Abstract
Advances in imaging methods have led to new capability to study muscle and tendon motion in vivo. Direct measurements of muscle and tendon kinematics using imaging may lead to improved understanding of musculoskeletal function. This review presents quantitative ultrasound methods for muscle dynamics that can be used to assess in vivo musculoskeletal function when integrated with other conventional biomechanical measurements.
Keywords: muscle elastography, muscle kinematics, contraction velocity, strain rate, vector Doppler Imaging
1. INTRODUCTION
Musculoskeletal movement is accomplished through a synergistic action of skeletal muscles, tendons, and ligaments. Musculo-tendon units (MTUs) are responsible for force production, joint stabilization, and energy storage and are determining factors of movement performance. Altered muscle function can lead to muscle injury, movement abnormalities (e.g., altered gait), and has also been implicated in impaired joint health (e.g., cartilage degeneration) (44). Conversely, abnormalities in the soft tissue neighborhood of muscle and fascia, such as myofascial trigger points, have been associated with chronic myofascial pain and limited joint range of motion. The pathophysiological mechanisms of many complex musculoskeletal problems (e.g., osteoarthritis, myofascial pain syndrome) remain poorly understood, in part because of the lack of robust measures to directly and concurrently quantify the multifactorial aspects of dynamic function of individual musculo-tendon units in vivo.
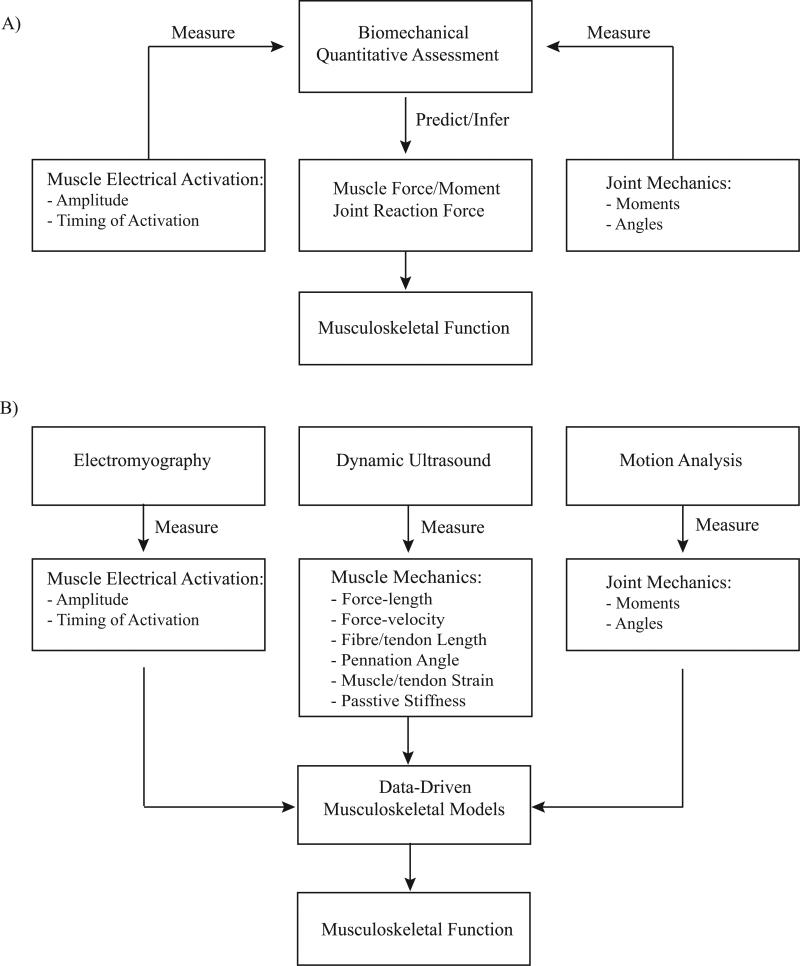
In the fields of exercise and sport sciences and biomechanics, the focus of dynamic assessment of human movement has typically been performed at the joint level (e.g., range of motion, joint kinematics). These measurements are used to either qualitatively or quantitatively infer the underlying function of the musculoskeletal system during dynamic tasks. In particular, important intrinsic parameters that cannot be measured experimentally, such as muscle force and moment as well as joint load, have been estimated from in vivo measurements using biomechanical analysis of musculoskeletal models, as shown in Fig. 1A. While computational models have provided insight into musculoskeletal function, the outcome is model-based estimation and subject to deviation from true values due to inherent limitations (e.g., muscle architecture is primarily based on cadaveric data).
Figure 1.
A) Traditional, and B) integrative biomechanical framework for assessment and quantification of dynamic musculoskeletal function.
If dynamic assessments of musculoskeletal function (e.g., contraction velocity, muscle strain development and viscoelasticity) can be conducted in vivo during natural movement tasks, the mechanical properties such as the force-length relationship can be directly quantified instead of being derived from animal and cadaveric data. Furthermore, muscles’ architectural parameters (i.e., pennation angle) are not constant during movement, and vary as a function of the kinematic configuration of the joint(s) that a muscle spans. Thus, a comprehensive characterization of dynamic muscle function requires in vivo measurement of these parameters as well. Lastly, as previously noted (1), inherent muscle and subject variations may also affect accuracy of the simulation, and consequently its inference. Therefore, subject-specific models that incorporate measurements of intrinsic muscle dynamics are more desirable in order to obtain consistent and realistic predictions.
Advanced imaging methods, such as ultrasonography (US) and Magnetic Resonance Imaging (MRI) have been applied to directly assess individual muscles in vivo and provide detailed information about muscle dynamics (20,44). In recent years, there has been increasing research efforts in developing dynamic measures of musculo-tendon mechanical properties during movement using cine phase-contrast MRI (2), cine DENSE MRI (45), and large-bore real-time MRI (19). A number of US-based methods, including our own, have been proposed to quantify the dynamic nature of muscle contraction (and tendon stretch) visible on real time sonography (10,11). Elastography methods can be used to objectively assess muscle viscoelastic properties. Further, assessment of muscle contraction velocity enables us to precisely determine the muscle contraction characteristics (eccentric versus concentric) at different time points during movement so that an individual muscle's function as an actuator, decelerator, or a stabilizer for that particular movement can be better understood (27). Muscle contraction velocity may provide evidence about muscle heterogeneity, which has not been extensively examined in vivo. In particular, muscle deformation across different dynamic behaviors, reflecting uniform or non-uniform muscle characteristics may be useful to quantify disparities between healthy and pathological conditions.
The purpose of this paper is to review quantitative techniques that build on the recent advances in using imaging for quantifying dynamic function of musculo-tendon units, and present applications of these methods relevant to exercise and sport sciences. We propose that a novel integrative approach of these dynamic muscle and tendon measures with conventional joint kinematics using mathematical models can lead to novel insight into normal musculoskeletal function, as well as the pathophysiological mechanisms underlying complex musculoskeletal disorders.
2. A NEW FRAMEWORK FOR INTEGRATING MUSCLE AND JOINT KINEMATICS
Normal and pathological muscle function can be differentiated by a number of intrinsic muscle properties, such as shortening velocities, and active and passive stiffness (10). We have developed techniques in our laboratories that quantify muscle-tendon dynamics in vivo including contraction velocity, strain development, and viscoelastic material properties using dynamic ultrasound imaging (15). We have also developed novel subject-specific biomechanical models to simulate normal and pathological movement (43). Incorporating in vivo musculotendinous measurements into traditional biomechanical analysis of dynamic tasks (Fig. 1B) will provide new insights into normal and abnormal musculoskeletal function at the individual level. For example, this framework can be used to test specific hypothesis about the role of asymmetries of muscle strength in a symptomatic patient after anterior cruciate ligament reconstruction. It also provides a quantitative approach to measure stretch-shortening cycle in vivo (that is, muscle lengthens before it shortens to generate faster movement) and study how it enhances athletic performance in sports involving plyometric and fast movements (20). In the following sections, we will describe our methods for measuring velocity, strain, stiffness of muscle and tendon and how they can be applied in exercise and sports sciences. We will also present our results and discuss technical considerations in integrating multiple measurements.
2.1 MEASUREMENT OF MUSCLE AND TENDON CONTRACTION VELOCITY AND STRAIN
Ultrasound imaging is uniquely suited to visualize and track real-time movement of muscles and tendons (10). Fascicles in muscle and collagen strands in tendons can be clearly envisioned using ultrasound imaging due to the differences in acoustic properties at the interfaces of different tissue types. As sound propagates through the tissue microstructure, constructive and destructive interference creates a speckle pattern that is a unique signature of the underlying tissue. The movement of these structures and the associated speckle patterns can be clearly observed and further analyzed as the muscle contracts (36).
There is a long history of estimating dynamic tissue motion using ultrasound, starting with the motion mode (or M-mode) display for tracking heart valve leaflets that is still in use today (38). More quantitative approaches have evolved that rely on signal and image processing to provide semi-automatic estimates of motion. The methods proposed in the literature for muscle and tendon tracking can be divided into three main approaches: (1) speckle-tracking methods that use cross-correlation on raw radiofrequency (RF) ultrasound data or envelope-detected gray scale (or B-mode) image data. These techniques have been widely used in both musculoskeletal (6) and cardiac (36) muscle motion tracking and estimation; (2) methods that track the muscle fascicles or anatomical features (27) and may use image processing for further semi-automatic analysis, and (3) tissue Doppler imaging techniques used in both cardiac and skeletal muscle (23) motion estimation. These methods, while being very promising, suffer from several limitations, including susceptibility to decorrelation (speckle tracking), user intervention (feature-based tracking), and dependence on insonation angle (tissue Doppler). In the following sections, we will describe methods that we have developed to overcome these limitations and accomplish robust tracking of muscle and tendon motion under a number of different conditions.
2.1.1 Tracking Muscle and Tendon Motion from Ultrasound Video Sequences
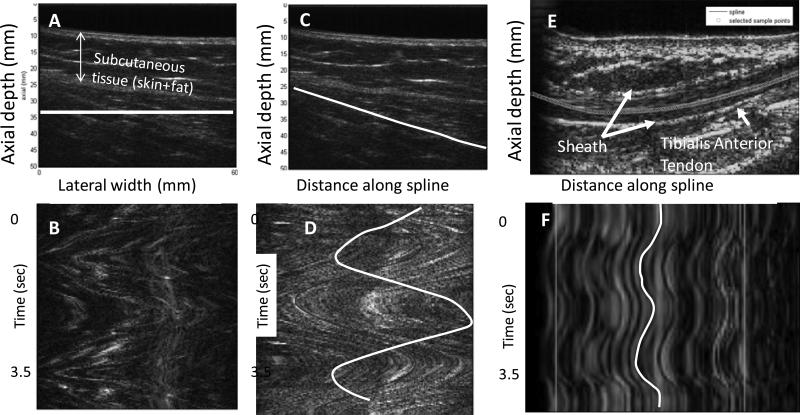
Deformation of muscle and tendon in vivo using B-mode ultrasound sequences have been previously reported (32). Typically, these approaches rely on visualizing the fascicles, aponeuroses, myotendinous junction or other visible landmarks. Once displacement is estimated, strain can be calculated by estimating differences in displacement between two points separated by a known distance. We have proposed a method for tracking muscle motion along the direction of the fascicles that can be broadly applicable even in situations where the entire muscle fascicle cannot be visualized (14,15). Our approach is inspired by the M-mode and kymography displays. The M-mode display has time on the horizontal axis, distance into tissue on the vertical axis and the image brightness corresponding to the strength of the echo. Moving tissue at different depths forms a trace on this display that reflects the waveform of tissue displacement. Such an image display, with time on one axis and a spatial dimension along another can be reconstructed from ultrasound video sequences and helps to visualize waveforms of motion. Given a sequence of gray scale images (2D + time), M-mode image displays can be derived by resampling pixels along an arbitrary line or curve in the 2D image (Fig. 2). This resampled M-mode (or curved M-mode) can be used to track motion along an arbitrary curve (14,15). Either manual operations or cross-correlation based image processing techniques can be exploited to estimate motion from these curved M-model images. The curves do not need to be fixed and can adapt to deforming muscle through image processing algorithms.
Figure 2.
A) Gray-scale B-mode image of rectus femoris muscle during repetitive flexion and extension. (B) M-mode image reconstructed by resampling pixels along the horizontal line indicated in white. The M-mode image shows the pattern of muscle motion back and forth over the course of the knee flexion and extension experiment. (C) A manually initialized curve indicating the approximate orientation of the fascicles. (D) M-mode image reconstructed by resampling pixels along the curved line in (C). The sinusoidal pattern of the muscle contraction and relaxation during knee flexion and extension is clearly visualized. A comparison of the patterns in (C) and (D) demonstrates that to obtain the true pattern of motion, the curve should be aligned with the fascicle direction. (E) B-mode image of the tibialis anterior tendon with a spline curve indicating the location of the tendon. (F) Resampled M-mode image of tibialis anterior tendon generated by sampling pixels along the curve through time. Two cycles of dorsiflexion and relaxation are shown. (Reproduced with permission from IEEE (17)).
2.1.2 Vector Tissue Doppler for Quantifying the Magnitude and Direction of Motion
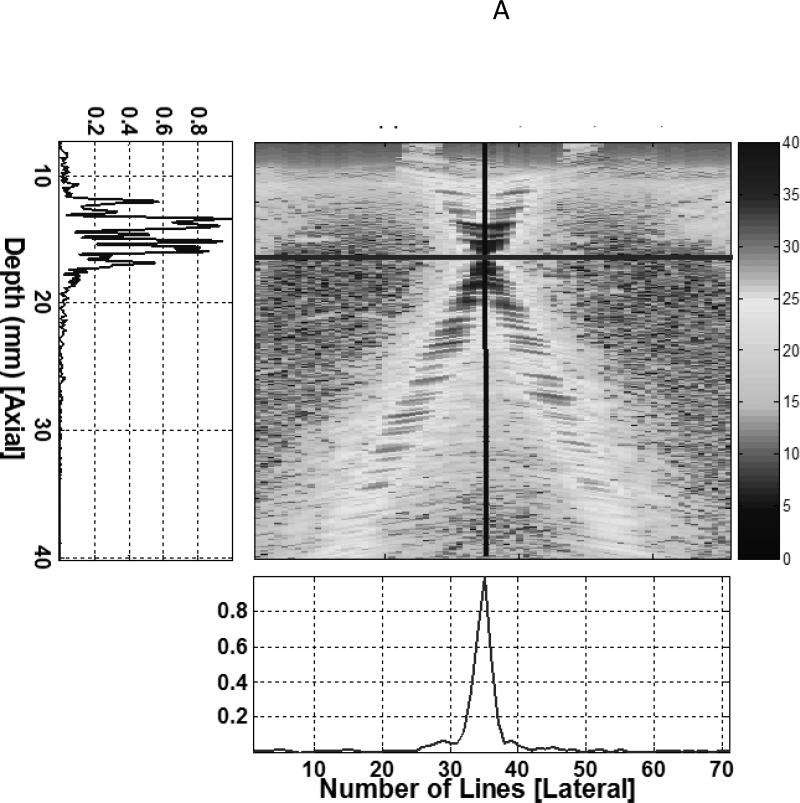
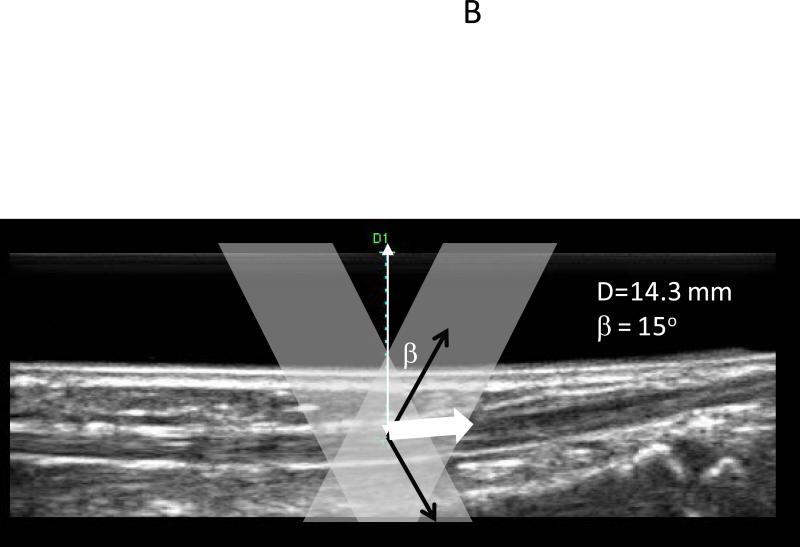
Image based methods are limited by the ability to visualize the underlying motion, and their temporal resolution is limited by the imaging frame rate. Velocity estimation methods that do not require visual tracking of the underlying features are attractive for applications where there is inadequate visualization or fast motion (16). Doppler methods have been used clinically for several decades to estimate blood flow velocities (26). Pulsed Doppler methods estimate the velocity of scatterers in a range gate by tracking the change of phase between consecutive pulse transmissions. Tissue Doppler utilizes this concept to measure tissue motion rather than blood velocities. Tissue Doppler methods have been used to measure skeletal muscle contractions (23), however only the component of the velocity along the path of the ultrasound beam can be estimated, and this is a major limitation of conventional Doppler. This limitation is relevant since muscle and tendon motion is typically along the skin surface and perpendicular to the direction of ultrasound propagation, most of the longitudinal musculotendinous motion is missed during motion estimation. Vector Doppler (13) is a technique that can overcome this limitation by utilizing two or more ultrasound beams steered at different angles and estimating the velocity components at multiple angles. These components can then be appropriately combined to get the velocity vector. We have developed a vector Doppler system for tracking muscle and tendon motion (14). We electronically split the transducer array into two apertures that were steered in different directions by programming the ultrasound transmission and reception parameters on an ultrasound system with a research interface (Sonix RP, Ultrasonix Corp., Vancouver, BC). This allows us to estimate two independent components of the underlying velocity, which are then combined in the correct tissue geometry to yield the velocity vector (Fig. 3).
Figure 3.
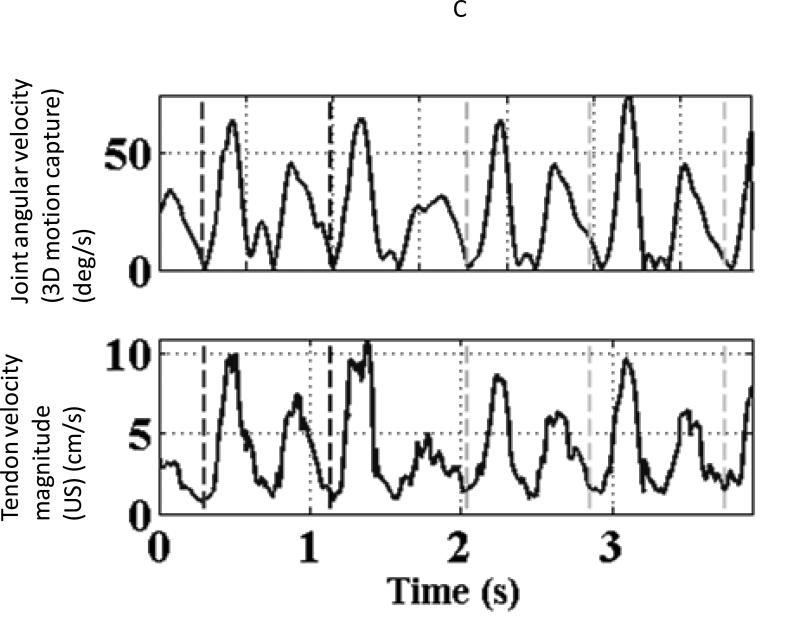
(A) The beam profiles for a vector Doppler imaging visualized using a beam profile phantom. The overlap region between the beams can be adjusted to different depths electronically by changing the separation between the two apertures. (B) B-mode image showing the anterior tibialis tendon visualized at a depth of 14 mm. The overlay denotes a schematic of the two-ultrasound beams used for vector Doppler imaging. The beams were steered at 15° with respect to the normal and velocity estimation was done using 2D Fourier transform. The individual velocity estimates (black arrows) along each beam can be combined geometrically to yield the resultant velocity vector (solid white arrow) (C) Joint angular velocities and corresponding velocity waveforms of the tibialis anterior tendon measured using vector Doppler during four cycles of dorsiflexion and relaxation. The peak magnitude of the velocity vector of the tendon is plotted, and this velocity magnitude was obtained from two separately measured components: axial (perpendicular to the ultrasound transducer) and lateral (parallel to the ultrasound transducer). The separate components are not shown. (Reproduced with permission from IEEE (14)).
The magnitude of the resultant velocity vector from the individual Doppler shift components along each beam is calculated as previously described (37) and has been validated (18). Once the velocity components are obtained, they are further processed to estimate the strain and strain rate. The lateral and axial strain rates are calculated using the spatial gradients in the lateral and axial velocities. The axial (across the fibers) and lateral (along the fibers) strain rates, with positive representing contraction and negative representing relaxation, may be calculated by integrating the axial and lateral velocities, respectively.
2.2 METHODS FOR MEASURING MUSCLE STIFFNESS
A number of methods for qualitatively and quantitatively mapping the mechanical properties of soft tissue have been introduced in the past two decades, primarily using MRI and US. The mapping of mechanical properties of tissue using imaging can broadly be referred to as elasticity imaging, although specific methods have been described using a number of different names, including strain imaging, transient elastography, sonoelastography, shear wave elastography imaging, acoustic radiation force imaging, among others (4,6). These methods have been reviewed elsewhere (39). Elasticity imaging methods are based on fundamental principles of material mechanics that define the relationships between stress, strain and modulus of elasticity, and the relationships between the speed propagation of elastic shear waves and shear modulus. All existing methods involve some perturbation of the material of interest and measurement of the resulting tissue response using imaging techniques to infer the underlying mechanical properties.
For an isotropic incompressible material undergoing small deformations in response to a quasi-static compression, the applied uniaxial stress (σ) is related to the induced uniaxial strain (ε) through the elastic (Young's) modulus (E). Ultrasound imaging can be used to estimate the induced deformation or strain by tracking tissue displacements and this method is referred to as strain imaging (40). In strain imaging, the distribution of stress in the tissue is unknown; therefore the Young's modulus of elasticity cannot be directly estimated. Furthermore, for heterogeneous tissue, the strain image is a manifestation of the overall distribution of material properties, and is not necessarily representative of the local elastic modulus. Strain imaging can be readily implemented in real time as we have shown in previous work (40), and many commercial ultrasound systems now implement strain imaging.
Quantitative elastography methods, such as magnetic resonance elastography (MRE) (7) and US shear wave elastography (35), attempt to estimate the speed of a propagating shear wave in soft tissue, which depends upon the underlying mechanical properties. For a linear elastic material under a harmonic excitation, the shear modulus (μ) can be derived from the propagation speed of the shear wave (vs). For viscoelastic materials, such as most soft tissue, the shear wave speed exhibits dispersion with the frequency of the harmonic excitation (12). This approach has been previously used to measure the mechanical properties of skeletal muscles (7,12,30). In our previous work (4), we have performed shear wave elastography in a commercial ultrasound system (Ultrasonix Sonix RP) to assess muscle stiffness.
3. APPLICATIONS TO EXERCISE AND SPORTS SCIENCES
Muscle contraction velocity, muscle strain, muscle volume, and cross-sectional area are important factors that determine muscle force production capability (44). In our previous work, we have reported that lower extremity mechanics is altered post-fatigue during sidestep cutting and crossover tasks (8,9). However, from our results and others it remains unclear if altered movement patterns post-fatigue are caused by a central or peripheral fatigue mechanism (8,9,33). To that purpose, vector Doppler methods can be applied to quantify peripheral muscle dynamic changes (e.g., contraction velocity, strain) during fatigue processes that may be leading to altered movement patterns, changes in patterns of recruitment among different muscle groups, and decreased muscle's ability to contract. We have also applied vector Doppler methods to quantify muscle contraction velocity and muscle strain during weight bearing activities to examine quadriceps femoris muscle dynamics (16). The aim was to comprehend the potential muscle contribution to increased knee joint moments (e.g., knee adduction moment) (31). This is not only particularly relevant in sports that may place individuals at higher risk for anterior cruciate ligament injuries, but also to those individuals that experience anterior cruciate ligament reconstruction (ACLr) and are more susceptible to early onset of knee osteoarthritis. The quadriceps atrophy subsequent to ACLr has been associated with the inability to maximize muscle force production to protect the knee joint from harmful forces (44). Since it is impossible to measure instantaneous muscle force in vivo, measurements of muscle strain, strain rate and instantaneous shortening/lengthening velocity using ultrasound can be used as surrogate measurements. Strain rate is the temporal derivative of strain, and the spatial derivative of the instantaneous velocity field. These measures of shortening velocity can be related to muscle force production through the force velocity curve of muscle fibers. The concept of strain and strain rate has been used in the myocardium as a surrogate for force production,(26) and a similar argument can be made in skeletal muscles as well.
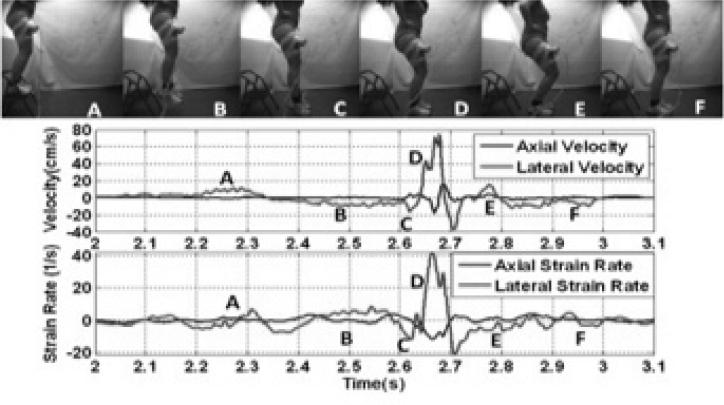
In our recent work, we have implemented the ultrasound imaging methods described above that enable the possibility to quantify quadriceps muscle dynamics during a weight-bearing task. We have quantified axial and lateral quadriceps femoris velocities, as well as axial and lateral quadriceps femoris strain in 8 subjects during a drop landing (15). Fig. 4 shows the lateral and axial velocities obtained using vTDI during a jump sequence. We found that the quadriceps femoris velocity was predominant in the lateral component during the flexion-extension phase, and its peak occurred immediately after ground impact. Strain rate occurred primarily in the lateral direction immediately post contact with the ground (40 1/s), and was higher than in the axial direction (−10 1/s). Our initial results further suggest that vTDI presents an opportunity to quantify in vivo quadriceps femoris contraction velocity and strain development to elucidate on the potential individual and collective contributions to altered movement patterns that may be involved in 1) increased knee loads that may lead to ACL injury, and 2) pathophysiological process contributing to the development of early knee OA post ACLr.
Figure 4.
The experimental setup for measuring the rectus femoris muscle velocities and strain rate during a drop-landing task, and representative values of the axial and lateral velocities, and strain rate during the drop-landing task. The top panel shows representative frames from a high speed motion capture showing different phases of the drop landing. The middle panel shows the measured axial (perpendicular to the transducer) and lateral (parallel to the ultrasound transducer) velocity waveforms of the rectus femoris muscle, measured using vector Doppler. The bottom panel shows the corresponding strain rates (spatial derivative of velocity). The different phases of the drop landing task are indicated with the letters A-F corresponding to the frames from the high speed video. (Reproduced with permission from IEEE (15)).
Plyometric exercises based on the stretch-shortening cycle (SSC) mechanism of musclotendonunits is used in athletic training to enhance performance (24). Energy that is stored in an actively lengthened musculo-tendon unit and then quickly released during active shortening provides greater force and increased speed during movement such as jumping and sprinting. SSC during athletic movement has been actively studied in the sports science and biomechanics communities (25,42) to understand its mechanism in specific muscles and specific sports. Most existing work on investigating SSC in vivo applied kinematic analysis. Muscle and tendon of the same MTU are not distinguished and simplified as of one point-to-point unit, the length change of which is estimated geometrically from tracked joint angles. It possesses obvious difficulty in studying the underlying mechanism of biarticular muscles, important in energy storage and transmission across two joints, (22) since muscle and tendon may maintain isometric length and lengthen at the same time, illustrated by ultrasound analysis (20). Our methodology based on vTDI and other ultrasound studies (28) offers a better methodology to quantify biarticular muscle SSC in athletic exercises by measuring local muscle and tendon deformation, impossible to do in conventional kinematic approaches. We have demonstrated the measurement of quadriceps femoris muscle deformation during drop jump, one common plyometric exercise. Combined with motion capture, our technique can be used to quantify SSC that can lead to improve training progress and methods.
Altered mechanical properties of muscle can also affect function. We have utilized shear wave imaging to understand the mechanical properties of the upper trapezius muscle in human subjects with myofascial pain syndrome (MPS). MPS is a common, non-articular musculoskeletal disorder that is characterized by myofascial trigger points (MTrPs). MTrPs are hard, palpable, discrete, localized nodules located within taut bands of skeletal muscle, which are painful on compression. MTrPs can be either active or latent, and are highly prevalent in selected populations: 85-93% of patients with chronic pain disorders presenting to specialty pain management centers have MPS (21). Further, MTrPs can impair athletic performance, such as upper trapezius trigger points for throwing athletes; quadriceps femoris trigger points for idiopathic knee (34). MTrPs do not only lead to impaired performance in sports, as they can increase the likelihood of other chronic problems (e.g., altered gait, posture). Despite its high prevalence, the underlying mechanisms of MPS are poorly understood. In particular, very little is known about the pathophysiology and soft tissue environment of the MTrP. Therefore, there is a clinical need within the sports science field to objectively quantify and evaluate the structural properties of skeletal muscle and the neighborhood of MTrPs in vivo, relate the local properties of the soft tissue milieu to dynamic function, and identify whether postural biomechanical factors play a role in perpetuating the MTrPs.
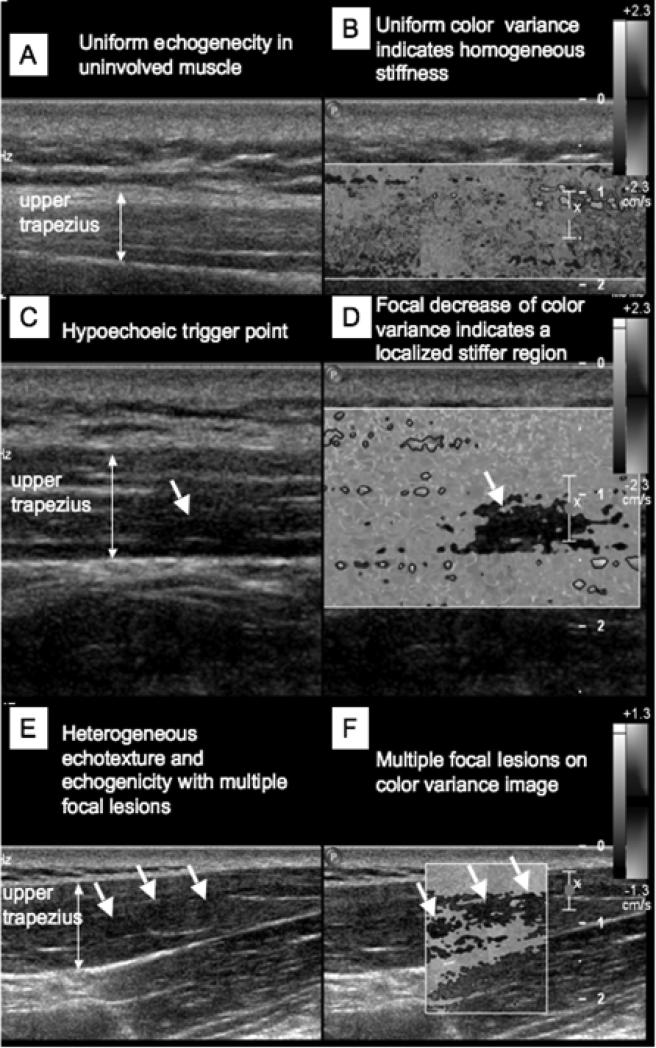
Using ultrasound elastography, we have visualized the location of suspected MTrPs that are correlated with the palpable nodules (Fig. 5) (41), and objectively described the MTrPs and their milieu in terms of their size (3) and mechanical properties (4). We have shown that the viscoelastic properties of the muscle can be quantitatively measured using off-the-shelf equipment using the principle of shear wave elastography by externally vibrating the muscle at different frequencies. There is a significant difference between the shear speeds in muscle with active MTrPs compared to normal muscle in frequencies above 150 Hz (4). These objective measures can be utilized as outcome measures to evaluate treatment efficacy, and assist with performance enhancement. By integrating measures of dynamic function of the affected muscle with biomechanical measures of respective body segments, we can investigate the role of biomechanical factors in the perpetuation and pathogenesis of MTrPs. The utilization of an integrative approach is not only beneficial for treatment of MTrPs, as well as to avoid impaired performance and long-term health problems in sports and exercise. Another example of the application of ultrasound to enable direct measurement of musculo-tendon biomechanics done by our group is tracking the tibialis anterior during dorsiflexion (17). The goal was to understand the role of tendon in functional improvements following treatments for foot drop such as functional electric stimulation of the peroneal nerve. In particular (17), we have developed ultrasound methods for tracking the tibialis anterior tendon in children with cerebral palsy and foot drop (Fig. 2 E and F).
Figure 5.
Simultaneous 2D grayscale and color variance imaging. (A and B) Normal upper trapezius muscle. The normal muscle appears isoechoic and has uniform color variance (C and D) Muscle with a palpable MTrP. A hypoechoic region and a well-defined focal decrease of color variance indicating a localized stiffer region are visible. (E and F) Muscle with palpable MTrPs. Multiple hypoechoic regions and multiple focal nodules are visible. (Reproduced with permission from APMR (41)).
Combining intrinsic dynamic muscle measures with conventional joint kinematics can provide insight into the dynamics of tendon. In particular, we have shown that vector Doppler imaging can be used to measure instantaneous acceleration of muscle during a tendon tap (29). By comparing the timing and direction of the muscle velocities, we were able to clearly differentiate between the initial passive stretch of the muscle immediately after a tendon tap and before the reflex activation. Based on these measurements, we could calculate the instantaneous velocity and acceleration of the passive muscle stretch induced by a patellar tendon tap, and showed that the acceleration values during a clinical patellar tendon tap are outside the ranges encountered during normal activities of daily living.
4. TECHNICAL CONSIDERATIONS
We have presented methods and novel applications of ultrasound imaging to quantify musculotendon function. Accuracy and precision of these measurements are important considerations. Since ultrasound enables direct visualization of moving anatomical features, the accuracy of the measurements has largely been taken for granted by researchers in the field. Phase contrast MRI has been used for estimating musculoskeletal kinematics (5), although no direct comparisons between ultrasound and phase-contrast MRI has been performed to date. Reproducibility and precision are the major issues when using dynamic ultrasound measurements, and considerable attention has been paid to these issues. There are a number of technical issues to consider when integrating ultrasound measurements with those acquired using other instruments. Ultrasound images represent tomographic slices through the anatomy of interest. The orientation of these slices depends upon the placement of the ultrasound probe with respect to the anatomy. There is no world coordinate system associated with ultrasound imaging unlike MRI. Ultrasound images are always referenced to the coordinate system associated with the transducer. If a handheld transducer is used for imaging, the coordinate system will be changing with movement of the transducer relative to the anatomy. Therefore, for quantitative assessment, placement of the transducer relative to the anatomy is an important consideration, especially if repeated measurements are being performed. Further, typically ultrasound images represent 2D slices through the body, whereas the motion is in 3D. As a result, the measures derived from ultrasound imaging only measure one or two components of the true 3D motion. In recent years, 3D ultrasound imaging using matrix arrays have been introduced, which might be able to estimate true 3D motion. The typical approach is to use anatomical landmarks to place the ultrasound transducer. For further quantification of the ultrasound imaging slice, a magnetic position tracking system (e.g., 3D TrakStar, Ascension Technologies Ltd) can be used, with one six-degree of freedom sensor placed on the ultrasound probe and a number of other sensors placed on anatomical landmarks.
During dynamic tasks, the ultrasound transducer is often attached to the anatomy of interest using an external cuff. While a properly designed cuff can minimize movement between the transducer and fixed anatomic landmarks, some motion is nevertheless inevitable due to the movement of the skin and underlying subcutaneous tissue especially for highly dynamic tasks. Even slight motion that alters the angulation of the imaging slice can lead to profound changes in the appearance of the ultrasound image and the speckle pattern. This is a major challenge for automatic and semi-automatic image analysis methods for quantifying motion based on the image sequence, and automated tracking algorithms can fail in the presence of significant out-of-plane motion. However, Doppler-based methods, as the ones we have implemented, are less sensitive to this motion, since it relies not on unique anatomical features, but on the phase shift of the ultrasound echoes due to the underlying motion. The transducer motion contributes to the variance of the Doppler estimate, and with proper analytic techniques could be modeled as an additional source of noise.
Another important consideration in utilizing multiple measurements is temporal synchronization between multiple instruments (e.g. motion capture, ultrasound). While motion capture and EMG systems have built-in capability to synchronize based on external triggers, many ultrasound instruments are not capable of being controlled externally. Using the timing of ultrasound acquisition as the master control can facilitate the synchronization. In our previous work (29), we have used a trigger from the ultrasound instrument to synchronize multiple instruments. A current alternative strategy that we are using is a pressure sensor instrumented to a keyboard that is used to control the ultrasound acquisition. This pressure sensor emits a transistor-transistor logic (TTL) signal to synchronize the ultrasound system with the other systems (i.e., motion capture). The data from the different instruments are acquired at different sampling rates, thus they need to be resampled to the same sampling rate for analysis. The choice of appropriate filtering during the resampling process is important to prevent any distortion of the temporal signals.
Using dynamically measured muscle contraction velocity and strain, dynamic architectural parameters such as pennation angle, and viscoelastic tissue properties, certain muscle mechanical characteristics such as fiber operating length and force-length curve can be determined for specific muscles of a subject and then integrated in musculoskeletal simulation with other available measurement as previously discussed. Developing integrated data-driven biomechanical models is nontrivial and time-consuming. It is informative to analyze inter-subject and intra-subject variations, and understand the underlying sources of error. The underlying reasons for between subject variability cannot be controlled, thus significant population variability is to be expected in the measurements. However with proper standardization, it is feasible to limit variability in repeated measurements on the same subject. With this in mind, subject-specific biomechanical models might have their greatest utility in longitudinal comparisons, for example before and after an intervention, where the subject acts as their own control. As part of the standardization process, careful attention should be paid to reproducibility of the spatial position and orientation of the ultrasound transducer, with respect to anatomical landmarks. In addition, when developing subject-specific models, it may not be realistic to examine all the muscles and tendons, therefore one needs to be cautious while integrating different data/parameter sources, either from literature or from subject-specific measurement, and justifying possible inconsistency and its influence when interpreting results.
5. SUMMARY
Musculoskeletal function has been primarily quantified through indirect measurements, inferred from computational models, or in non-dynamic conditions. This limitation deters the assessment and integrative understanding of the pathophysiological process of complex disorders. While MRI measures can provide detailed information about muscle structures, its ability to quantify muscle dynamics (e.g., contraction velocity, strain) during natural activities (e.g., gait) remains limited. In this review, we have presented methods to assess in vivo muscle dynamics using ultrasound imaging, and how it can be integrated with other traditional measures (e.g., motion analysis, electromyography). Our novel integrative concept can be utilized to quantify musculoskeletal function both directly from experimental data, and as well to serve as inputs for data-driven biomechanical models providing subject-specific information (e.g., muscle properties). Future efforts should implement this framework due to its potential to enhance our understanding of musculoskeletal function and consequently the pathophysiological process of chronic pathologies. Ultrasound imaging instruments continue to be miniaturized, and now portable probes that plug into standard universal serial bus (USB) interfaces of laptop computers and smartphones are commercially available. These advances will further overcome the logistical difficulties of tethering instruments to subjects during highly dynamic tasks, and further facilitate the use of imaging for the study of musculoskeletal function.
Acknowledgments
Funding: This research was supported in part by grant #1R01-AR057348 from National Institutes of Arthritis and Musculoskeletal and Skin Diseases at the NIH, and grant #0953652 from the National Science Foundation.
REFERENCES
- 1.Arnold EM, Delp S. Fiber operating lengths of human lower limb muscles during walking. Phil Trans R Soc B. 2011;366(1570):1530–9. doi: 10.1098/rstb.2010.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asakawa DS, Nayak KS, Blemker SS, et al. Real-time imaging of skeletal muscle velocity. Journal of magnetic resonance imaging : JMRI. 2003;18(6):734–9. doi: 10.1002/jmri.10422. [DOI] [PubMed] [Google Scholar]
- 3.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30(10):1331–40. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballyns JJ, Turo D, Otto P, et al. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med. 2012;31(8):1209–19. doi: 10.7863/jum.2012.31.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behnam AJ, Herzka DA, Sheehan FT. Assessing the accuracy and precision of musculoskeletal motion tracking using cine-PC MRI on a 3.0T platform. J Biomech. 44(1):193–7. doi: 10.1016/j.jbiomech.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PG, Alsousou J, Cooper A, Thompson MS, Noble JA. The AutoQual ultrasound elastography method for quantitative assessment of lateral strain in post-rupture Achilles tendons. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Basford J, An KN. Ability of magnetic resonance elastography to assess taut bands. Clin Biomech (Bristol, Avon) 2008;23(5):623–9. doi: 10.1016/j.clinbiomech.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes N, Greska E, Kollock R, Ambegaonkar J, Onate JA. Changes in lower extremity biomechanics due to a short-term fatigue protocol. Journal of athletic training. 2013;48(3):306–13. doi: 10.4085/1062-6050-48.2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes N, Quammen D, Lucci S, Greska E, Onate J. A functional agility short-term fatigue protocol changes lower extremity mechanics. Journal of sports sciences. 2012;30(8):797–805. doi: 10.1080/02640414.2012.671528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin NJ, af Klint R, Grey MJ, Sinkjaer T. Ultrasonography as a tool to study afferent feedback from the muscle-tendon complex during human walking. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 2011;21(2):197–207. doi: 10.1016/j.jelekin.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Cronin NJ, Avela J, Finni T, Peltonen J. Differences in contractile behaviour between the soleus and medial gastrocnemius muscles during human walking. The Journal of experimental biology. 2013;216(Pt 5):909–14. doi: 10.1242/jeb.078196. [DOI] [PubMed] [Google Scholar]
- 12.Deffieux T, Montaldo G, Tanter M, Fink M. Shear wave spectroscopy for in vivo quantification of human soft tissues visco-elasticity. IEEE transactions on medical imaging. 2009;28(3):313–22. doi: 10.1109/TMI.2008.925077. [DOI] [PubMed] [Google Scholar]
- 13.Dunmire B, Beach KW, Labs K, Plett M, Strandness DE., Jr Cross-beam vector Doppler ultrasound for angle-independent velocity measurements. Ultrasound Med Biol. 2000;26(8):1213–35. doi: 10.1016/s0301-5629(00)00287-8. [DOI] [PubMed] [Google Scholar]
- 14.Eranki A, Bellini L, Prosser L, et al. Measurement of tendon velocities using vector tissue Doppler imaging: a feasibility study. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2010;2010(5310-3) doi: 10.1109/IEMBS.2010.5626323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eranki A, Cortes N, Ferenček ZG, Kim JJ, Sikdar S. Real-time measurement of rectus femoris muscle kinematics during drop jump using ultrasound imaging: A Preliminary study. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2012;2012(1):4851–4. doi: 10.1109/EMBC.2012.6347080. [DOI] [PubMed] [Google Scholar]
- 16.Eranki A, Cortes N, Ferenček ZG, Sikdar S. A Novel Application of Musculoskeletal Ultrasound Imaging. J Vis Exp. 2013;79(e50595) doi: 10.3791/50595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eranki A, Otto P, Curatalo L, et al. Measurement of tendon velocities using vector tissue Doppler imaging and curved M-mode in patients with cerebral palsy. Ultrasonics Symposium (IUS), 2011 IEEE International. 2011:676–9. [Google Scholar]
- 18.Eranki A, Sikdar S. Experimental characterization of a vector Doppler system based on a clinical ultrasound scanner. Conf Proc IEEE Eng Med Biol Soc. 2009:2260–3. doi: 10.1109/IEMBS.2009.5334972. [DOI] [PubMed] [Google Scholar]
- 19.Fiorentino N, Lin JS, Ridder KB, Guttman MA, McVeigh ER, Blemker SS. Rectus femoris knee muscle moment arms measured in vivo during dynamic motion with real-time magnetic resonance imaging. J Biomech Eng. 2013;135(4):044501. doi: 10.1115/1.4023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc Biol Sci. 2001;268(1464):229–33. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412–20. doi: 10.1007/s11916-001-0052-8. [DOI] [PubMed] [Google Scholar]
- 22.Gregoire L, Veeger HE, Huijing PA, van Ingen Schenau GJ. Role of mono- and biarticular muscles in explosive movements. International journal of sports medicine. 1984;5(6):301–5. doi: 10.1055/s-2008-1025921. [DOI] [PubMed] [Google Scholar]
- 23.Grubb NR, Fleming A, Sutherland GR, Fox KA. Skeletal muscle contraction in healthy volunteers: assessment with Doppler tissue imaging. Radiology. 1995;194(3):837–42. doi: 10.1148/radiology.194.3.7862989. [DOI] [PubMed] [Google Scholar]
- 24.Hay JG. Length changes of muscle-tendon units during athletic movements. In: Nigg BM, Macintosh BR, Mester J, editors. Biomechanics and Biology of Movement. Human Kinetics; 2000. [Google Scholar]
- 25.Hay JG, Thorson EM, Kippenhan BC. Changes in muscle-tendon length during the take-off of a running long jump. Journal of sports sciences. 1999;17(2):159–72. doi: 10.1080/026404199366262. [DOI] [PubMed] [Google Scholar]
- 26.Heimdal A, Stoylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1998;11(11):1013–9. doi: 10.1016/s0894-7317(98)70151-8. [DOI] [PubMed] [Google Scholar]
- 27.Ichinose Y, Kawakami Y, Ito M, Kanehisa H, Fukunaga T. In vivo estimation of contraction velocity of human vastus lateralis muscle during “isokinetic” action. J Appl Physiol. 2000;88(3):851–6. doi: 10.1152/jappl.2000.88.3.851. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa M, Niemela E, Komi PV. Interaction between fascicle and tendinous tissues in short-contact stretch-shortening cycle exercise with varying eccentric intensities. J Appl Physiol. 2005;99(1):217–23. doi: 10.1152/japplphysiol.01352.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lebiedowska MK, Sikdar S, Eranki A, Garmirian L. Knee joint angular velocities and accelerations during the patellar tendon jerk. J Neurosci Methods. 2011;198(2):255–9. doi: 10.1016/j.jneumeth.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Levinson SF, Shinagawa M, Sato T. Sonoelastic determination of human skeletal muscle elasticity. J Biomech. 1995;28(10):1145–54. doi: 10.1016/0021-9290(94)00173-2. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd DG, Buchanan TS, Besier TF. Neuromuscular biomechanical modeling to understand knee ligament loading. Med Sci Sports Exerc. 2005;37(11):1939. doi: 10.1249/01.mss.0000176676.49584.ba. [DOI] [PubMed] [Google Scholar]
- 32.Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586(1):71–81. doi: 10.1113/jphysiol.2007.139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean SG, Samorezov JE. Fatigue-induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc. 2009;41(8):1661–72. doi: 10.1249/MSS.0b013e31819ca07b. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen BM. Myofascial trigger point, falls in the elderly, idiopathic knee pain and osteoarthritis: an alternative concept. Medical hypotheses. 2013;80(6):806–9. doi: 10.1016/j.mehy.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Nordez A, Hug F. Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J Appl Physiol. 2010;108(5):1389–94. doi: 10.1152/japplphysiol.01323.2009. [DOI] [PubMed] [Google Scholar]
- 36.Notomi Y, Lysyansky P, Setser RM, et al. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45(12):2034–41. doi: 10.1016/j.jacc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- 37.Pastorelli A, Torricelli G, Scabia M, Biagi E, Masotti L. A real-time 2-D vector Doppler system for clinical experimentation. IEEE transactions on medical imaging. 2008;27(10):1515–24. doi: 10.1109/TMI.2008.927337. [DOI] [PubMed] [Google Scholar]
- 38.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 39.Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. An Overview of Elastography - an Emerging Branch of Medical Imaging. Curr Med Imaging Rev. 2011;7(4):255–82. doi: 10.2174/157340511798038684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shamdasani V, Bae U, Sikdar S, et al. Research interface on a programmable ultrasound scanner. Ultrasonics. 2008;48(3):159–68. doi: 10.1016/j.ultras.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90(11):1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thelen DG, Chumanov ES, Best TM, Swanson SC, Heiderscheit BC. Simulation of biceps femoris musculotendon mechanics during the swing phase of sprinting. Med Sci Sports Exerc. 2005;37(11):1931–8. doi: 10.1249/01.mss.0000176674.42929.de. [DOI] [PubMed] [Google Scholar]
- 43.Wei Q, Sueda S, Pai DK. Physically-based modeling and simulation of extraocular muscles. Progress in biophysics and molecular biology. 2010;103(2-3):273–83. doi: 10.1016/j.pbiomolbio.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005;38(4):685–93. doi: 10.1016/j.jbiomech.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhong X, Epstein FH, Spottiswoode BS, Helm PA, Blemker SS. Imaging two-dimensional displacements and strains in skeletal muscle during joint motion by cine DENSE MR. J Biomech. 2008;41(3):532–40. doi: 10.1016/j.jbiomech.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]