Abstract
The retromer complex is well known to mediate retrograde transport from endosomes to the Golgi. In a recent issue of Neuron, Choy et al. (2014) identify a function for retromer in supporting fast, local delivery of neurotransmitter receptors from endosomes to the dendritic plasma membrane.
A defining feature of eukaryotic cells is the compartmentalization of their cytoplasm into an array of membrane-bound organelles. Various transport pathways connect these organelles, allowing cargo transfer from one compartment to another. These pathways include transport from the endoplasmic reticulum towards the Golgi complex, the trans-Golgi network (TGN), endosomes, lysosomes, and the plasma membrane (“anterograde” transport), as well as transport in the opposite direction (“retrograde” transport). As a functional necessity, some proteins undergo sequential anterograde and retrograde transport. Well-characterized examples of these proteins are the transmembrane mannose 6-phosphate receptors (MPRs) that carry acid hydrolases from the TGN to endosomes. After releasing the hydrolases into the endosomal lumen for eventual transport to lysosomes, the unoccupied receptors return to the TGN to mediate further rounds of sorting. This latter process is critically dependent on a heteropentameric complex named “retromer”. The retromer complex can be subdivided into two subcomplexes: a Vps26-Vps29-Vps35 “core” trimer and a Snx1/Snx2-Snx5/Snx6 “sorting nexin” dimer. Retromer is recruited to endosomal membranes by interactions between the core trimer and the GTP-bound form of the Rab7 GTPase as well as interactions between the sorting nexin dimer and phosphatidylinositol-3 phosphate in the membrane. The core then binds the MPRs as well as other recycling proteins, leading to their sorting into membrane tubules for retrograde transport to the TGN (Figure 1, left; Seaman, 2012). Mounting evidence, however, points to an additional requirement of retromer for transport of some proteins from endosomes to the plasma membrane (Figure 1, left; Temkin et al., 2011; Seaman, 2012). This requirement could be indirect, involving intermediate passage through the TGN, or it could reflect direct delivery to the plasma membrane. In an article published recently in Neuron, Choy et al. (2014) present compelling new evidence for a function of retromer in fast, local delivery of various neurotransmitter receptors from endosomes to adjacent plasma membrane domains in neuronal dendrites.
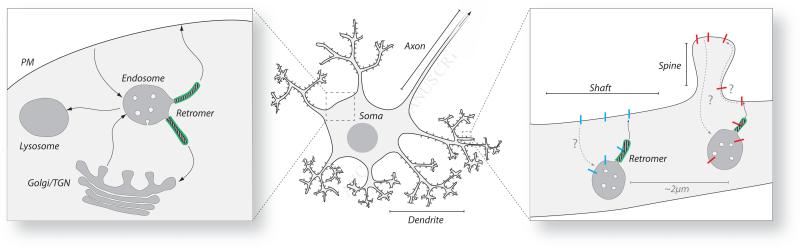
Figure 1. The Role of Retromer in Neurons.
Left: Simplified view of endosomal trafficking pathways throughout the cell. The classical retromer pathway can be seen from the endosome to the TGN, as well as the pathway identified by Choy et al. (2014) from the endosome to the plasma membrane. Retromer tubules are shown in green.
Center: Structure and domains of a neuron.
Right: Model of dendritic receptor delivery to plasma membrane by retromer. β2ARs (blue) are delivered and function at the shaft, whereas neurotransmitter receptors such as AMPA- or NMDA-type glutamate receptors (red) function at the spine. Retromer tubules are shown in green.
The ability to transport proteins to their correct intracellular locations is particularly critical in neurons, which are highly polarized cells with long axons and extensively branched dendrites projecting from their soma (Figure 1, center). Each of these structures not only represents a distinct domain of the neuronal cytoplasm but also comprises specialized subdomains such as the axon terminals and the dendritic shafts and spines (Figure 1, right). The process of synaptic transmission involves the exocytic release of neurotransmitters from the axon terminals of one neuron and their binding to cognate receptors on the dendritic shaft or spines of another neuron. Examples of post-synaptic neurotransmitter receptors are inhibitory GABA receptors that localize to dendritic shafts, and excitatory AMPA- and NMDA-type glutamate receptors that localize to dendritic spines. Clustering of these receptors at their corresponding post-synaptic sites is dependent on interactions with underlying protein scaffolds that provide dynamic anchoring while regulating synaptic transmission (Choquet and Triller, 2013). How receptors reach these sites, however, is an issue that remains poorly understood. Three mechanisms have been proposed for the delivery of receptors to post-synaptic sites: (i) vesicular transport to the plasma membrane of the soma followed by long-range lateral diffusion to the dendritic plasma membrane, (ii) vesicular transport from the soma to the dendrites and direct fusion into post-synaptic sites, and (iii) vesicular transport from the soma to the dendrites and fusion at extra-synaptic shaft locations followed by short-range lateral diffusion to post-synaptic sites (Choquet and Triller, 2013). Choy et al. (2014) provide support for aspects of the third mechanism by identifying a population of retromer-decorated endosomes located at ~2 m intervals along the dendrites, from which neurotransmitter receptors are inserted into the adjacent plasma membrane of the shaft (Figure 1, right). The high density of retromer-containing endosomes coupled with their limited movement stochastically results in an even distribution along the dendrites, ensuring a quick and effective insertion of receptors with limited reliance on diffusion (Choy et al., 2014). Several neurotransmitter receptors, including β2-adrenergic receptors (β2ARs) and AMPA receptors, are delivered to these endosomes following their internalization from the dendritic surface. β2ARs can be subsequently recycled to the adjacent shaft surface without complete fusion of the endosomes with the plasma membrane. Importantly, delivery of β2ARs to the shaft and the activity of the AMPA and NMDA receptors at their corresponding post-synaptic sites (i.e., spines) are dependent on retromer (Choy et al., 2014). Previous studies had shown that retromer mediates long-range retrograde transport of AMPA receptors from the dendrites back to TGN elements in the soma as a way of regulating synaptic strength in Caenorhabditis elegans (Zhang et al., 2012). The findings by Choy et al. (2014) differ from these previous observations in that receptor transport is short-range and anterograde, and that the TGN is not apparently involved. Thus, retromer-containing endosomes are key components of an organellar system that ensures local, regulated traffic of neurotransmitter receptors within the dendritic arbor.
The study by Choy et al. (2014) raises further questions about retromer-containing endosomes in neuronal dendrites. How do neurotransmitter receptors internalized from the spines or the shaft reach such endosomes? Do newly-synthesized receptors coming from the soma enter those endosomes en route to the dendritic surface? Could there be TGN-like elements akin to “Golgi outposts” (Horton et al., 2005) in close proximity to such endosomes? How can retromer mediate both retrograde and anterograde transport from dendritic endosomes? Could differential association of retromer with other components such as the WASH complex (Seaman et al., 2013) determine the destination of the transport intermediates? Does anterograde delivery to the dendritic shaft occur by a kiss-and-run mechanism, or via vesicular or short tubular intermediates? Addressing these questions would not only shed light on basic biological processes involving retromer but also contribute to explaining the pathogenesis of some neurodegenerative disorders. Indeed, a missense mutation in Vps35 has been shown to cause an autosomal-dominant, late-onset form – and possibly some sporadic cases – of Parkinson's disease (Zimprich, et al., 2011). In addition, polymorphisms in the genes encoding Snx1, as well as the retromer-associated Snx3 and Rab7a proteins, have been associated with Alzheimer's disease (Vardarajan, et al., 2012). Although defects in retrograde transport from endosomes to the TGN could underlie the development of these diseases, the findings by Choy et al. (2014) suggest an alternative explanation based on defective anterograde transport of neurotransmitter receptors or other proteins from endosomes to the plasma membrane in neuronal dendrites. Further research into the functions of retromer could reveal additional causative factors in this class of neurodegenerative disorders, potentially opening new avenues for their prevention and treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Choquet D, Triller A. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Choy RW, Park M, Temkin P, Herring BE, Marley A, Nicoll RA, von Zastrow M. Neuron. 2014;xx:xxx–xxx. doi: 10.1016/j.neuron.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Seaman MN. J Cell Sci. 2012;125:4693–4702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Gautreau A, Billadeau DD. Trends Cell Biol. 2013;23:522–528. doi: 10.1016/j.tcb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. Nat Cell Biol. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarajan BN, Bruesegem SY, Harbour ME, St George-Hyslop P, Seaman MNJ, Farrer LA. Neurobiol Aging. 2012;33:2231.e15–2231.e30. doi: 10.1016/j.neurobiolaging.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Isack NR, Glodowski DR, Liu J, Chen CC, Xu XZ, Grant BD, Rongo C. J Cell Biol. 2012;196:85–101. doi: 10.1083/jcb.201104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, et al. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]