Abstract
Objective
The aims of this study were to (i) detect the presence and edge frequency (fe) of a cochlear dead region in the ear with residual acoustic hearing for bimodal cochlear implant (CI) users, and (ii) determine whether amplification based on the presence or absence of a dead region would improve speech understanding and sound quality.
Design
Twenty two listeners with a CI in one ear and residual acoustic hearing in the non-implanted ear were tested. Eleven listeners had a cochlear dead region in the acoustic-hearing ear and eleven did not. Dead regions were assessed with the threshold equalizing noise (TEN) and the sweeping noise, psychophysical tuning curve (SWPTC) tests. Speech understanding was assessed with monosyllabic words and the AzBio sentences at +10 dB signal-to-noise ratio. Speech and music quality judgments were obtained with the Judgment of Sound Quality questionnaire.
Results
For this population, using shifted tips of the PTCs as a basis for diagnosis, the TEN had high sensitivity (0.91) and poor specificity (0.55). The value of fe was lower when estimated with the SWPTC test than with the TEN test. For the listeners with cochlear dead regions, speech understanding, speech quality and music quality were best when no amplification was applied for frequencies within the dead region. For listeners without dead regions, speech understanding was best with full-bandwidth amplification and was reduced when amplification was not applied when the audiometric threshold exceeded 80 dB HL.
Conclusion
Our data suggest that, to improve bimodal benefit for listeners who combine electric and acoustic stimulation, audiologists should routinely test for the presence of cochlear dead regions and determine amplification bandwidth accordingly.
INTRODUCTION
Cochlear implant (CI) listeners who have low-frequency acoustic hearing in the non-implanted ear can benefit significantly from combining acoustic stimulation in that ear with the stimulation provided by the CI (e.g., Ching et al. 2004; Kiefer et al. 2004; Kong, Stichney & Zeng 2005; Mok et al. 2006; Gifford et al. 2007; Dorman et al. 2008; Zhang et al. 2010). For brevity, we refer to this as bimodal stimulation. Both speech understanding and sound quality are improved significantly by bimodal stimulation, relative to listening via the CI alone. However, considerable variability remains in individual benefit. One reason for the variability in benefit is differences in basic auditory function in the ear with acoustic hearing (Gantz 2009; Golub et al. 2012; Zhang et al. 2012). In this paper we investigate the possibility that a portion of the variability is due to the presence or absence of a cochlear dead region in that ear.
A cochlear dead region is a region in the cochlea where there are few or no functioning inner hair cells and/or auditory neurons, so that a tone producing peak vibration in that region is detected via inner hair cells and neurons adjacent to the dead region, via “off-frequency listening” (Moore 2001). The incidence of dead regions in listeners with moderate-to-profound high-frequency hearing loss is quite high (Ching et al. 1998; Hogan & Turner 1998; Vinay & Moore 2007). It has been reported in several studies that the amplification of frequencies well inside a high-frequency dead region usually (1) does not improve speech understanding and may degrade speech understanding due to downward spread of masking; and (2) creates increased feedback and unwanted distortion (e.g., Yanz 2002; Hornsby & Ricketts, 2006; Gordo & Lorio 2007; Moore 2009; Cox et al. 2011).
Users of bimodal stimulation typically have a steeply sloping hearing loss in the acoustically stimulated ear, with thresholds in the mild-to-moderate range for frequencies up to 500 Hz and in the severe-to-profound range for frequencies of 1000 Hz and above. A dead region is common in ears with this configuration of hearing loss (Preminger, Carpenter & Ziegler 2005; Vinay & Moore 2007). It is reasonable to assume that success in hearing aid fitting, and as a consequence, bimodal benefit, could be improved if clinicians were able to identify bimodal users for whom broad-bandwidth amplification is contraindicated due to the presence of a dead region.
Methods of Identifying a Dead Region
Although a cochlear dead region is often associated with a steeply sloping hearing loss, the presence and edge frequency, fe, of a dead region cannot be accurately identified from the amount of hearing loss or the slope of the audiogram (Moore et al. 2000; Summers et al. 2003; Vinay & Moore, 2007). There may be only a moderate (even mild) hearing loss at fe, since the audiometric threshold is determined by off-frequency listening. The ‘gold standard’ method for identifying a dead region is the psychophysical tuning curve (PTC). PTCs can be used both to determine the presence/absence of a dead region and to determine the value of fe (Moore 2001; Moore & Alcantara 2001). When the signal frequency falls in a high-frequency dead region, the tip of the PTC is shifted below the signal frequency. However, precautions need to be taken to prevent the PTCs from being influenced by the detection of beats and combination tones, which can lead to a shifted tip of the PTC even when a dead region is not present (Kluk & Moore, 2004, 2005).
Measurement of PTCs using traditional methods requires specialized software/hardware and is time intensive, taking two or more hours per signal frequency. Thus, the use of traditional PTCs is not clinically feasible. To facilitate clinical identification of a cochlear dead region, Moore et al. (2000, 2004) developed the threshold-equalizing noise (TEN) test. The TEN test is based on measurement of the threshold for detecting a pure tone presented in a band-limited noise. The TEN is designed to produce the same masked threshold for all signal frequencies for listeners without dead regions; the signal threshold is typically equal to the nominal level of the TEN, specified as dB/ERBN. A signal threshold that is 10 dB or more above the TEN level is taken as indicating the presence of a dead region at the signal frequency. Although high consistency between the results of the TEN and PTC tests has been reported for a small number of hearing-impaired listeners (Moore et al. 2000; Moore & Alcantara 2001), the sensitivity and specificity of the TEN test are still unknown. Additional limitations of the TEN test are that (1) the test gives only a rough indication of fe; and (2) the outcome can be inconclusive when hearing loss is severe or profound. In response to these limitations, Sęk and his co-workers (Sęk et al., 2005; Sęk & Moore, 2011) developed an efficient method for measuring PTCs - the sweeping psychophysical tuning curve (SWPTC) test. The SWPTC test is also based on measurement of the threshold for detecting a pure tone presented in a band-limited noise. The band of noise masker sweeps in centre frequency and the masker levels are determined using a Békésy method where signal detection is at threshold. When the signal frequency falls within a DR, the SWPTC tedt gives PTCs with shifted tips. The edge frequency of DR is where the shifted tip of PTC is located and the DR extends upwards from the shifted tip of PTC. The SWPTC test takes just 8–10 minutes per ear, making it clinically practical.
In the research presented here, we (i) determined the presence/absence of a cochlear dead region for the ear with residual acoustic hearing of listeners receiving bimodal stimulation, using the TEN and the SWPTC tests, (ii) determined the sensitivity and specificity of the TEN test using the results of the SWPTC test as the reference, and (iii) assessed whether amplification bandwidth based on the presence or absence of dead regions would improve speech understanding and sound quality. The working hypotheses were that: (1) the determination of cochlear dead regions would be possible with either the TEN or SWPTC tests, and (2) providing amplification for frequencies within a dead region would yield significantly poorer bimodal benefit and sound quality than limiting the bandwidth to the “live” region.
METHOD
Listeners
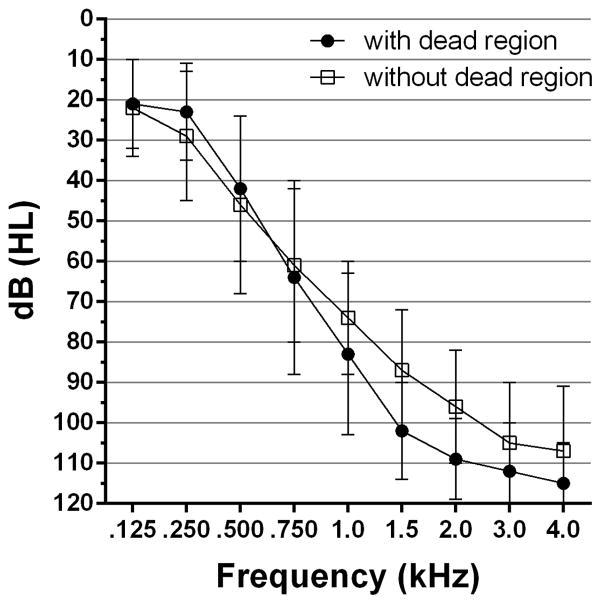
Twenty two recipients of bimodal stimulation participated. Eleven had a cochlear dead region within the region of measurable thresholds and eleven did not. Audiometric thresholds for the two groups of listeners are shown in Figure 1. The mean thresholds did not differ significantly (p > 0.05) at any frequency across the two groups.
Figure 1.
Audiometric thresholds for listeners with and without a dead region.
The listeners ranged in age from 36 to 82 years with a mean age of 61 years. Fifteen were male and 7 were female. The duration of hearing loss for the aided ear ranged from 4–56 years. All listeners made full-time use of the hearing aid in the non-implanted ear. Ten listeners used Cochlear Corporation CIs, 5 used Advanced Bionics CIs and 7 used MED-EL CIs. Five listeners used short electrode arrays with 500–700 Hz low-frequency cutoff for the most apical electrode and the remainder used long electrode arrays with 250–300 Hz low-frequency cutoff for the most apical electrode. All five listeners did not have a cochlear dead region within the region of measurable thresholds. The possible effect of electrode array type on the assessment of speech understanding and sound quality perception was latter analyzed within the group of listeners with dead region using the electrode type (short vs. long) as a between-subject factor. The results showed no significant between-subject effects of array type (p > 0.05).
Assessment of Dead Regions
Each listener was evaluated with both the TEN(HL) (Moore et al., 2004) and SWPTC (Sek and Moore, 2011) tests. The TEN(HL) test stimuli were replayed from a compact disc to an audiometer (Grason-Stadler GSI 61, Eden Prairie, MN) and delivered monaurally via an insert earphone (Etymotic Research 3A, Elk Grove Village, IL) as described by Moore et al. (2004). Audiometric thresholds at 500, 1000, 1500, 2000, 3000, 4000 Hz were established, first in quiet, and then in the presence of the TEN. The test tones were generated from the TEN-test compact disk (Moore et al., 2004). The level of the TEN was typically set at 10 dB above threshold level at the test frequency or at the threshold level if it was equal to or above 80 dB HL (Moore et al., 2004). The presence/absence of dead regions was determined using the criteria proposed by Moore et al., namely a masked threshold that was at least 10 dB above the absolute threshold and at least 10 dB above the level of the TEN. The value of fe was estimated as the lowest frequency at which an abnormal TEN-test result was obtained. Since the lowest test frequency used in the TEN(HL) test is 500 Hz, this meant that the estimated value of fe could not be below 500 Hz.
SWPTCs were obtained using stimuli presented via Sennheiser HD250 linear II headphones (Wedemark, Germany), following the procedure described by Sek & Moore (2011). For each participant, the signal frequency was one octave above the lowest frequency at which the absolute threshold was 80 dB HL or more (where typically the TEN-test outcome can be inconclusive). The absolute threshold for detecting a pure tone at the selected signal frequency was estimated using the forced-choice method implemented in the SWPTC software. The signal level was set 10 dB above the estimated absolute threshold. The masker center frequency was slowly swept from below to above the signal frequency (upward sweep) or above to below the signal frequency (downward sweep), and the masker level was adjusted to track the level required just to mask the signal, using a Békésy tracking method. The listener was asked to press the space bar on a keyboard when the signal was heard (in which case the masker level increased) and to release the space bar when the signal could not be heard (in which case the masker level decreased). The rate of change of the masker level, the masker bandwidth, and the starting and ending frequencies were automatically set by the SWPTC software (Sek & Moore, 2011). The tip frequency of PTC was determined by a double-regression analysis implemented in the software (Sek & Moore, 2011). The final tip frequency was determined from the average of two upward and two downward sweeps. A shift of the tip frequency relative to the signal frequency of 20% or more was taken as indicating that the signal frequency fell within a dead region. When the tip of the PTC was shifted, the value of fe was taken as the frequency at the shifted tip.
Assessment of Speech Understanding
Speech understanding was assessed using consonant-nucleus-consonant (CNC) monosyllabic words (Peterson & Lehiste 1962) and sentences from the AzBio lists (Spahr et al. 2012) presented in speech-shaped noise at +10-dB signal-to-noise ratio. Stimulus presentation was controlled by MATLAB scripts. The signals were presented via Sennheiser HD 250 Linear II headphones, which have large ear muffs that were placed over the CI processors. In the implant-only condition, the non-implanted ear was plugged (EAR Classic, Indianapolis, IN) and muffed (UltraSonic, Elvex, Bethel, CT). For the acoustic-only condition, the listeners’ processors were removed.
Listeners were tested using their ‘everyday’ CI speech-coding program. Only one subject had a feature of directional microphone activated in a separate program, which was not used during the assessment of speech understanding and sound quality perception. The presentation level of the signal delivered to the implanted ear was verified as ‘comfortably loud’ by each listener. To accommodate the different degrees of hearing loss in the non-implanted ear, acoustic signals delivered to the non-implanted ear were subjected to the frequency-gain characteristic prescribed by the NAL-R formula (Byrne and Dillon, 1986). The maximum gain was limited to 50 dB. The acoustic signals delivered to the implanted ear were not modified using the NAL rule.
The final presentation level of the acoustic stimuli was adjusted to equate the loudness of the electric and acoustic signals. This was accomplished by alternating the presentation of a signal to the CI with the presentation of an amplified signal to the earphone in the full-bandwidth condition. Listeners used a response card to indicate whether the sound presented through the earphone was louder or softer than the signal presented via the CI. The response card contained a continuous scale, labeled with ‘softer’ and ‘louder’ at the end points and ‘same’ at the midpoint. The overall gain applied to the signal presented via the earphone was adjusted (typically within a range of ±5 dB around the gain prescribed by the NAL-R formula) until the listener reported similar loudness in the two ears. The same gain adjustment was used when the acoustic stimuli were lowpass filtered. To establish a baseline from which to assess the effects of amplification adjustments, all listeners were first tested using bimodal stimulation with full-bandwidth amplification of the acoustic stimuli. For the listeners with dead regions, the stimuli were presented in two other conditions. In one, no gain was provided at and above the value of fe determined using the TEN(HL) test. In the other, no gain was provided at and above the value of fe determined using the SWPTC test. For the listeners without dead regions, a single extra condition was used in which no gain was provided for frequencies at which audiometric thresholds were ≥ 80 dB HL. The 80-dB-rule-of-thumb method is based on previous reports that a dead region is typically present at frequencies for which audiometric thresholds are greater than or equal to 80 dB HL (Moore et al., 2000, 2001, 2004; Summers, 2004).
Assessment of Sound Quality
For listeners with a dead region, sound quality for speech and music was assessed subjectively via the Judgment of Sound Quality (JSQ) questionnaire (Gabrielsson et al. 1988). The JSQ evaluates eight dimensions of sound quality including softness, brightness, clarity, fullness, nearness, loudness, spaciousness and “total impression” using a 10-point continuous rating scale. The final scores for each condition were estimated by averaging the rating scores across all eight dimensions. The JSQ was administered to assess subjective estimates of sound quality for the following conditions: (1) CI plus full-bandwidth amplification; (2) CI plus amplification for frequencies up to the value of fe determined using the SWPTC test. The speech signal for JSQ administration was connected discourse from a male speaker from the Speech Intelligibility Rating test (Cox and McDaniel, 1989). The music passage for JSQ administration was Beethoven’s Symphony Number 9 (Choral); Allegro ma non torppo, un poco maestro and Molto vivace, performed by the Dresden Symphonic Orchestra and Choir. The 60-second music samples were equated in peak level (to within ±2 dB) and the variation in rms sound level within the samples was 30 dB over 30 seconds. The speech and music signals were presented via the CI at 60 dB SPL and via Sennheiser HD 250 Linear II headphones at levels adjusted to give equal loudness of the electric and acoustic signals in a manner similar to that described for “Assessment of speech understanding”.
RESULTS
Edge Frequencies of Dead Regions
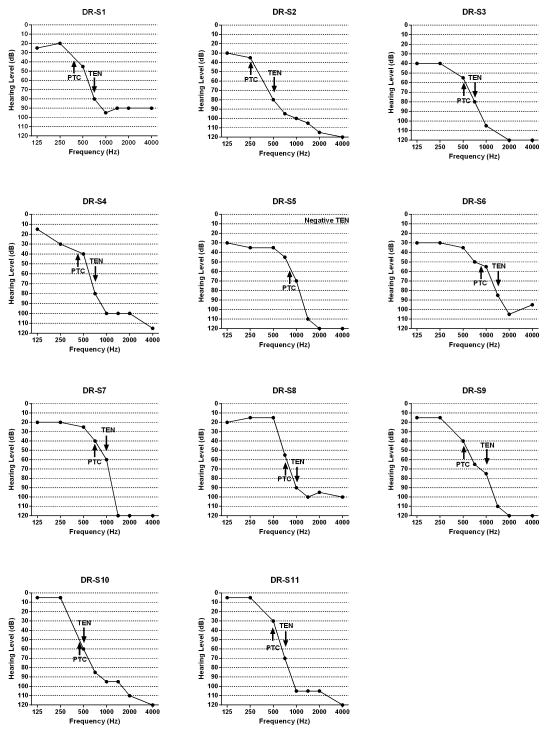
For each listener with a dead region, the values of fe as determined by the TEN and SWPTC tests are shown by arrows in Figure 2 and are given in Table 1. The values of fe were consistently lower for the SWPTC than for the TEN test. This is as expected, since for the TEN test the “true” value of fe for a high-frequency dead region lies between the lowest frequency at which the criteria are met and the next lowest test frequency. The difference between the two estimates varied widely, from less than 50 Hz to one octave. The largest discrepancies occurred when the value of fe estimated using the SWPTC test fell below 500 Hz. There are bound to be discrepancies in such cases, since the lowest test frequency implemented using the TEN (HL) test is 500 Hz, so the estimated fe cannot fall below 500 Hz. Other versions of the TEN test allow the use of lower test frequencies (Moore et al., 2010). Table 1 also shows the frequencies determined by the “80-dB-HL-rule-of-thumb method” used for indicating the presence of DRs (Moore, et al., 2000, 2001, 2004; Summers, et al., 2003; Summers, 2004). The value of fe determined using the TEN test was consistent with the frequency determined by the “80-dB-HL-rule-of-thumb” method for only 5 out of 11 listeners, meaning that the “80-dB-HL-rule-of-thumb” method is not appropriate for estimating fe, especially for listeners with steeply sloping hearing loss.
Figure 2.
Audiograms of listeners with dead regions and values of fe as determined by the SWPTC and TEN tests.
TABLE 1.
Values of fe determined by the TEN and SWPTC tests for listeners with a dead region, and frequencies determined by the “80-dB-HL-rule-of-thumb method”.
| Listener | TEN (Hz) | SWPTC (Hz) | 80-dB-rule-of-thumb method (Hz) |
|---|---|---|---|
| S1 | 750 | 407 | 750 |
| S2 | 500 | 252 | 500 |
| S3 | 750 | 514 | 750 |
| S4 | 750 | 427 | 750 |
| S5 | Negative | 854 | 1500 |
| S6 | 1500 | 918 | 1500 |
| S7 | 1000 | 725 | 1500 |
| S8 | 1000 | 769 | 1500 |
| S9 | 1000 | 496 | 1500 |
| S10 | 500 | 450 | 750 |
| S11 | 750 | 491 | 1000 |
Sensitivity and Specificity of the TEN test
Using results for the SWPTC test as the ‘gold standard’, the sensitivity and specificity of the TEN test were determined. Sensitivity was the probability of a positive TEN test result, given that the listener had a dead region in the frequency range of residual hearing. Specificity was the probability of a negative TEN test result, given that the listener did not have a dead region. The results are shown in Table 2. The sensitivity of the TEN test was 91%, but the specificity was only 55%.
Table 2.
Data on which the estimates of sensitivity and specificity are based. Sensitivity and specificity of TEN test using the SWPTC test as the standard were 0.91 and 0.55, respectively.
| TEN positive | TEN negative | |
|---|---|---|
| Listeners with a dead region | 10 (true positive) | 1 (false negative) |
| Listenerss without a dead region | 5 (false positive) | 6 (true negative) |
Speech Recognition
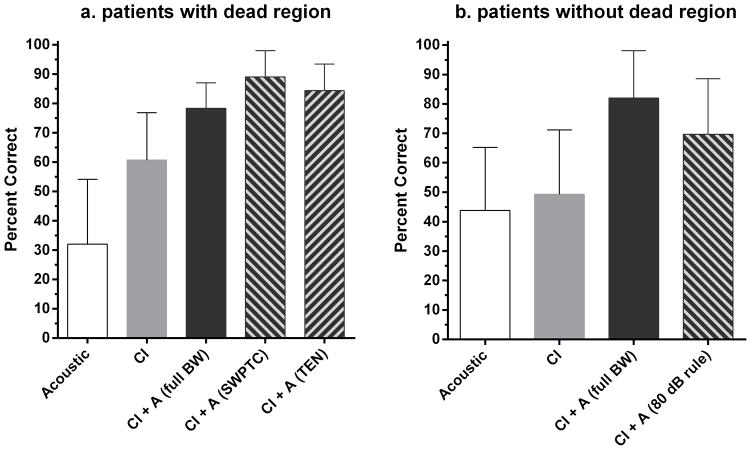
Figure 3a shows mean speech recognition scores (in percent correct) for each condition for CNC words for listeners with a dead region. The scores are shown for (i) acoustic only stimulation with full-bandwidth amplification (32% correct), (ii) CI only stimulation (61% correct), (iii) CI plus full-bandwidth amplification (78% correct), (iv) CI plus amplification for frequencies up to the value of fe determined by the SWPTC test (89% correct) and (v) CI plus band-limited amplification for frequencies up to the value of fe determined by the TEN test (84% correct). A repeated-measures analysis of variance (ANOVA) with the Greenhouse-Geisser correction showed a main effect of condition (F(4,54) = 39.02, p < 0.0001). Post-hoc testing using all-pairwise multiple comparisons with the Holm-Sidak statistic (also used for subsequent post-hoc multiple comparisons) indicated that scores were higher for CI plus full-bandwidth amplification than for CI only (p < 0.001). Thus, there was significant bimodal benefit. Scores were higher for both bimodal conditions with band-limited amplification than for bimodal stimulation with full-bandwidth amplification (p < 0.001). Thus, avoiding amplification for frequencies falling in the dead region yielded significantly better performance. Scores were slightly but significantly higher when the upper frequency limit of amplification was based on the value of fe determined using the SWPTC test than when it was based on the value obtained using the TEN test (p < 0.05).
Figure 3.
Figure 3a,b. Percent correct recognition of CNC words by listeners with and without a dead region. Error bars show ±1 standard deviation.
Figure 3b shows percent correct scores for CNC words for listeners without dead regions for (i) acoustic only stimulation with full-bandwidth amplification (44% correct), (ii) CI only stimulation (49 % correct), (iii) CI plus full-bandwidth amplification (82% correct), and (iv) CI plus acoustic amplification restricted to frequencies for which audiometric thresholds were ≤ 80 dB HL (70% correct). A repeated-measures ANOVA showed a main effect of condition (F(3,43) = 16.51, p = 0.0002). Post-hoc tests showed that scores were significantly higher for CI plus full-bandwidth amplification than for CI alone (p < 0.001), indicating a bimodal benefit. Scores with band-limited amplification were significantly poorer than scores obtained with full-bandwidth amplification (p < 0.001). Thus, for listeners without dead regions, reducing amplification for frequencies where thresholds were ≥ 80 dB HL reduced speech recognition performance.
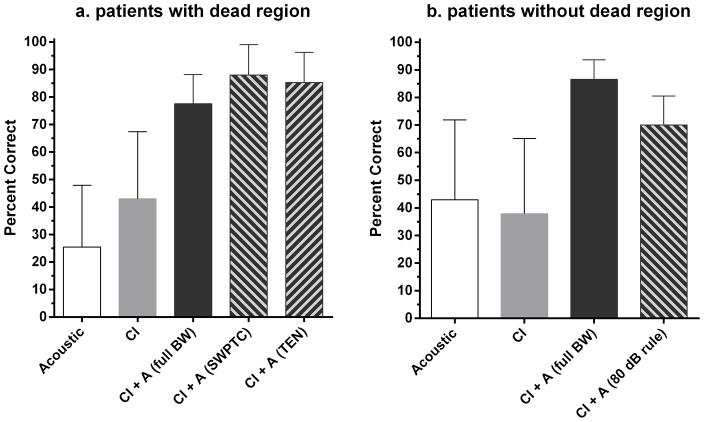
Figure 4a shows mean percent correct scores for each condition for AzBio sentences at +10 dB SNR for listeners with a dead region. The conditions were (i) acoustic only stimulation for full-bandwidth amplification (25% correct), (ii) CI only stimulation (43% correct), (iii) CI plus full-bandwidth amplification (77% correct), (iv) CI plus amplification for frequencies up to the value of fe determined by the SWPTC test (88% correct) and (v) CI plus amplification for frequencies up to the value of fe determined by the TEN test (85% correct). A repeated-measures ANOVA showed a significant main effect of condition (F(4, 54) = 33.7, p < 0.0001). Post-hoc tests showed that scores were higher for CI plus full-bandwidth amplification than for CI only (p < 0.001), indicating a significant bimodal benefit. Scores were higher for both listening conditions using amplification for frequencies up to fe than for full-bandwidth amplification (p < 0.001). Thus, avoiding amplification of frequencies in the dead region significantly improved performance. Scores obtained with the two methods for limiting bandwidth (fe for TEN versus fe for SWPTC) were not significantly different (p > 0.05).
Figure 4.
Figure 4a,b. Percent correct recognition of AzBio sentences at +10 SNR by listeners with and without a dead region. Error bars show ±1 standard deviation.
Figure 4b shows percent correct scores for AzBio sentences at +10 dB SNR for listeners without dead regions for (i) acoustic only stimulation for full-bandwidth amplification (43% correct), (ii) CI only stimulation (38% correct), (iii) CI plus full-bandwidth amplification (86 % correct), and (iv) CI plus amplification for frequencies where audiometric thresholds were ≤ 80 dB HL (70% correct). A repeated-measures ANOVA showed a main effect of condition (F(3,43) = 15.16, p = 0.0005). Post-hoc tests showed that scores were significantly higher for CI plus full-bandwidth amplification than for CI alone, indicating significant bimodal benefit (p < 0.001). Bimodal scores with band-limited amplification were lower than scores with full-bandwidth amplification (p < 0.001). Thus, for listeners without dead regions, reducing amplification for frequencies where audiometric thresholds were ≥ 80 dB HL led to significantly poorer speech recognition.
Sound Quality
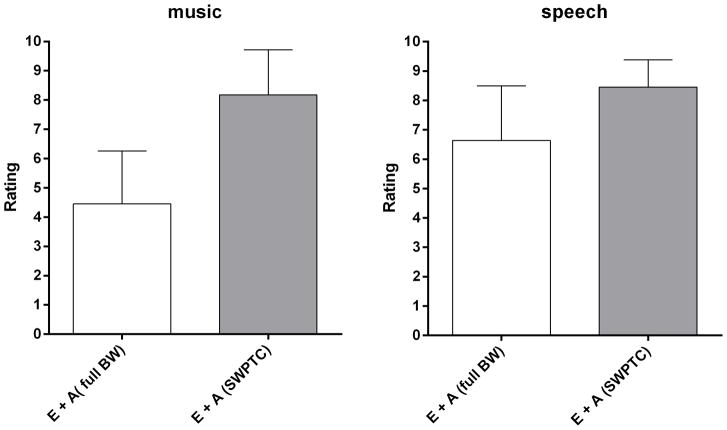
Estimates of sound quality for bimodally presented speech and music for listeners with dead regions are shown in Figure 5. For music, avoiding amplification for frequencies above fe (as determined using the SWPTC test) significantly improved sound quality (mean score = 8.2) compared to full-bandwidth amplification (mean score = 4.4) (t10 =5.31, p < 0.0003). For speech, a significant, but smaller, improvement in sound quality was found for the band-limited condition (mean score = 8.5) versus the full-bandwidth condition (mean score = 6.6) (t10 = 4.10, p = 0.002).
Figure 5.
Sound quality judgments for music and speech for each test condition for listeners with a dead region. Error bars show ±1 standard deviation.
DISCUSSION
In the near future, as the criteria for cochlear implantation become less conservative, a majority of CI recipients will have low-frequency acoustic hearing in either one or both ears. These listeners typically have a dead region, or regions, in the parts of the non-implanted cochlea responding to medium and high frequencies. Amplification of frequencies inside the dead region(s) sufficient to restore audibility, may cause downward spread of masking, unwanted distortion, and increased acoustic feedback (e.g., Yanz 2002; Hornsby & Ricketts, 2006; Gordo & Lorio 2007; Moore 2009; Cox et al. 2011), which may compromise the benefit of combining the amplified, acoustic signal and the electric signal. At issue in this project was the frequency range over which acoustic amplification should be provided to yield the best speech understanding and speech/sound quality for listeners receiving bimodal stimulation.
For listeners with dead regions, significantly better speech understanding and speech quality was found when the acoustic amplification was not applied for frequencies falling in the dead region(s). The magnitude of the improvement was, on average, 10 to 11 percentage points. The improvement in speech quality with band-limited amplification was also significant, with scores increasing from 6.6 to 8.5 on a 10-point scale. The gain in music quality with band-limited amplification was much greater, from 4.4 to 8.2. These findings are consistent with results from previous studies (e.g., Yanz 2002; Hornsby & Ricketts, 2006; Hornsby & Ricketts, 2006; Gordo & Lorio 2007; Moore 2009; Cox et al. 2011; Hornsby et al., 2011) showing that if sufficient gain is applied to the high-frequency components of speech, those components may mask the lower-frequency components due to downward spread of masking (e.g., Yanz 2002; Hornsby & Ricketts, 2006; Gordo & Lorio 2007; Moore 2009; Cox et al. 2011). In this case of bimodal stimulation, this may limit the bimodal benefit.
For listeners without cochlear dead regions, reducing amplification for frequencies where audiometric thresholds were ≥ 80 dB HL impaired performance. A similar result was reported by Neuman and Svirsky (2013), who investigated how the amplification bandwidth of the hearing aid fitting affected the speech recognition of bimodal users. They recommended providing amplification across as wide a frequency region as permitted by audiometric thresholds in the HA used by bimodal users. However this study did not test the presence or absence of dead regions. In the present study, full-bandwidth amplification provided significantly better speech understanding, by 13 to 16 percentage points, depending on the type of test material. This means that it is important to determine whether or not a candidate for bimodal stimulation has a dead region. Currently, neither the TEN test nor PTCs are routinely applied in clinical evaluations of such candidates. The “80-dB-rule-of thumb” method has been applied routinely in hearing aid fittings for listeners with sharply sloping hearing loss, including for CI listeners with residual, low-frequency acoustic hearing. Our results indicate that, for ears without dead regions, bimodal benefit can be reduced by limiting amplification to frequencies where audiometric thresholds are ≤ 80 dB HL.
In summary, the findings of the present study emphasize the importance of a clinical measure of dead regions in the best-practice recommendations for hearing-aid fitting for bimodal listeners in audiology clinics in order to maximize the EAS benefit. If the “80-dB-HL-rule-of-thumb” method (the most commonly used mothed in clinics) is used to fit hearing aids in cases of bimodal stimulation, the bimodal benefit is likely to be below what could be achieved. For listeners with dead regions, reducing amplification for frequencies above fe is likely to be beneficial, whereas for listeners without read regions, amplification over the widest frequency range possible is likely to be beneficial. Therefore, the diagnosis of cochlear dead regions is important to maximize bimodal benefit. It seems reasonable to recommend that audiologists test for dead regions when programming hearing aids for CI listeners with residual, low-frequency acoustic hearing.
For this sample of listeners, the TEN test had high sensitivity but poor specificity when using the results of the SWPTC test as a reference. The value of fe of the dead region as estimated by the TEN test was typically higher than the value fe estimated by the SWPTC test. However, this discrepancy could be reduced if a version of the TEN test with a wider range of signal frequencies were used. At present is seems reasonable to recommend that the TEN test is used as an initial screening tool, and to get a rough estimate of the value of fe, and the SWPTC test is used to confirm the diagnosis and to obtain a more accurate estimate of fe (Malicka et al., 2010; Moore and Malicka, 2013).
Finally, the use of the SWPTC test to assess the presence and edge frequency of dead regions may prove cost effective in evaluating whether a potential candidate for bimodal stimulation should actually receive a hearing aid. Listeners with a cochlear dead region starting at a frequency where the hearing loss is only mild may not benefit from amplification. Indeed, a hearing aid could be both costly and counterproductive. Without amplification, listeners with this hearing configuration can avoid amplification in the low-frequency range but still benefit from their native, low-frequency hearing (Zhang et al. 2010. On the other hand, individuals with greater hearing losses at the edge frequency might benefit from an acoustic hearing aid, but might reject an aid if full-bandwidth amplification is provided, due to poor sound quality and cochlear-level distortion; but these same individuals could derive significantly higher levels of speech understanding and perceived sound quality with individually determined amplification. These findings warrant a larger scale assessment of the clinical relevance of dead regions in order to maximize the bimodal benefit for unilateral implant recipients with residual acoustic hearing. Indeed, such an assessment has the potential to further the field with respect to a personalized approach to best practices for hearing healthcare management.
Supplementary Material
Acknowledgments
This research was supported by NIDCD F32 DC010937 to the first author, R01-DC010821 to the second author and R01-DC009404 to the third author.
References
- Byrne D, Dillon H. The National Acoustic Laboratories’ (NAL) new procedure for selecting the gain and frequency response of a hearing aid. Ear Hear. 1986;7:257–265. doi: 10.1097/00003446-198608000-00007. [DOI] [PubMed] [Google Scholar]
- Ching T, Dillon H, Byrne D. Speech recognition of hearing-impaired listeners: Predictions from audibility and the limited role of high-frequency amplification. J Acoust Soc Am. 1998;103:1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- Ching T, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Cox RM, Alexander GC, Johnson J, et al. Cochlear dead regions in typical hearing aid candidates: prevalence and implications for use of high-frequency speech cues. Ear Hear. 2011;32:339–348. doi: 10.1097/AUD.0b013e318202e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RM, McDaniel DM. Development of the Speech Intelligibility Rating (SIR) test for hearing aid comparisons. J Speech Hear Res. 1989;32:347–352. doi: 10.1044/jshr.3202.347. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Gifford R, Spahr A, et al. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol Neurootol. 2008;13:105–112. doi: 10.1159/000111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsson A, Schenkman BN, Hagerman B. The effects of frequency responses on sound quality judgments and speech intelligibility. J Speech Hear Res. 1988;31:166–177. doi: 10.1044/jshr.3102.166. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Hansen MR, Turner CW, et al. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14(Suppl 1):32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, McKarns S, et al. Combined electric and contralateral acoustic hearing: word and sentence intelligibility with bimodal hearing. J Speech Lang Hear Res. 2007;50:835–843. doi: 10.1044/1092-4388(2007/058). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub JS, Won JH, Drennan WR, et al. Spectral and temporal measures in hybrid cochlear implant users: on the mechanism of electroacoustic hearing benefits. Otol Neurotol. 2012;33:147–53. doi: 10.1097/MAO.0b013e318241b6d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordo A, Iorio MCM. Dead regions in the cochlea at high frequencies: Implications for the adaptation to hearing aids. Rev Bras Otorinolaringol. 2007;73:299–307. doi: 10.1016/S1808-8694(15)30072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan CA, Turner CW. High-frequency audibility: Benefits for hearing-impaired listeners. J Acoust Soc Am. 1998;104:432–441. doi: 10.1121/1.423247. [DOI] [PubMed] [Google Scholar]
- Hornsby BW, Ricketts TA. The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding. II. Sloping hearing loss. J Acoust Soc Am. 2003;119:1752–1763. doi: 10.1121/1.2161432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby BW, Ricketts TA. The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding. II. Sloping hearing loss. J Acoust Soc Am. 2006;119:1752–1763. doi: 10.1121/1.2161432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby BW, Johnson EE, Picou E. Effects of degree and configuration of hearing loss on the contribution of high- and low-frequency speech information to bilateral speech understanding. Ear Hear. 2011;32:543–555. doi: 10.1097/AUD.0b013e31820e5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer J, Gstoettner W, Baumgartner, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124:272–280. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- Kluk K, Moore BCJ. Factors affecting psychophysical tuning curves for normally hearing subjects. Hear Res. 2004;194:118–134. doi: 10.1016/j.heares.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kluk K, Moore BCJ. Factors affecting psychophysical tuning curves for hearing-impaired subjects. Hear Res. 2005;200:115–131. doi: 10.1016/j.heares.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Kong YY, Stickney GS, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Malicka AN, Munro KJ, Baker RJ. Diagnosing cochlear dead regions in children. Ear Hear. 2010;31:238–246. doi: 10.1097/AUD.0b013e3181c34ccb. [DOI] [PubMed] [Google Scholar]
- Mok M, Grayden D, Dowell R, et al. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J Speech Lang Hear Res. 2006;49:338–351. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- Moore BCJ. Dead regions in the cochlea: Diagnosis, perceptual consequences, and implications for the fitting of hearing aids. Trends Amplif. 2001;5:1–34. doi: 10.1177/108471380100500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ. Dead zones: What are they and what do you do about them? Hear J. 2009;62:10–14. [Google Scholar]
- Moore BCJ, Alcantara JI. The use of psychophysical tuning curves to explore dead regions in the cochlea. Ear Hear. 2001;22:268–278. doi: 10.1097/00003446-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Malicka AN. Cochlear dead regions in adults and children: Diagnosis and clinical implications. Sem Hear. 2013;34:37–50. [Google Scholar]
- Moore BCJ, Glasberg BR, Stone MA. New version of the TEN test with calibrations in dB HL. Ear Hear. 2004;25:478–487. doi: 10.1097/01.aud.0000145992.31135.89. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Huss M, Vickers DA, et al. A test for the diagnosis of dead regions in the cochlea. Br J Audiol. 2000;34:205–224. doi: 10.3109/03005364000000131. [DOI] [PubMed] [Google Scholar]
- Neuman AC, Svirsky MA. Effect of hearing aid bandwidth on speech recognition performance of listeners using a cochlear implant and contralateral hearing aid (bimodal hearing) Ear Hear. 2013;34:553–561. doi: 10.1097/AUD.0b013e31828e86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory test. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Preminger JE, Carpenter R, Ziegler CH. A clinical perspective on cochlear dead regions: Intelligibility of speech and subjective hearing aid benefit. J Am Acad Audiol. 2005;16:600–613. doi: 10.3766/jaaa.16.8.9. [DOI] [PubMed] [Google Scholar]
- Sęk A, Alcántara JI, Moore BCJ, et al. Development of a fast method for determining psychophysical tuning curves. Int J Audiol. 2005;44:408–420. doi: 10.1080/14992020500060800. [DOI] [PubMed] [Google Scholar]
- Sęk A, Moore BCJ. Implementation of a fast method for measuring psychophysical tuning curves. Int J Audiol. 2011;50:237–42. doi: 10.3109/14992027.2010.550636. [DOI] [PubMed] [Google Scholar]
- Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2011;33:112–117. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers V. Do tests for cochlear dead regions improve hearing aid fitting? J Acoust Soc Am. 2004;115(4):1420–1423. doi: 10.1121/1.1649931. [DOI] [PubMed] [Google Scholar]
- Summers V, Molis MR, Musch H, et al. Identifying dead regions in the cochlea: Psychophysical tuning curves and tone detection in threshold-equalizing noise. Ear Hear. 2003;24:133–142. doi: 10.1097/01.AUD.0000058148.27540.D9. [DOI] [PubMed] [Google Scholar]
- Vinay &, Moore BCJ. Prevalence of dead regions in subjects with sensorineural hearing loss. Ear Hear. 2007;28:231–241. doi: 10.1097/AUD.0b013e31803126e2. [DOI] [PubMed] [Google Scholar]
- Yanz JL. Cochlear dead regions and practical implications for high frequency amplification. Hear Rev. 2002;9:58–63. [Google Scholar]
- Zhang T, Dorman MF, Spahr AJ. Information from the voice fundamental frequency (F0) region accounts for the majority of the benefit when acoustic stimulation is added to electric stimulation. Ear Hear. 2010;31:63–69. doi: 10.1097/aud.0b013e3181b7190c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Sphar AJ, Dorman MF, Saoji A. Relationship between auditory function of nonimplanted ears and bimodal benefit. Ear and Hearing. 2012;34(2):133–41. doi: 10.1097/AUD.0b013e31826709af. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.