Abstract
Response to endotoxins is an important part of the organismal reaction to Gram-negative bacteria and plays a critical role in sepsis and septic shock, as well as other conditions such as metabolic endotoxemia. Humans are generally more sensitive to endotoxins when compared with experimental animals such as mice. Inflammatory caspases mediate endotoxin-induced IL-1β secretion and lethality in mice, and caspase-4 is an inflammatory caspase that is found in the human, and not mouse, genome. To test whether caspase-4 is involved in endotoxin sensitivity, we developed a transgenic mouse expressing human caspase-4 in its genomic context. Caspase-4 transgenic mice exhibited significantly higher endotoxin sensitivity, as measured by enhanced cytokine secretion and lethality following LPS challenge. Using bone marrow–derived macrophages, we then observed that caspase-4 can support activation of caspase-1 and secretion of IL-1β and IL-18 in response to priming signals (LPS or Pam3CSK4) alone, without the need for second signals to stimulate the assembly of the inflammasome. These findings indicate that the regulation of caspase-1 activity by human caspase-4 could represent a unique mechanism in humans, as compared with laboratory rodents, and may partially explain the higher sensitivity to endotoxins observed in humans. Regulation of the expression, activation, or activity of caspase-4 therefore represents targets for systemic inflammatory response syndrome, sepsis, septic shock, and related disorders.
Introduction
Early detection and host immune responses to invading pathogens are mediated by a family of pattern recognition receptors. Endotoxins such as LPS are detected by a subset of pattern recognition receptors, termed TLRs, which are responsible for the recognition of extracellular pathogen-specific moieties (pathogen-associated molecular patterns) or the host's damage-associated molecular patterns. Pathogen-associated molecular patterns are nucleic acids and proteins unique to bacteria and viruses, such as LPS, ssRNA, and flagellin (1), whereas damage-associated molecular patterns are often intracellular proteins released as a result of injury and tissue damage (2). Activation of TLRs initiates intracellular signaling cascades involving intracellular adaptors (i.e., MyD88, Toll/IL-1R domain–containing adapter protein [TIRAP], Toll/IL-1R domain–containing adapter-inducing IFN-β [TRIF], and Toll/IL-1R domain–containing adapter-inducing IFN-β–related adaptor molecule), leading to activation of transcription factors such as NF-κB, followed by induction of cytokines and IFNs (3). Nucleotide-binding oligomerization domain–like receptors (NLRs) are intracellular multiprotein complexes mediating the sensing of intracellular microbial pathogens, leading to the activation of caspase-1, the maturation of a subset of cytokines including IL-1β and IL-18 (1, 4, 5), and lytic cell death, termed pyroptosis (1, 6, 7). IL-1β is an important cytokine regulating a wide array of immune and physiological responses (8) and genetic aberrations leading to dysregulation of IL-1β production, and signaling has been implicated in autoinflammatory syndromes (9). Recent studies have highlighted the significance of NLRs in response to metabolites such as fatty acid, cholesterol, and β-amyloid and underscored the importance of inflammasome signaling in conditions including sepsis, diabetes, atherosclerosis, and Alzheimer's disease (10–15).
The mouse has been a useful model system to understand the molecular and cellular basis of human immune diseases. However, it has been shown that there are considerable differences between human and mouse immunity (16). Endotoxin sensitivity is one such example, and evidence indicates that humans are among the species that are thought to be most sensitive to endotoxins, whereas rodents are highly resistant (17, 18). Recent studies further highlighted the profound genomic difference between mouse and human immune responses to immunological stimuli such as endotoxins and trauma (19). Mouse models of endotoxemia led to the cytokine theory of disease and the development of widely used biological anti-inflammatory therapeutics, particularly in diseases of chronic autoimmunity, such as rheumatoid arthritis (20, 21). However, species differences may have contributed to difficulties in predicting the clinical and therapeutic outcomes in acute or chronic inflammatory settings using data obtained from mouse models. There is therefore a need for improved animal models that better represent how human cells and molecules function in the immune system in response to given stimuli in vivo, therefore providing more reliable models for understanding pathobiology and with predictive validity.
Caspase-4 belongs to a family of inflammatory caspases that play important roles in secretion of IL-1β and IL-18, crucial cytokines for controlling inflammatory responses. Activation of these caspases is also associated with cell death. Caspase-1 directly catalyzes cleavage of pro–IL-1β and IL-18 both in mouse and human. Murine caspase-11 was demonstrated to mediate LPS-induced lethality and has been shown to mediate caspase-1 activation in response to LPS, as well as in response to certain pathogens such as Escherichia coli and Citrobacter rodentium (6, 7, 22, 23). In contrast, the human genome contains caspase-4 and -5 in the syntenic region of murine caspase-11, likely as a result of duplication of the ancestral gene of caspase-11. Unlike caspase-11 and -5, for which expression is normally low and induced by LPS, caspase-4 expression is relatively constitutive, indicating differing functions of these caspases (24, 25). In addition, although both caspase-4 and -5 have been shown to mediate inflammasome activation (26, 27), the amino acid identity between caspase-11 and caspase-4 or -5 is <60% (24), consistent with the possibility that they may carry out unique functions. However, functional analysis of caspase-4 has not been carried out in the in vivo context.
In this report, we characterized the role of caspase-4 in response to LPS. Our results demonstrated that caspase-4 transgenic mice displayed evidence of increased caspase-1 activity in response to LPS in vivo, followed by secretion of additional multiple cytokines. Using bone marrow–derived macrophages (BMDMs), we observe that caspase-4 can efficiently link TLR stimulation to caspase-1 activation, which results in secretion of IL-1β and IL-18. In summary, our results indicate that the presence of caspase-4 in humans constitutes an important part of enhanced LPS sensitivity seen in humans.
Materials and Methods
Animals
All animal experiments were performed according to the guidelines of the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. Caspase-4 transgenic mice were generated by pronuclear injection of the RP11 693N9 bacterial artificial chromosome fragment containing the entire caspase-4 gene including 6.8 kb of upstream sequences containing the promoter region (Children's Hospital Oakland Research Institute, Oakland, CA) into B6C3 hybrids. The bacterial artificial chromosome also carries the long isoform of caspase-12, resulting from a rare single-nucleotide variant found in a small proportion of humans of African ancestry (28). This long isoform of human caspase-12 is catalytically inactive and cannot participate in processing events but acts (as does murine caspase-12) to suppress LPS-induced cytokine secretion and NF-κB activation (28). Caspase-12–deficient mice are more sensitive to LPS-induced lethality and secrete higher IL-1β and IL-18 by acting as an inhibitor of caspase-1 (29). Although we could not confirm protein expression due to a lack of human-specific caspase-12 Ab, the mRNA expression was reduced in the brain after LPS challenge. These findings would indicate that the presence of caspase-12 cannot explain the phenotypes we observed and may actually ameliorate those phenotypes we observed. Founders with caspase-4 expression were backcrossed to the C57BL/6J background for at least six generations. Caspase-4 transgenic mice were genotyped by quantitative PCR (qPCR) using the following universal probe library and primer pairs: probe 72, 5′-CACATACCACCAACAACTCTCAA-3′ and 5′-GCCCACCATAGAACGACTGT-3′. Alternatively, genotyping was performed by conventional PCR using primers 5′-AAGGCAACCACAGAAAAAAGCCACT-3′ and 5′-CTTTTTTATTGGGGGATATTTGGTC-3′. The caspase-1/11 double knockout (dKO) mouse (NOD.129S2(B6)-Casp1tm1Sesh/LtJ) was purchased from The Jackson Laboratory (Bar Harbor, ME) and backcrossed to C57BL/6J for at least six generations. This strain has been shown to be deficient in inducing caspase-11 (6, 30) and therefore indicated as Casp1/Casp11 dKO throughout the manuscript. Casp1/Casp11 dKO mice were genotyped using probe 117, 5′-AAGTGCCCAAGCTTGAAAGA-3′ and 5′-AATGGGAATGCCCTGTTATTT-3′, for the wild-type (WT) allele and 5′-GAAGAGATGTTACAGAAGCC-3′ and 5′-GCGCCTCCCCTACCCGG-3′ primers for the mutant allele. Caspase-11–deficient caspase-4 transgenic mice were generated from at least four generations of backcrossing 129P3/J-derived caspase-11 null alleles onto caspase-4 transgenic mice in the C57BL/6J background. Caspase-11 null alleles were genotyped using the following primer/UPL probe sets: probe 38, 5′-AGCAATATATGACACTTTCTCTCTTCACAGA-3′ and 5′-CAAAGAGATGACAAGAGCAAGCATGT-3′, for the WT allele, and probe 38, 5′-AGCAATATATGACACTTTCTCTCTTCTCAC-3′ and 5′-CAAAGAGATGACAAGAGCAAGCATGT-3′, for the null allele. Nlrp3-deficient mice (B6N.129-Nlrp3tm2Hhf/J) were obtained from The Jackson Laboratory and backcrossed to a caspase-4 transgenic mouse in the C57BL/6J background. Genotyping was performed using the following primer sets: 5′-CGTGTAGCGACTGTTGAGGT-3′ and 5′-CACCCTGCATTTTGTTGTTG-3′ for WT and 5′-CGTGTAGCGACTGTTGAGGT-3′ and 5′-GCTACTTCCATTTGTCACGTCC-3′ for the mutant allele. Because they were of the 129 congenic background, they were genotyped for caspase-11 to ensure the absence of caspase-11 spontaneous mutation.
Reagents, plasmids, cells, and Abs
Abs used in this study were as follows; anti–caspase-4 (4B9; MBL International, Woburn, MA), anti–caspase-1 (M20; Santa Cruz Biotechnology, Dallas, TX), anti–caspase-3 (9662; Cell Signaling Technology, Danvers, MA) anti–caspase-11 (17D9; eBioscience, San Diego, CA), anti-actin (A5060; Sigma-Aldrich, St. Louis, MO), anti–IL-1β (3ZD; Biological Resources Branch Frederick Cancer Research & Development Center, Frederick, MD), anti-murine IL-1β (AF-401-NA; R&D Systems, Minneapolis, MN), anti-mouse IL-18 (39-3F; MBL International), anti–inducible NO synthase (2977; Cell Signaling Technology); and anti-human CX3CR1 (AB1891; Millipore, Billerica, MA). LPS E. coli 055:B5 (L2880; Sigma-Aldrich), cholera toxin B (C9903; Sigma-Aldrich), Pam3CSK4 (tlrl-pms; Invivogen, San Diego, CA), LPS-EB Ultrapure (tlrl-3pelps; Invivogen), and the Mouse TLR1-9 Agonist kit (tlrl-kit1mw; Invivogen) were used for macrophage stimulation and i.p. injection. Mouse TNF-α, IFN-β (produced in mammalian cells), and IFN-γ were from eBioscience, PBL Biomedical (Piscataway, NJ), and Biovision (Milpitas, CA), respectively. ATP (A2383; Sigma-Aldrich) and inducible NO synthase inhibitors 2-ethyl-2-thiopseudourea (3441821; Millipore) and 1400W (100050; EMD) were used for BMDM treatments. Palmitate-BSA was prepared according to a study previously described (31). Various tissue lysates were prepared by homogenizer in RIPA buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Nonidet P-40, 1% deoxycholate, and protease inhibitor mixture (Roche Applied Science, Indianapolis, IN). After homogenization, lysates were centrifuged at 100,000 × g for 30 min, and supernatants were collected for immunoblot analysis. Nitrite/nitrate was quantitated using a Biovision colorimetric assay kit (Biovision) based on the Griess reagent. Luminex multiple cytokine ELISAs were performed using a Milliplex MAP mouse cytokine panel (MPXMCYTO70; Millipore). Murine caspase-1 and human caspase-4 cDNA were cloned from splenic total RNA of caspase-4 transgenic mouse into pCMV-myc vector and fully sequenced. Catalytic mutations for caspase-1 (C284A) and caspase-4 (C285A) were introduced by site-directed mutagenesis. Caspase activation and recruitment domain (CARD) of caspase-4 was created by deleting amino acid residues from 1–89. For cotransfection, 1 μg plasmids for each construct was mixed with Lipofectamine LTX (Life Technologies, Grand Island, NY) and applied to one well in a six-well plate.
qPCR
Total RNAs were isolated from whole brains of mice using the RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA were synthesized using the Superscript3 First Strand Synthesis kit (Life Technologies). qPCR was performed using the Universal ProbeLibrary (Roche Applied Science), and the following primer sets and probes were used for each gene: 18s: probe 48, 5′-gcaattattccccatgaacg-3′ and 5′-gggacttaatcaacgcaagc-3′; Gapdh: probe 29, 5′-gccaaaagggtcatcatctc-3′ and 5′-cacacccatcacaaacatgg-3′; Rpl13a: probe 41, 5′-acaagaaaaagcggatggtg-3′ and 5′-gccccaggtaagcaaactt-3′; IL-1α: probe 52, 5′- ttggttaaatgacctgcaaca-3′ and 5′- gagcgctcacgaacagttg-3′; IL-1β: probe 38, 5′- agttgacggaccccaaaag-3′ and 5′-agctggatgctctcatcagg-3′; TNF-α: probe 49, 5′- tcttctcattcctgcttgtgg-3′ and 5′-caccccgaagttcagtagaca-3′; and caspase-4: probe 29, 5′- ctctgaggctctttccaacg-3′ and 5′-ttccaacaccttaagtggcttt-3′. Data were analyzed using qBase plus (Biogazelle, Ghent, Belgium) and normalized to geometric mean of Gapdh, 18s, and Rpl13a.
Serum collection
Sera were prepared from blood collected from the submandibular vein using appropriate mouse lancets. Blood was allowed to coagulate for 30 min at room temperature, after which it was centrifuged at 13,000 × g for 10 min. Supernatants were carefully collected and kept in a −80°C freezer until assay.
Cell cultures
Human monocyte-derived macrophages were purchased from AllCells (PB-MDM-001F; Alameda, CA) and cultured in RPMI 1640 supplemented with 10% FBS/penicillin/streptomycin and 10 ng/ml human M-CSF (R&D Systems). BMDM cultures were prepared according to previous reports (6, 32). Briefly, bone marrow cells were isolated from 8–12-wk-old mice and cultured in RPMI 1640 supplemented with 10% FBS/penicillin/streptomycin/10% L929 cell-conditioned medium for 6 to 7 d. For cytokine ELISA assays, 2 × 105 cells/well of a 96-well plate were treated in quadruplicate with indicated concentrations of LPS or Pam3CSK4 for 24 h. Medium used for BMDM stimulation was Opti-MEM, unless otherwise indicated. Alternatively, BMDMs were stimulated with 100 ng/ml LPS for 4 h and treated with 5 mM ATP or 500 μM palmitate-BSA for 24 h. Mouse IL-1β, TNF-α (eBioscience), and IL-18 (MBL International) ELISAs were performed according to the manufacturer's instructions. For immunoblot analysis of supernatants, collected supernatants were centrifuged once at 500 × g, and 100% trichloroacetic acid was added to the supernatants to 30% final concentration. Proteins were precipitated by centrifugation at 100,000 × g for 15 min, with a subsequent wash with 100% ethanol. The precipitates were boiled in SDS loading buffer for 10 min for SDS-PAGE analysis. ATP concentration in supernatants was analyzed using an ATP determination kit (A22066; Invitrogen).
Statistics
All statistics were performed using SPSS version 20 (IBM Corporation, Somers, NY). Data were first tested for homogeneity of variance for parametric tests including t test and ANOVA. Post hoc tests were performed using the Bonferroni procedure where indicated, and nonparametric analyses were used, including Mann–Whitney and Kruskal–Wallis tests.
Results
Constitutive and induced expression of human caspase-4 in transgenic mice
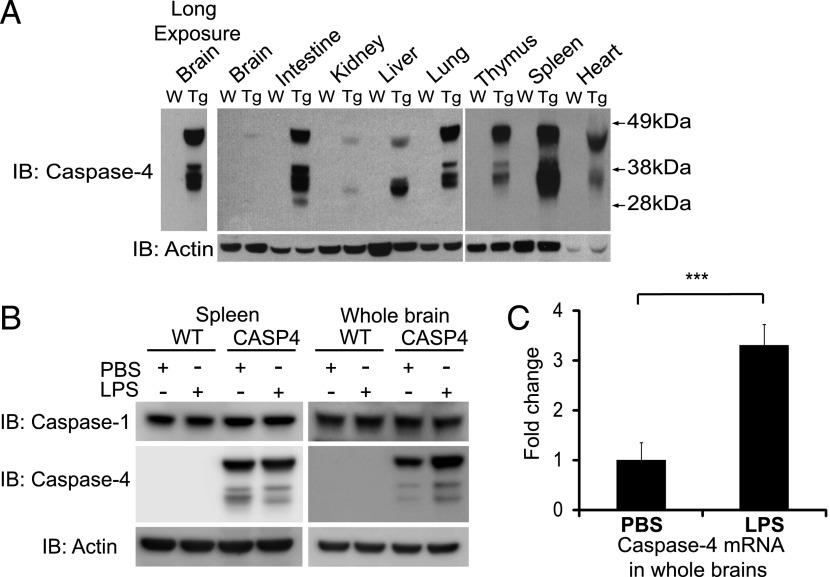
Two founder lines that were positive by PCR analysis for the human CASP4 gene were tested for caspase-4 expression across different tissues by immunoblot analysis, and both showed similar levels of caspase-4 expression (Fig. 1A). Distribution of caspase-4 was examined in tissues dissected with or without extensive transcardial perfusion with PBS to eliminate contribution of blood cells, and both approaches produced similar results (representative data after PBS perfusion is shown in Fig. 1A). Caspase-4 was constitutively expressed in lymphoid tissues including spleen and thymus consistent with a role in immune function. This distribution pattern mirrored a previous study analyzing caspase-4 mRNA expression in human tissues (25). In addition, tissues such as intestine and lung, which provide a first line of defense to potential invading pathogens, showed high levels of caspase-4 expression. In the brain, caspase-4 was expressed only weakly, as compared with most peripheral tissues, and was detectable only after longer exposure (Fig. 1A). In addition, the expression level of caspase-4 in mouse BMDMs was higher than in cultured human macrophages (Supplemental Fig. 1A).
FIGURE 1.
Caspase-4 expression in the transgenic mice and its regulation by LPS. (A) The indicated tissues were isolated from WT (W) and caspase-4 transgenic (Tg) mice after extensive perfusion with PBS, and lysates were analyzed by immunoblot (IB) with specific Abs to caspase-4 and actin. Results representative of two independent experiments are shown. Left panel, Longer exposure of immunoblot for the brain is shown. (B) Lysates from WT or caspase-4 transgenic (CASP4) mice injected with PBS or 5 mg/kg of LPS for 6 h were subjected to immunoblotting with anti–caspase-1, caspase-4, and actin Abs. Results representative of three independent experiments are shown. (C) Caspase-4 transgenic mice were injected with PBS (n = 8) or LPS (n = 7), and mRNA extracted from whole brains was analyzed by qPCR for caspase-4 mRNA. Data were normalized to PBS-injected group, and Student t test was used for statistical analysis. Error bars represent SEM. ***p < 0.001.
The majority of tissues showed multiple isoforms of caspase-4, which are likely derived from at least two known alternative transcripts (α: NM_001225; and γ: NM_033306) as well as alternative translational initiation (data not shown). Expression of caspase-4 was inducible 6 h after i.p. injection of LPS (5 mg/kg) in a tissue-specific manner. For example, we found that caspase-4 protein expression remained unaltered in spleen (Fig. 1B), whereas, in contrast, it was induced in the brain (Fig. 1B). qPCR analysis confirmed the induction of caspase-4 mRNA in whole brain following i.p. injection of LPS (Fig. 1C).
A role for caspase-4 in LPS-induced cytokine induction and lethality
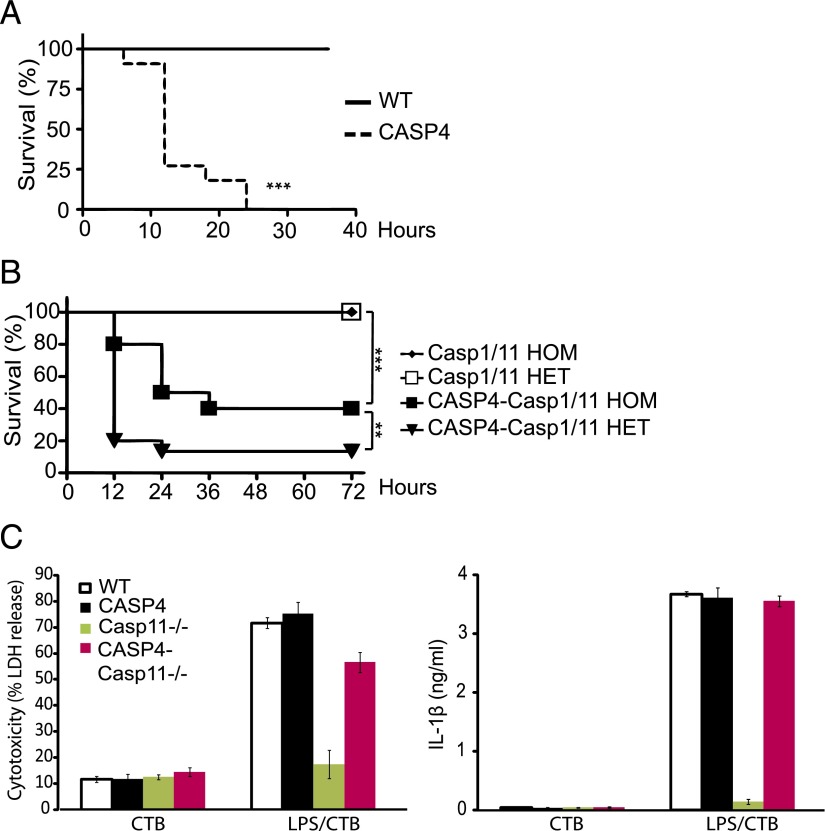
Inflammatory caspases, including caspase-1 and -11, have been implicated as mediators of IL-1β secretion and lethality in the murine model of LPS-induced endotoxemia and septic shock (22, 33). We therefore tested whether caspase-4 transgenic mice were sensitive to endotoxins using either lethal (40 mg/kg) or sublethal (5 mg/kg) doses of LPS. Even the lower dose of LPS was lethal to caspase-4 transgenic mice within 24 h, conditions in which none of the WT littermates died (Fig. 2A [5 mg/kg], Supplemental Fig. 1B [40 mg/kg]). To understand the role of caspase-1 and -11 in this caspase-4–sensitive lethality, we expressed the caspase-4 transgene on either a homozygous or heterozygous caspase-1/11–deficient background and tested LPS sensitivity. Although the caspase-4–sensitive lethality showed some dependence on caspase-1 and -11 expression, caspase-4 expression was sufficient to lead to significant lethality (50% within 24 h) even in the absence of caspase-1 and -11 (Fig. 2B). Recently, intracellular LPS has been shown to mediate activation of caspase-11 and subsequent cytotoxicity in BMDMs and lethality in mice (34, 35). We therefore introduced the 129P3/J line–derived caspase-11 spontaneous mutation onto a caspase-4 transgenic mouse in the C57BL/6J background (CASP4-Casp11−/−) and tested whether caspase-4 could complement cytotoxicity of intracellular LPS using BMDMs from CASP4-Casp11−/− mice. Consistent with our finding that caspase-4 could confer lethality independent of caspase-11, a similar level of cytotoxicity and IL-1β secretion was induced in CASP4-Casp11−/− BMDMs compared with WT and CASP4 BMDMs using intracellular LPS delivered with cholera toxin B (Fig. 2C).
FIGURE 2.
Caspase-4 contributes to endotoxin-induced lethality. (A) LPS (5 mg/kg) was injected into WT and caspase-4 transgenic (CASP4) mice (n = 11/genotype), and survival was monitored every 6 h for the first 2 d and afterward once a day for 7 d. Results for the first 36 h are shown. All WT animals survived for the entire period. (B) LPS (10 mg/kg) was injected into mice, and survival was monitored every 12 h for up to 7 d. The first 72 h are shown (n = 10–17/genotype). The Casp1/11 homozygous (HOM) and heterozygous (HET) animals survived for the entire period. For (A) and (B), Kaplan-Meier survival tests were used for statistical analysis. (C) Lactate dehydrogenase (LDH) activity and IL-1β secretion were measured 24 h after treating BMDMs from the indicated genotypes with cholera toxin B (CTB) (20 μg/ml) alone or together with ultrapure LPS (105 EU) derived from O111:B4 E. coli serotype. **p < 0.01, ***p < 0.001.
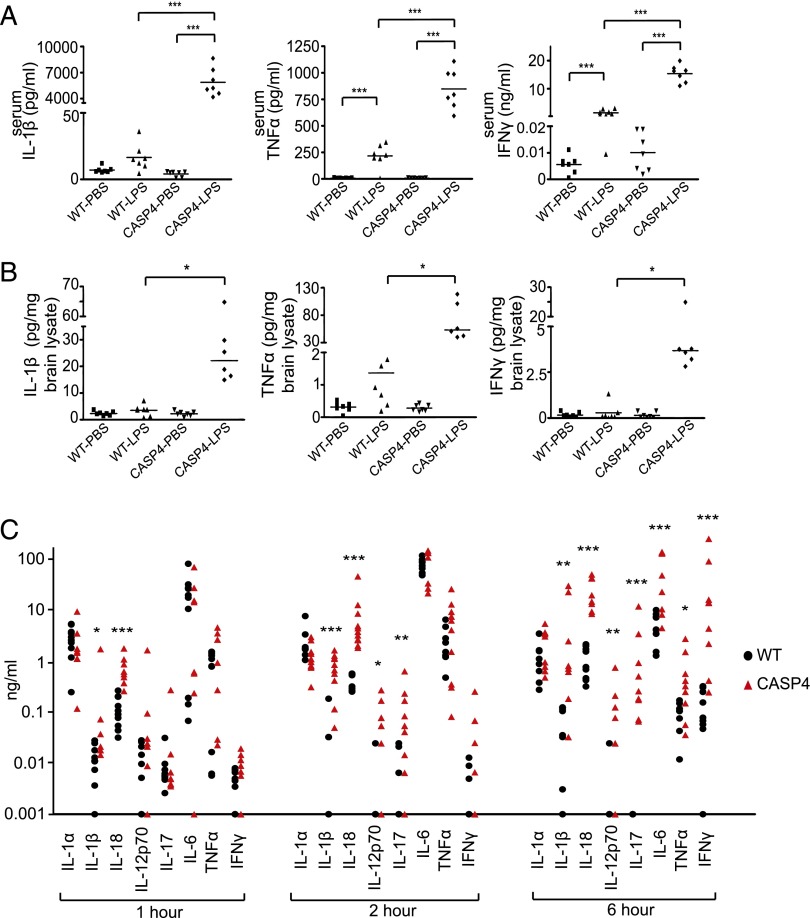
Because inflammatory caspases regulate processing of IL-1β and IL-18 for their maturation and secretion, we next tested whether caspase-4 contributes to secretion of cytokines. Interestingly, we found that not only IL-1β, but also TNF-α and IFN-γ were profoundly elevated in sera from LPS-challenged caspase-4 transgenic mice (Fig. 3A). In contrast, TGF-β levels were significantly reduced in LPS-challenged caspase-4 transgenic mice (Supplemental Fig. 2A). In the brain, where caspase-4 was expressed at low levels, peripheral injection of LPS upregulated mRNA (Supplemental Fig. 2B) and protein levels (Fig. 3B, Supplemental Fig. 2C) of several proinflammatory cytokines, including IL-1β, TNF-α, and IFN-γ, indicating that peripheral exposure to endotoxins can acutely contribute to brain inflammation.
FIGURE 3.
Caspase-4 contributes to endotoxin-induced proinflammatory cytokine secretion. LPS (5 mg/kg) or PBS were injected i.p., and sera (A) or cortical brain lysates (B) were collected after 6 h to determine cytokine levels (n = 6–8/genotype). For (A), ANOVAs with Bonferroni post hoc test are shown. For (B), Kruskal–Wallis nonparametric one-way ANOVA with pairwise comparisons was performed. (C) LPS (0.2 mg/kg) was injected and sera collected at 1, 2, and 6 h (n = 8–11/genotype). Serum cytokines were quantitated by multiplex ELISA, and Mann–Whitney nonparametric test was used for statistical analysis. *p < 0.05, **p < 0.01, ***p < 0.001.
We analyzed the kinetics of cytokine secretion by challenging mice with a lower dose of LPS (0.2 mg/kg, a dose that led to lethality in <10% of caspase-4 transgenic mice in our preliminary tests). IL-1β and IL-18 were significantly elevated at 1 h post–LPS injection (Fig. 3C, left panel), followed by a subsequent increase in IL-12p70 and IL-17 at 2 h (Fig. 3C, middle panel). Other proinflammatory cytokines, such as IFN-γ, IL-6, and TNF-α, were significantly increased after 6 h (Fig. 3C, right panel).
Priming alone stimulates IL-1β and IL-18 secretion from macrophages in caspase-4 transgenic mice
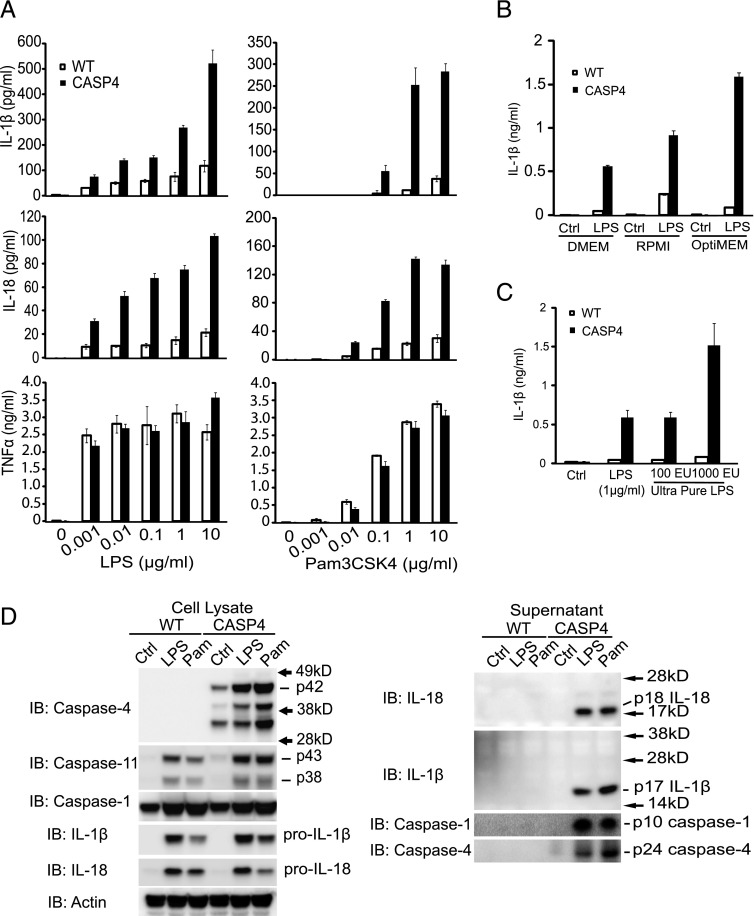
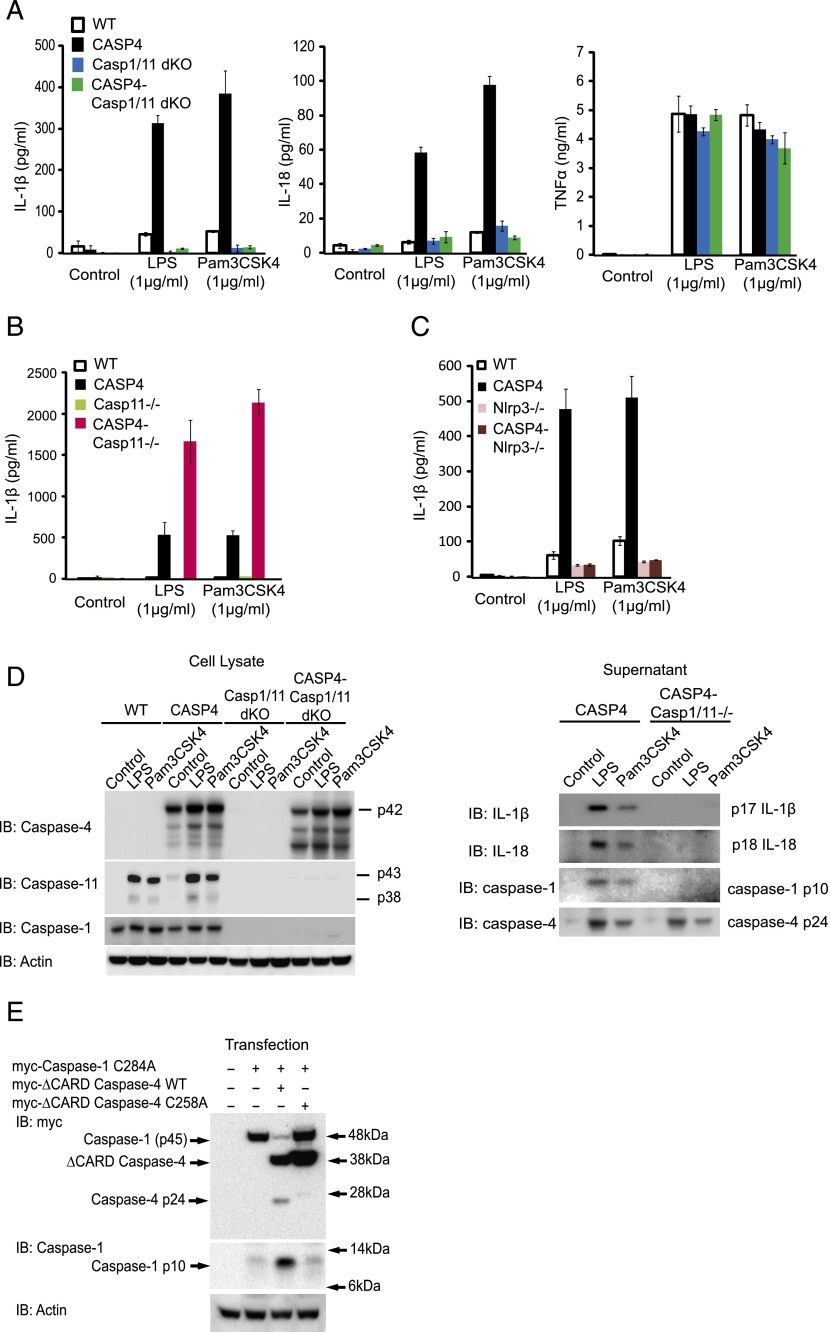
Recent studies have indicated that caspase-4 is a component of the inflammasome, activating caspase-1 (27). For efficient secretion of IL-1β, mouse WT BMDMs require both a priming signal such as LPS and a second signal (such as ATP or saturated fatty acids) and subsequent activation of caspase-1. We did not find any difference in IL-1β secretion between WT and CASP4 BMDMs when they were primed with LPS and stimulated with either ATP or a fatty acid (palmitate-BSA) (Supplemental Fig. 3A). However, in CASP4 BMDMs, we observed that using a priming signal alone was sufficient to produce higher IL-1β. Hence, when BMDMs from WT or caspase-4 transgenic mice were stimulated with increasing doses of LPS or Pam3CSK4 (Fig. 4A), or other TLR ligands for 24 h (Supplemental Fig. 3B), CASP4 BMDMs secreted dramatically higher levels of IL-1β or IL-18 as compared with WT BMDMs. This effect was not the result of factors in the culture medium, as culturing with DMEM, RPMI 1640, and OptiMEM all showed higher IL-1β secretion in CASP4 BMDMs (Fig. 4B). In addition, this enhancement was not likely due to contamination with TLR ligands or other compounds, as ultrapure LPS alone also induced significantly higher level of IL-1β secretion in CASP4 BMDMs (Fig. 4C). Furthermore, the secretion was unlikely due to increased cell lysis as lactate dehydrogenase activity in the supernatants were not different across genotypes (Supplemental Fig. 3C). Increased IL-1β secretion was not due to increased production of ATP, as we observed that ATP secretion was actually reduced in CASP4 BMDMs (Supplemental Fig. 3D). Also, the induction of mRNAs for cytokines such as IL-1β, TNF-α, and IL-6 did not differ between WT and CASP4 BMDMs (Supplemental Fig. 3E).
FIGURE 4.
Caspase-4 mediates IL-1β and IL-18 secretion after stimulation with TLR ligands. (A) BMDMs from WT or caspase-4 transgenic (CASP4) mice were stimulated with the indicated concentration of LPS or Pam3CSK4 for 24 h, and the levels of IL-1β, IL-18, and TNF-α in supernatants were determined by ELISA. Error bars represent SEM. (B) WT or CASP4 BMDMs were treated with LPS (1 μg/ml) in the indicated medium for 24 h. IL-1β was quantitated by specific ELISA assay. (C) BMDMs from the indicated genotypes of mice were stimulated with LPS or ultrapure LPS for 24 h. Levels of IL-1β in supernatants were determined by ELISA. (D) Immunoblot (IB) analyses of lysates (left panel) and supernatants (right panel) from WT and CASP4 BMDMs treated for 24 h with control medium (Ctrl) or 1 μg/ml of LPS or Pam3CSK4 (Pam). Data representative of three independent experiments are shown.
Immunoblot analysis revealed comparable levels of caspase-1, caspase-11, IL-1β, and IL-18 in cell lysates from WT and CASP4 BMDMs (Fig. 4D, left panel). However, secreted IL-1β and IL-18 were detectable only in the supernatants of CASP4 BMDMs, together with activated caspase-1 and caspase-4 (Fig. 4D, right panel).
Caspase-4–associated secretion of cytokines requires caspase-1
To explore the role of other inflammatory caspases in caspase-4–mediated IL-1β and IL-18 secretion, we tested their secretion in BMDMs deficient in caspase-1 and -11. In the absence of caspase-1 and -11, secretion of IL-1β and IL-18 were abolished (Fig. 5A). At the same time, the secretion of TNF-α was not affected (Fig. 5A), indicating that caspase-1 and/or -11 are specifically required for secretion of IL-1β and IL-18 from CASP4 BMDMs. We next wanted to assess whether caspase-11 was required for the secretion of IL-1β and IL-18 induced by caspase-4. When we used CASP4-Casp11−/− BMDMs, the secretion of IL-1β after LPS or Pam3CSK4 stimulation was not abolished by the Casp11−/− genotype, indicating that caspase-11 was dispensable (Fig. 5B, Supplemental Fig. 4A). In addition, the Nlrp3 inflammasome was specifically required for the effect of caspase-4, as IL-1β secretion was completely abolished in the absence of Nlrp3 (Fig. 5C, Supplemental Fig. 4B). To study whether caspase-4 exerts its effect via activation of caspase-1, we analyzed cell lysates and supernatants of BMDMs treated with vehicle, LPS, or Pam3CSK4 (Fig. 5D). Active fragments of IL-1β and IL-18 were seen in CASP4 BMDMs, but not in CASP4–Casp1/11 dKO. However, the cleaved fragment of caspase-4 was still detected in CASP4–Casp1/11 dKO, indicating that activation of caspase-4 does not require caspase-1. To test whether caspase-4 can directly cleave caspase-1, myc-tagged caspase-1 full-length catalytic mutant was cotransfected with myc-tagged WT or catalytic mutant (C258A) caspase-4 without CARD domain (ΔCARD) in 293T cells. Reduction of full-length caspase-1 (p45) with concomitant increase of active caspase-1 fragment (p10) was observed only when WT but not mutant caspase-4 was cotransfected, supporting the role for caspase-4 in processing and activating caspase-1 (Fig. 5E).
FIGURE 5.
CASP4 BMDMs secrete IL-1β and IL-18 in response to LPS in a caspase-1–dependent manner. (A) BMDMs prepared from WT, caspase-4 transgenic (CASP4), caspase-1/11 dKO (Casp1/11 dKO), or caspase-4 transgenic/caspase-1/11 dKO (CASP4-Casp1/11 dKO) mice were stimulated with 1 μg/ml of LPS or Pam3CSK4 for 24 h. IL-1β, IL-18, and TNF-α in supernatants were measured by ELISA. Representative result of three independent experiments is shown. (B) Similarly to (A), BMDMs from WT, CASP4, caspase-11 knockouts (Casp11−/−), or caspase-4 transgenic/caspase-11 knockouts (CASP4-Casp11−/−) mice were stimulated with control medium, LPS, or Pam3CSK4, and IL-1β was determined by ELISA. Representative result of three independent experiments is shown. Error bars represent SEM. (C) BMDMs from WT, CASP4, Nlrp3 knockouts (Nlrp3−/−), or caspase-4 transgenic/Nlrp3 knockouts (CASP4-Nlrp3−/−) mice were stimulated with control medium, LPS, or Pam3CSK4, and IL-1β was determined by ELISA. Representative result of two independent experiments is shown. Error bars represent SEM. (D) Cell lysates (left panel) and supernatants (right panel) from the indicated genotypes of BMDMs were subjected to immunoblot (IB) analysis for indicated proteins. Data are representative results of two experiments. (E) Plasmids containing caspase-1 and -4 constructs were cotransfected as indicated into 293T cells for 24 h, and cell lysates were analyzed by immunoblots with anti-myc, caspase-1, or actin Abs. Data representative of three independent experiments are shown.
Discussion
Mice have been known to be resistant to endotoxins compared with humans (17). For example, the doses of LPS required to stimulate serum IL-6 secretion in human and in mouse have been shown to be 2 and 500 ng/kg, respectively (36). The goal of this study was to investigate molecules responsible for the high endotoxin sensitivity in humans. We focused on caspase-4, as it is a gene found in humans and a close family member of inflammatory caspases such as caspase-1 and -11 in mouse. We made use of genetic manipulations of these proteins in a way to better understand aspects of the human endotoxin sensitivity, and our resultant data showed that caspase-4 is important to enhanced endotoxin response, such as that seen in humans.
Expression of caspase-4 alone significantly elevated LPS-induced secretion of proinflammatory cytokines and led to rapid lethality. Recent studies have indicated that LPS-induced cytotoxicity mediated by caspase-11 plays a major role in LPS-induced lethality in mice (34, 35). In agreement with this, we found that intracellular LPS could induce cytotoxicity in CASP4 BMDMs in the absence of caspase-11 (Fig. 2C). In addition, we observed that caspase-4 stimulated IL-1β and IL-18 secretion in response to TLR ligands in macrophage cultures. Consistent with this, LPS-injected caspase-4 transgenic mice secreted higher levels of IL-1β and IL-18 initially, and interestingly, in contrast to in vitro assay, other cytokines were significantly induced, indicating the presence of complex cytokine cross-stimulation between cells and tissues in vivo. More recently, secretion of IL-1β and IL-18 has been shown to contribute to lethality induced by low doses of LPS in mice (37), although they may not mediate lethality to high doses of LPS (14). Our data showing that the secretion of IL-1β and IL-18 represents an immediate early response in caspase-4 transgenic mice may partially explain the mechanism of the increased lethality (Fig. 3C).
Using BMDMs, we found that many TLR ligands can by themselves stimulate secretion of IL-1β and IL-18 in CASP4 BMDMs (Supplemental Fig. 3B). However, there are different responses depending on the receptor. Although some TLR ligands (i.e., Pam3CSK4, LPS, flagellin, FSL1, and ssRNA40) potently promoted both IL-1β and IL-18 secretion, ODN1826 was not effective, despite being able to induce expression of both cytokines. Polyinosinic-polycytidylic acid was potent in inducing IL-18 secretion, but not in IL-1β secretion, and this could be partly due to its inability to induce IL-1β. It has been known that different TLRs engage different intracellular adaptors (3), and although the precise caspase-4 pathway remains to be elucidated, this may explain the differential effects of TLR ligands. TNF-α, which has been recently shown to act on the caspase-4 pathway (38), was not able to stimulate secretion of both cytokines despite being able to induce them. IFN-β was recently shown to induce activation of caspase-11 (23). In our study, however, IFN-β or IFN-γ had no effect on secretion of IL-1β and IL-18 from CASP4 BMDMs.
Our study highlighted the functional conservation as well as divergence between caspase-4 and -11, despite their sequence similarity and evolutionary origin. We confirmed that caspase-4 was able to induce lethality by LPS (Fig. 2B) and complement cytotoxicity in BMDMs lacking caspase-11 (Fig. 2C). For the secretion of IL-1β, however, not only was caspase-11 dispensable, but also inhibitory for caspase-4–mediated secretion of IL-1β. This could be explained by previous as well as current observations that caspase-11 and -4 used the same inflammasome scaffold such as caspase-1 and Nlrp3 (6, 23, 27). We speculate that both caspases compete for the Nlrp3 inflammasome, and in the absence of caspase-11, more Nlrp3 inflammasome is available for caspase-4 activating caspase-1. Interestingly, we found that the secretion of IL-1β was specific to LPS stimulation. TNF-α, which induces expression of IL-1β, was not able to stimulate secretion in CASP4 BMDMs with or without classical Nlrp3 ligands such as ATP, aluminum hydroxide, and palmitate (Supplemental Fig. 4C). These data suggest that the activation of caspase-4 is unique to TLR stimulation.
Caspase-1 processes pro–IL-1β and IL-18 to mature active forms (39, 40). Previous genetic and biochemical investigations of rare hereditary autoinflammatory syndromes have revealed that genes that are frequently mutated, including NLRP3, MEFV, and IL1RN, are often regulators of caspase-1/IL-1β signaling (9), and patients suffer a wide spectrum of multisystem abnormalities characterized by recurrent fever, skin rashes, anemia, hearing loss, and developmental delay. Although these syndromes are effectively treated with pharmacological blockade of IL-1β, more recent clinical trials have shown that type 2 diabetes, rheumatoid arthritis, and gout can also be treated with this approach (21), supporting the notion that IL-1β has diverse physiological functions, and its dysregulation could be detrimental to human health. Furthermore, recent animal studies have underscored the importance of Nlrp3 and caspase-1 activation in a diverse array of pathophysiologies (10–12, 15). Our current finding that caspase-4 enhances IL-1β secretion in an Nlrp3- and caspase-1–dependent manner suggests that caspase-4 could be an important therapeutic target for treating a subset of autoinflammatory diseases.
Endotoxins are major components of the cell wall of Gram-negative bacteria. With the recent advance in our understanding of the role played by intestinal and other microbiota in human health (41), the importance of understanding human immune response to endotoxins goes beyond just bacteremia and sepsis. Many environmental factors appear to influence our health through intestinal microbiota. For example, a high-fat diet increases blood endotoxin level in both mice and humans in a process that leads to metabolic endotoxemia (42, 43). Consumption of alcohol also leads to elevated endotoxins in blood (44). This metabolically induced endotoxemia and subsequent chronic inflammation have been postulated to be important risk factors for many metabolic syndromes (45). Given the importance of the endotoxin response and activation of caspase-1/IL-1β signaling, our finding of caspase-4 mediating activation of caspase-1 and IL-1β and IL-18 secretion in response to TLR ligands may be of importance in metabolic endotoxemia. More broadly, epidemiological studies have suggested that systemic inflammation and elevated cytokines, including IL-1β, could be key contributing factors to several pathophysiologies including Alzheimer's disease, insulin resistance, and type 2 diabetes (46–49). In addition, endotoxin produces various cognitive and emotional disturbances in humans (50).
Although we aimed to model human endotoxin response in mouse by genetic modification of caspase-4 genes, human innate immunity to LPS is likely to be more complex than can be explained by caspase-4. Recent studies have highlighted that human monocytes, but not macrophages, can secrete high levels of IL-1β in response to TLR ligands alone (51, 52). These observations in monocytes could be explained by caspase-4. However, we and others observed that human macrophages do not secrete IL-1β after LPS treatment alone (data not shown) (51), despite expressing caspase-4. This raises an interesting question regarding how caspase-1 is regulated in human macrophages. It is possible that caspase-1 activation in human is more tightly regulated than in mouse by other genetic elements. Indeed, the human genomic region harboring inflammatory caspases (chromosome 11q22) contains genes that are absent in the mouse syntenic region (chromosome 9 A1). These genes (CARD16, CARD17, and CARD18 or COP, INCA, and ICEBERG, respectively), believed to be generated by gene duplication, encode only the CARD domain and have been shown to be negative regulators of caspase-1 (53, 54). Further study will be necessary to define in what cells and tissues and which pathways these CARD only proteins are involved.
In summary, our data are consistent with a model in which the presence of caspase-4 mediating the activation of caspase-1 in response to LPS makes human cells particularly sensitive to endotoxins as reflected in IL-1β and IL-18 secretion and downstream sequelae. In contrast, the absence of caspase-4 in mice provides protection for this species. The results suggest that caspase-4 transgenic mice are an improved model for the study of human endotoxin responses and that caspase-4 represents an important potential therapeutic target.
Acknowledgments
We thank Ona Bloom (Feinstein Institute for Medical Research) for comments on the manuscript.
This work was supported by the Icahn School of Medicine at Mount Sinai Alzheimer Disease Research Center (Dr. Mary Sano, principal investigator; O.B. and J.D.B., project leaders; U01 P50 AG005138-28). G.V. was also supported by a Manasaki Foundation scholarship. J.D.B. is the G. Harold and Leila Y. Mathers Professor of Geriatrics and Adult Development.
The online version of this article contains supplemental material.
- BMDM
- bone marrow–derived macrophage
- CARD
- caspase activation and recruitment domain
- dKO
- double knockout
- NLR
- nucleotide-binding oligomerization domain–like receptor
- qPCR
- quantitative PCR
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lamkanfi M., Dixit V. M. 2012. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28: 137–161 [DOI] [PubMed] [Google Scholar]
- 2.Krysko D. V., Garg A. D., Kaczmarek A., Krysko O., Agostinis P., Vandenabeele P. 2012. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12: 860–875 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T., Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34: 637–650 [DOI] [PubMed] [Google Scholar]
- 4.Franchi L., Muñoz-Planillo R., Núñez G. 2012. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathinam V. A., Vanaja S. K., Fitzgerald K. A. 2012. Regulation of inflammasome signaling. Nat. Immunol. 13: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., et al. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121 [DOI] [PubMed] [Google Scholar]
- 7.Broz P., Ruby T., Belhocine K., Bouley D. M., Kayagaki N., Dixit V. M., Monack D. M. 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490: 288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello C. A. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastner D. L., Aksentijevich I., Goldbach-Mansky R. 2010. Autoinflammatory disease reloaded: a clinical perspective. Cell 140: 784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., Brickey W. J., Ting J. P. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12: 408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., et al. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T. C., et al. 2013. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493: 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9: 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamkanfi M., Sarkar A., Vande Walle L., Vitari A. C., Amer A. O., Wewers M. D., Tracey K. J., Kanneganti T. D., Dixit V. M. 2010. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185: 4385–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandanmagsar B., Youm Y. H., Ravussin A., Galgani J. E., Stadler K., Mynatt R. L., Ravussin E., Stephens J. M., Dixit V. D. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17: 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestas J., Hughes C. C. 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172: 2731–2738 [DOI] [PubMed] [Google Scholar]
- 17.Brade H. 1999. Endotoxin in Health and Disease. Marcel Dekker, Inc., New York [Google Scholar]
- 18.Wichterman K. A., Baue A. E., Chaudry I. H. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J. Surg. Res. 29: 189–201 [DOI] [PubMed] [Google Scholar]
- 19.Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., Richards D. R., McDonald-Smith G. P., Gao H., Hennessy L., et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110: 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracey K. J. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117: 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinarello C. A., Simon A., van der Meer J. W. 2012. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11: 633–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S., Miura M., Jung Y. K., Zhu H., Li E., Yuan J. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92: 501–509 [DOI] [PubMed] [Google Scholar]
- 23.Rathinam V. A., Vanaja S. K., Waggoner L., Sokolovska A., Becker C., Stuart L. M., Leong J. M., Fitzgerald K. A. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150: 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Miura M., Jung Y-k., Zhu H., Gagliardini V., Shi L., Greenberg A. H., Yuan J. 1996. Identification and characterization of Ich-3, a member of the interleukin-1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J. Biol. Chem. 271: 20580–20587 [DOI] [PubMed] [Google Scholar]
- 25.Lin X. Y., Choi M. S., Porter A. G. 2000. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. J. Biol. Chem. 275: 39920–39926 [DOI] [PubMed] [Google Scholar]
- 26.Martinon F., Burns K., Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10: 417–426 [DOI] [PubMed] [Google Scholar]
- 27.Sollberger G., Strittmatter G. E., Kistowska M., French L. E., Beer H. D. 2012. Caspase-4 is required for activation of inflammasomes. J. Immunol. 188: 1992–2000 [DOI] [PubMed] [Google Scholar]
- 28.Saleh M., Vaillancourt J. P., Graham R. K., Huyck M., Srinivasula S. M., Alnemri E. S., Steinberg M. H., Nolan V., Baldwin C. T., Hotchkiss R. S., et al. 2004. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429: 75–79 [DOI] [PubMed] [Google Scholar]
- 29.Saleh M., Mathison J. C., Wolinski M. K., Bensinger S. J., Fitzgerald P., Droin N., Ulevitch R. J., Green D. R., Nicholson D. W. 2006. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440: 1064–1068 [DOI] [PubMed] [Google Scholar]
- 30.Kang S. J., Wang S., Hara H., Peterson E. P., Namura S., Amin-Hanjani S., Huang Z., Srinivasan A., Tomaselli K. J., Thornberry N. A., et al. 2000. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149: 613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olofsson C. S., Salehi A., Holm C., Rorsman P. 2004. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J. Physiol. 557: 935–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weischenfeldt J., Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc. 2008 doi: 10.1101/pdb.prot5080. 2008: pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 33.Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J., et al. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80: 401–411 [DOI] [PubMed] [Google Scholar]
- 34.Hagar J. A., Powell D. A., Aachoui Y., Ernst R. K., Miao E. A. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341: 1250–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayagaki N., Wong M. T., Stowe I. B., Ramani S. R., Gonzalez L. C., Akashi-Takamura S., Miyake K., Zhang J., Lee W. P., Muszyński A., et al. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249 [DOI] [PubMed] [Google Scholar]
- 36.Copeland S., Warren H. S., Lowry S. F., Calvano S. E., Remick D., Inflammation and the Host Response to Injury Investigators 2005. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12: 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanden Berghe T., Demon D., Bogaert P., Vandendriessche B., Goethals A., Depuydt B., Vuylsteke M., Roelandt R., Van Wonterghem E., Vandenbroecke J., et al. 2014. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am. J. Respir. Crit. Care Med. 189: 282–291 [DOI] [PubMed] [Google Scholar]
- 38.Nickles D., Falschlehner C., Metzig M., Boutros M. 2012. A genome-wide RNA interference screen identifies caspase 4 as a factor required for tumor necrosis factor alpha signaling. Mol. Cell. Biol. 32: 3372–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267: 2000–2003 [DOI] [PubMed] [Google Scholar]
- 40.Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., Quintal L., Sekut L., Talanian R., Paskind M., et al. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386: 619–623 [DOI] [PubMed] [Google Scholar]
- 41.Sommer F., Bäckhed F. 2013. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 11: 227–238 [DOI] [PubMed] [Google Scholar]
- 42.Erridge C., Attina T., Spickett C. M., Webb D. J. 2007. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 86: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 43.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., et al. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772 [DOI] [PubMed] [Google Scholar]
- 44.Rao R. 2009. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50: 638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manco M. 2009. Endotoxin as a missed link among all the metabolic abnormalities in the metabolic syndrome. Atherosclerosis 206: 36, author reply 37. [DOI] [PubMed] [Google Scholar]
- 46.Holmes C., Cunningham C., Zotova E., Woolford J., Dean C., Kerr S., Culliford D., Perry V. H. 2009. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73: 768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan B. B., Schmidt M. I., Pankow J. S., Ballantyne C. M., Couper D., Vigo A., Hoogeveen R., Folsom A. R., Heiss G., Atherosclerosis Risk in Communities Study 2003. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52: 1799–1805 [DOI] [PubMed] [Google Scholar]
- 48.Mehta N. N., McGillicuddy F. C., Anderson P. D., Hinkle C. C., Shah R., Pruscino L., Tabita-Martinez J., Sellers K. F., Rickels M. R., Reilly M. P. 2010. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59: 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pussinen P. J., Havulinna A. S., Lehto M., Sundvall J., Salomaa V. 2011. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34: 392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. 2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58: 445–452 [DOI] [PubMed] [Google Scholar]
- 51.Netea M. G., Nold-Petry C. A., Nold M. F., Joosten L. A., Opitz B., van der Meer J. H., van de Veerdonk F. L., Ferwerda G., Heinhuis B., Devesa I., et al. 2009. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113: 2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward J. R., West P. W., Ariaans M. P., Parker L. C., Francis S. E., Crossman D. C., Sabroe I., Wilson H. L. 2010. Temporal interleukin-1beta secretion from primary human peripheral blood monocytes by P2X7-independent and P2X7-dependent mechanisms. J. Biol. Chem. 285: 23147–23158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Druilhe A., Srinivasula S. M., Razmara M., Ahmad M., Alnemri E. S. 2001. Regulation of IL-1beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 8: 649–657 [DOI] [PubMed] [Google Scholar]
- 54.Lamkanfi M., Denecker G., Kalai M., D’hondt K., Meeus A., Declercq W., Saelens X., Vandenabeele P. 2004. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1beta generation. J. Biol. Chem. 279: 51729–51738 [DOI] [PubMed] [Google Scholar]