/2010/721219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mercier JG, Hokanson JF, Brooks GA. Effects of cyclosporine A on skeletal muscle mitochondrial respiration and endurance time in rats. Am J Respir Crit Care Med. 1995;151:1532–1536. doi: 10.1164/ajrccm.151.5.7735611. [DOI] [PubMed] [Google Scholar]
- 80.Gabe SM, Bjarnason I, Tolou-Ghamari Z, et al. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology. 1998;115:67–74. doi: 10.1016/s0016-5085(98)70366-x. [DOI] [PubMed] [Google Scholar]
- 81.Vary TC. IGF-I stimulates protein synthesis in skeletal muscle through multiple signaling pathways during sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;290:R313–R321. doi: 10.1152/ajpregu.00333.2005. [DOI] [PubMed] [Google Scholar]
- 82.Tinti F, Mitterhofer AP, Muiesan P. Liver transplantation: role of immunosuppression, renal dysfunction and cardiovascular risk factors. Minerva Chir. 2012;67:1–13. [PubMed] [Google Scholar]
- 83.Miyabara EH, Conte TC, Silva MT, et al. Mammalian target of rapamycin complex 1 is involved in differentiation of regenerating myofibers in vivo. Muscle Nerve. 2010;42:778–787. doi: 10.1002/mus.21754. [DOI] [PubMed] [Google Scholar]
- 84.Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. 2001;7(3):213–219. doi: 10.1053/jlts.2001.22184. [DOI] [PubMed] [Google Scholar]
- 85.van den Ham EC, Kooman JP, Christiaans MH, van Hooff JP. Relation between steroid dose, body composition and physical activity in renal transplant patients. Transplantation. 2000;69:1591–1598. doi: 10.1097/00007890-200004270-00013. [DOI] [PubMed] [Google Scholar]
- 86.Braith RW, Mills RM, Welsch MA, Keller JW, Pollock ML. Resistance exercise training restores bone mineral density in heart transplant recipients. J Am Coll Cardiol. 1996;28:1471–1477. doi: 10.1016/s0735-1097(96)00347-6. [DOI] [PubMed] [Google Scholar]
- 87.Braith RW, Magyari PM, Fulton MN, Aranda J, Walker T, Hill JA. Resistance exercise training and alendronate reverse glucocorticoid-induced osteoporosis in heart transplant recipients. J Heart Lung Transpl. 2003;22:1082–1090. doi: 10.1016/s1053-2498(02)01184-1. [DOI] [PubMed] [Google Scholar]
- 88.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–1384. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation. 2000;69:1364–1369. doi: 10.1097/00007890-200004150-00026. [DOI] [PubMed] [Google Scholar]
- 90.Nickkholgh A, Schneider H, Encke J, Buchler MW, Schmidt J, Schemmer P. PROUD: effects of preoperative long-term immunonutrition in patients listed for liver transplantation. Trials. 2007;8:20. doi: 10.1186/1745-6215-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kyle UG, Nicod L, Raguso C, Hans D, Pichard C. Prevalence of low fat-free mass index and high and very high body fat mass index following lung transplantation. Acta Diabetol. 2003;40:S258–S260. doi: 10.1007/s00592-003-0080-4. [DOI] [PubMed] [Google Scholar]
- 92.Kyle UG, Nicod L, Romand JA, Slosman DO, Spiliopoulos A, Pichard C. Four-year follow-up of body compostion in lung transplant patients. Transplantation. 2003;75:821–828. doi: 10.1097/01.TP.0000054689.50879.36. [DOI] [PubMed] [Google Scholar]
- 93.Coroas AS, Oliveira JG, Sampaio S, et al. Body composition assessed by impedance changes very early with declining renal graft function. Nephron Physiol. 2006;104:115–120. doi: 10.1159/000095540. [DOI] [PubMed] [Google Scholar]
- 94.Harada H, Nakamura M, Hotta K, et al. Percentages of water, muscle, and bone decrease and lipid increases in early period after successful kidney transplantation: a body composition analysis. Transpl Proc. 2012;44:672–675. doi: 10.1016/j.transproceed.2011.12.010. [DOI] [PubMed] [Google Scholar]