Summary
Interleukin-15 (IL-15) exerts many biological functions essential for the maintenance and function of multiple cell types. Although its expression is tightly regulated, IL-15 upregulation has been reported in many organ-specific autoimmune disorders. In celiac disease, an intestinal inflammatory disorder driven by gluten exposure, the upregulation of IL-15 expression in the intestinal mucosa has become a hallmark of the disease. Interestingly, because it is overexpressed both in the gut epithelium and in the lamina propria, IL-15 acts on distinct cell types and impacts distinct immune components and pathways to disrupt intestinal immune homeostasis. In this article, we review our current knowledge of the multifaceted roles of IL-15 with regards to the main immunological processes involved in the pathogenesis of celiac disease.
Keywords: IL-15, celiac disease, tissue, autoimmunity, cytotoxic T cells, loss of oral tolerance
Introduction
Since its discovery in 1994 (1–3), the role of IL-15 has expanded tremendously from a T-cell growth factor to pleiotropic cytokine that acts on virtually each cell type of the innate and adaptive immune system. For a long time, IL-15 was viewed as a cytokine that primarily plays a role in immune homeostasis, namely in NK cell and memory CD8+ T-cell homeostasis. However, the fact that multiple reports depict the overexpression of IL-15 in tissues targeted by autoimmune processes poses the question of whether IL-15 may play a role in tissue immunity and be implicated in the development of organ-specific autoimmune disorders. Celiac disease (CD) is a T-cell-mediated intestinal disorder induced by dietary gluten that is a unique disease model to study the pathogenesis of autoimmune disorders in humans. Since IL-15 was first proposed to play a key role in CD pathogenesis (4–6), numerous studies have confirmed its role in multiple phases of the disease and expanded its impact on multiple cell types and immunological responses. In this review, we discuss the role of IL-15 in the pathogenesis of organ-specific autoimmunity using CD as a model, but first we highlight some general key features of IL-15 biology and CD pathogenesis.
Overview of IL-15 biology
IL-15 signaling and expression
IL-15 belongs to the family of Type I cytokines encompassing IL-2, IL-4, IL-7, IL-9, IL-21, and IL-15 (7–9). It shares the γc chain (CD132) of its heterotrimeric receptor with all members of the γc family cytokines (10) and the β chain (IL-2/15Rβ or CD122) with IL-2 (11). The interaction of IL-15 with IL-2/15Rβ chain and γc chain leads to the phosphorylation of Jak1 and Jak3, respectively, and to the activation of STAT5 (12–14). The Jak-STAT signaling pathway supports T-cell and natural killer (NK) cell homeostasis and expansion. In addition, IL-15 triggers other signaling pathways in T lymphocytes by promoting the phosphorylation of the Src family cytoplasmic tyrosine kinases, Lck and Syk (15–17), and by activating the phosphatidylinositol-3-kinase (PI3K), the kinase AKT (18, 19) and the Ras/Raf/MEK/mitogen-activated protein kinase (MAPK) (20–23) pathways that lead to mitogenic and anti-apoptotic signals (reviewed in 24). Unlike IL-2, IL-15-driven proliferation of T lymphocytes requires FKBP12 (12-Kda FK506-binding protein)-mediated p70S6 kinase (25). In addition, the recruitment of TRAF2 and Syk to the cytoplasmic tail of IL-15Rα chain has been shown to mediate IL-15 signaling in fibroblasts and neutrophils (26, 27).
IL-15 is a unique cytokine, because it is not secreted and can be upregulated on the surface of all cell types under conditions of inflammation and stress. Its expression on the cell surface requires the ‘private’ IL-15Rα chain. IL-15 is bound to IL-15Rα intracellularly during synthesis in the endoplasmic reticulum, shuttled to the surface, and is presented in trans to responder cells expressing the other IL-15R subunits, IL-15Rβ and γc; thus, IL-15 signaling acts in a cell contact-dependent manner (28–30). IL-15Rα stabilizes binding and greatly enhances the affinity of IL-15 for IL-2Rβ (31). Although IL-15 transpresentation represents the main mechanism by which IL-15 interacts with its receptor in vivo, alternative mechanisms have been proposed. For instance, cis-presentation represents another mechanism that involves soluble IL-15 binding to IL-15Rα allowing signaling of adjacent IL-2Rβ/γc on the same cell (32–34). Murine and human IL-15 and IL-15Rα can exist not only in membrane bound but also in a soluble form. Thus, the abundance of soluble IL-15Rα/IL-15 complexes that are cleaved from the surface of cells (35–37) suggests that IL-15 complexed to IL-15Rα could also mediate IL-15 responses. Nevertheless, it still remains unclear whether cis-presentation or stimulation by soluble IL-15Rα/IL-15 complexes are active mechanisms in vivo (38). Expression of IL-15 is tightly regulated at the level of transcription, translation, and intracellular trafficking, avoiding excessive protein production and secretion (39). The translation of IL-15 mRNA into protein is limited by the presence of multiple AUG initiation sites in the 5′-UTR region, a long signal peptide, and a negative regulatory element in the C-terminus of the IL-15 mature protein coding sequence (39, 40). Alternative splicing also controls IL-15 expression. Distinct IL-15 isoforms encoding the same mature protein that use different signal peptides are generated by alternative splicing. These different signal peptides drive the trafficking of IL-15 to distinct intracellular compartments where IL-15 isoforms are differentially translated (41–45). However, it is unknown whether expression of IL-15 isoforms contributes to tissue-specific regulatory functions. In addition, multiple isoforms of IL-15Rα contribute to IL-15 regulation. Splice variants of IL-15Rα in human monocytes and dendritic cells have been shown to determine the mode of action of IL-15, by either preventing the release of IL-15/IL-15Rα heterodimers from cell membranes thereby favoring transpresentation, or by promoting the release of IL-15 as a soluble secreted cytokine that can act on neighboring cells in a paracrine fashion (46). Therefore, IL-15 expression is fine-tuned at multiple levels to ensure that the cytokine can undertake its numerous functions. The fact that IL-15 acts mostly in a cell contact-dependent manner and that IL-2 preferentially signals via the high affinity IL2Rα-IL2Rβ-γc receptor may explain why these two cytokines that share a common signaling model yet promote different, and even opposing, outcomes. For instance, it is striking to note that inflammation and autoimmunity are associated with IL-2 deficiency (47–50) but that a dysregulated increase in IL-15 expression is observed in many inflammatory autoimmune diseases (51).
Both stromal cells and antigen-presenting cells mediate IL-15 transpresentation depending on the tissue of residence, their location within the tissue, and the responder cell (38). IL-15 expression by both hematopoietic cells and non-hematopoietic cells, i.e. medullary thymic epithelial cells, hepatic stellate cells and bone marrow stromal cells, is involved in the development and survival of naive CD8+ T cells, invariant NKT cells, and NK cells (52–58). Macrophages and dendritic cells are critically involved in IL-15 transpresentation to memory CD8+ T cells, hepatic invariant NKT cells, and differentiated NK cells (35, 52, 59–65). Thus, distinct stages of lymphocyte differentiation require IL-15 transpresentation by different cell types, which include both hematopoietic and non-hematopoietic cells (38).
In the gut, IL-15 expression is influenced by innate immune signaling. Indeed, TLR4 activation was shown to upregulate IL-15 on dendritic cells (35), and intestinal epithelial cells (IECs) require MyD88 for the expression of IL-15 and to promote the maintenance of intraepithelial lymphocytes (IELs) in an IL-15-dependant manner (66). This suggests that the microbiota, in the absence of overt inflammation, could continuously stimulate MyD88 signaling and hence contribute to the constitutive intestinal expression of IL-15 during steady state conditions. Furthermore, it has been suggested that Nod2 signaling might maintain the expression of IL-15 via recognition of the microbiota, as reduced IL-15 expression contributes to the loss of IELs in NOD2-deficient mice (67). Finally, consumption of a diet high in polyunsaturated fat leads to a decrease in IL-15 expression and concomitant reduction in IELs (68). Nevertheless, whether a direct association exists between diet, microbiota, and IL-15 expression has yet to be determined.
Role of IL-15 in immune homeostasis
The critical multifaceted roles of IL-15 during immune homeostasis are well established. IL-15 regulates adaptive memory CD8αβ TCRαβ T cells, as well as innate and innate-like lymphocytes. Its role in B-cell biology under physiological conditions is still under investigation.
Extensive characterization of mice deficient in IL-15 or in its private receptor α chain (IL-15Rα) demonstrated that IL-15 is required for the development, maintenance, and expansion of memory CD8+ T cells (38, 69–76), NK cells (77), and invariant NKT cells (49, 63, 70, 72, 78). IL-15 promotes the survival of CD8+ T cells by increasing the expression of the anti-apoptotic molecule Bcl-2 (79). In addition to its extensive role in promoting cell development and survival, it has been shown that IL-15 induces iNKT and NK cells activation (35, 53, 80–84) and increases the cytotoxicity of NK cells (85).
IELs in the small intestine represent a heterogeneous population of T cells composed mainly of TCRαβ and TCRγδ CD8+ T cells residing within the intestinal epithelium whose main role is to maintain the integrity of the epithelial layer by eliminating infected cells and promoting epithelial repair (86). In mice, IL-15 and IL-15Rα expression on intestinal epithelial cells (IECs) was shown to be critical for the survival and development of innate-like T-cell lymphocytes, i.e. CD8αα+ TCRαβ+ T cells and TCRγδ+ T cells (38, 49, 63, 64, 70, 72, 87–89). Furthermore, TCRγδ+ IELs were also shown to be expanded in transgenic mouse models where IL-15 is overexpressed in IECs (90). The mechanism underlying IL-15-mediated survival of unconventional IELs involves the activation of the Jak3-Jak1-PI3K-Akt-ERK pathway to upregulate Bcl-2 and Mcl-1 (79, 88, 91, 92). Additionally, it has been suggested that IL-15 can regulate the generation of the restricted TCR variable gamma-region gene repertoire of TCRγδ+ IELs (93), yet the exact role that IL-15 plays on γδ T cells is unclear. IL-15 does not seem to be critical for the survival of TCRαβ+ CD8αβ+ T cells whose numbers are maintained in the absence of IL-15Rα (72, 88). The limited expression of IL-2/15Rβ expression on CD8αβ T cells that reside within the intestinal epithelium could provide an explanation as to why IL-15 is less critical for this subset of IELs in mouse (38), yet the signals required for the survival of this subset of IELs remain to be determined. Nevertheless, it was shown that human CD8αβ+ IELs respond to IL-15 and its presence increases their cytolytic properties (6, 94, 95) and upregulates the expression of NK receptors (5, 6, 96), suggesting that IL-15 may impact on CD8αβ+ IELs function under inflammatory conditions. Furthermore, it was shown that TCRγδ+ IELs are expanded in intestinal organ cultures treated with IL-15 (97). IL-15 has also been recently implicated in the activation of human intraepithelial Type 1 innate lymphoid cells by promoting the production of IFN-γ (98). This latter observation is in line with the critical protective role played by IL-15 in defense mechanisms against invading pathogens, especially in the gut (99, 100). Although mice deficient in IL-15 or IL-15Rα exhibit normal numbers of B lymphocytes (70, 72, 101), in vitro studies also suggest that IL-15 can modulate B-cell activities by promoting the differentiation and proliferation of activated human B cells as well as immunoglobulin production (102, 103). Finally, besides its wide range of activities on lymphocytes, IL-15 has an impact on dendritic cells, neutrophils, and mast cells by preventing their apoptosis (104–107).
IL-15 overexpression in organ-specific autoimmune disorders other than celiac disease
In accordance with its essential role in regards to the control of immune homeostasis, IL-15 expression is tightly regulated at the translational, transcriptional, and intracellular trafficking levels and coordinated with cellular fate in myeloid vs. lymphoid cells (39, 108). Removal of these control mechanisms results in abnormal IL-15 expression in multiple cell types, whose 9 detrimental impact is exemplified in many autoimmune disorders and chronic inflammatory diseases. More specifically, IL-15 is upregulated in tissue cells that are targeted by an autoimmune process such as rheumatoid arthritis (109–114), multiple sclerosis (115, 116, 120), psoriatic arthritis or psoriasis (117–119), systemic lupus erythematosus (121–123), and type-1 diabetes (124). Furthermore, increased levels of IL-15 and/or IL-15-IL15Rα complexes have also been documented in the serum of patients with systemic lupus erythematosus (121, 123) or type-1 diabetes (124, 125). There is also dysregulated expression of IL-15 and IL-15Rα in the mucosal tissues of patients with inflammatory bowel disease (IBD) (126–130).
Mechanisms invoked to explain the role of IL-15 in organ-specific autoimmune disorders involve facilitating the maintenance of CD8+ memory T-cell survival including that of self-reactive memory T cells (9, 51), bystander activation with secretion of additional inflammatory cytokines by neighboring cells (51), activation of B cells (129), and upregulation of the activating NK receptor on CD8+ T cells (5, 6, 96, 131) and CD4+ T cells (132, 133).
Overview of celiac disease
CD is an inflammatory disorder with autoimmune features that occurs in genetically susceptible individuals expressing HLA-DQ2 or HLA-DQ8 molecules. CD patients develop inflammatory T-cell and antibody responses against dietary gluten, a protein present in wheat, rye, and barley (134). In addition, CD patients develop autoantibodies specific for the enzyme tissue transglutaminase (TG2). The disease is the ‘tip of an iceberg’ that includes a much larger undiagnosed population with various aspects of dysregulation of adaptive and innate immunity in response to gluten (135). The typical histopathological picture of CD is a small intestine enteropathy that is characterized by crypt hyperplasia, a massive increase in IELs, and villous atrophy as a consequence of surface IEC destruction. CD can be classified as classical or potential, depending on the presence of histological abnormalities in duodenal biopsies (136). Potential CD is defined by the presence of inflammatory anti-gluten immune response and anti-TG2 antibodies in the absence of villous atrophy, and therefore represents an incomplete, less severe form of CD. In contrast, classical active CD is characterized by the presence of villous atrophy (4, 137, 138), even though the identification of mucosal abnormalities upon intestinal biopsy is no longer required for diagnosis when anti-TG2-antibodies are detected (136). Withdrawal of gluten from the diet is classically associated with normalization of serology and progressive recovery of villous structures (139–143). However, while full recovery tends to occur in children with CD, more than 40% of adult CD patients maintain some level of histological anomalies on a gluten free diet (GFD) (144, 145). Furthermore, despite adherence to GFD, adult CD patients can develop a severe complication called refractory celiac disease (RCD), viewed as an early stage of enteropathy-associated T-cell lymphoma and characterized by severe villous atrophy and the presence of IELs with an abnormal phenotype (146–149).
IL-15 expression in celiac disease
The chronic upregulation of IL-15 in the epithelium (5, 150) and in the intestinal lamina propria (LP) (150, 151) is a hallmark of the disease and correlates with the degree of mucosal damage (152). Interestingly, the pattern of IL-15 overexpression differs between potential CD patients, active CD patients, and patients undergoing GFD. While most active CD patients have increased levels of IL-15 both in the intestinal LP and in the epithelium (Fig. 1), IL-15 is not upregulated in IECs in potential CD patients (authors’ unpublished data), potentially suggesting that it may be required for development of villous atrophy. Conversely, a high number of CD patients on a GFD maintain high levels of IL-15 expression in the epithelium (Fig. 1), suggesting that dysregulated expression of IL-15 in the epithelium is insufficient to induce villous atrophy. We discuss below, how, according to its location, IL-15 impacts distinct immune components and pathways to disrupt intestinal immune homeostasis.
Fig. 1. Patients with active celiac disease have high IL-15 expression both in the intestinal lamina propria and epithelium, while patients on a gluten free diet only maintain high expression in the epithelium.

Representative pictures of IL-15 immunohistochemistry staining are shown. Duodenal formalin fixed paraffin embedded sections were obtained from control non celiac subjects (control, left panel), untreated (active CD, middle panel), and treated celiac disease patients (GFD, right panel). Epithelial expression was assessed semi-quantitatively looking at staining intensity and localization (typically being stronger at the villous tip and then reducing its intensity going towards the crypts). The rate of IL-15 positive cells on the total number of infiltrating mononuclear cells in the lamina propria was assessed by two independent investigators in a double-blind set. Upregulation of IL-15 can be observed in both the small intestinal epithelium and lamina propria of active CD patients. Interestingly, celiac patients on a gluten-free diet (GFD) seem to retain only the epithelial but not the lamina propria IL-15 overexpression.
Role of IL-15 in celiac disease pathogenesis
Because it is upregulated both in the epithelium and in the LP, IL-15 acts on distinct cell types and promotes the dysregulation of multiple immune mechanisms in the small intestine that together contribute to CD pathogenesis (Fig. 2).
Fig. 2. Multifaceted roles of interleukin-15 (IL-15) in celiac disease pathogenesis.
IL-15 impacts distinct cell types to mediate its pathogenic effects. (i) In the lamina propria, IL-15 endows mucosal dendritic cells with inflammatory properties in a c-Jun N-terminal kinase (JNK)-dependent manner, and subsequently with the ability to prevent the differentiation of regulatory T cells and to promote inflammatory Th1 cell responses leading to the loss of oral tolerance. (ii) IL-15 renders effector T cells resistant to the suppressive functions of regulatory T cells through a mechanism involving JNK and phosphatidylinositol 3 kinase (PI3K). (iii) IL-15 impacts on intraepithelial lymphocytes by inducing the expression of NKG2D. The synergy between IL-15 and NKG2D cytolytic signaling pathway promotes the binding of the NKG2D-DAP10 complex to distinct adaptor proteins including PI3K whose activation promotes the phosphorylation of the mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), and JNK. This leads to cPLA2 activation, which in turn critically regulates NKG2D-mediated degranulation and cytolysis, and induces the release of arachidonic acid, a precursor of the pro-inflammatory compounds called leukotrienes. (iv) In patients with refractory sprue, IL-15 leads to the expansion and survival of an abnormal subset of CD3− intraepithelial lymphocytes by activating an anti-apoptotic cascade involving the phosphorylation of Jak3 and STAT5.
Role of IL-15 in loss of oral tolerance
Gluten is a unique protein due to its high content in proline and glutamine residues, and therefore it is a very good substrate for TG2. Prolines prevent gluten from being digested by intestinal enzymes (153), and the presence of a high frequency of glutamines and their spacing with proline (154), make the glutamines within gluten a good target for deamidation by TG2. Hence, the unique amino-acid composition of gluten allows for the generation of long peptides with negative charges that have a relatively high affinity for CD-predisposing HLA-DQ2 (155) and HLA-DQ8 (156), when TG2 is activated (157). However, the generation of peptides able to bind to HLA-DQ2 and HLA-DQ8 does not explain why inflammatory and not regulatory T-cell responses are induced. Indeed, unlike in healthy individuals where regulatory mechanisms allow the intestinal immune response to remain unresponsive to innocuous food antigens [an active process called oral tolerance (158)], CD patients exhibit a loss of oral tolerance manifested by HLA-DQ2 or HLA-DQ8-restricted anti-gluten inflammatory CD4+ T cells secreting interferon-γ (IFN-γ) and IL-21 in the small intestinal mucosa (159–162). Because IL-15 has pro-inflammatory properties and is highly upregulated in the LP of CD patients (150, 151), where dendritic cells taking up dietary antigens reside (163, 164), we hypothesized that IL-15 signaling in dendritic cells may lead to the induction of inflammatory T cell responses against gluten and the loss of oral tolerance (165, 166). Using an HLA-DQ8 mouse model overexpressing IL-15 in the LP but not in the intestinal epithelium, we showed that IL-15 in combination with retinoic acid altered the tolerogenic phenotype of intestinal dendritic cells, hence preventing the generation of inducible Foxp3+ Treg cells to dietary gluten and promoting the development of a TH1 inflammatory immune response to orally ingested gluten (165). It is important to note, however, that the location of IL-15 overexpression critically determines on which cells it acts and what the pathological impact is. For instance, IL-15 overexpression in the intestinal epithelium in our hands is not associated with the loss of oral tolerance (165, authors’ unpublished data), and potentially explains why ovalbumin-fed mice overexpressing IL-15 in the epithelium but not in the LP show no decrease in Foxp3+ T cells expressing the T-cell-receptor specific for ovalbumin (167). Furthermore, a defect in inducible regulatory T cells can be easily missed if one does not strictly control for TCR specificity using RAG-deficient TCR transgenic T cells (167), because regulatory Foxp3+ T cells without defined specificity are attracted, as are other effector T cells, to inflamed tissues. This explains why Foxp3+ T cells are paradoxically increased in the tissues of autoimmune disorders (168–172) and inflammatory bowel disease (173–179). To which degree Foxp3+ T cells recruited to inflamed tissues encounter their (self) antigen and are activated remains to be determined. Finally, in addition to losing oral tolerance, mice overexpressing IL-15 in the LP produce anti-TG2 IgG and IgA antibodies (165). Interestingly, no villous atrophy was observed in these mice, supporting the concept that in absence of the epithelial stress associated with IL-15 overexpression in the epithelium, adaptive anti-gluten immunity is insufficient to induce tissue damage. Hence, events leading to sustained or repetitive IL-15 upregulation in the LP have the potential to lead to the constitution of a growing inflammatory effector and memory pool of gluten-specific T cells that can result in the development of potential, but not active, CD.
Role of IL-15 in blocking the ability Foxp3+ regulatory T cells to regulate effector T-cell responses
Sallustro and colleagues (180), looking at effector and Foxp3+ T cells in the synovia of juvenile arthritis patients, first proposed that the presence of IL-15 could interfere with the regulatory properties of Foxp3+ T cells. It was later shown that IL-15 blocks the ability of transforming growth factor (TGF-β) to suppress activation of human mucosal T lymphocytes by activating c-Jun N-terminal kinase (JNK) and subsequently impairing Smad-3-dependent TGF-β signaling (181). Disruption of TGF-β signaling likely results in increased proliferation and production of inflammatory cytokines, ultimately promoting sustained intestinal inflammation (152). In addition, by activating the PI3K pathway, IL-15 renders effector CD8+ T cells unresponsive to the suppressive effect of Foxp3+ regulatory T cells (182). This may be by the same mechanism that IL-15 impairs the ability of regulatory T cells isolated from the blood and intestinal biopsies of CD patients to block effector CD4+ T cells in vitro (183). Interestingly, while IL-15 alters the response of effector T cells to regulatory T cells, it does not alter the intrinsic regulatory properties of Foxp3+ T cells (184). Altogether, this may explain why despite the increase in Foxp3+ T cells in inflamed tissues of patients with CD, autoimmune disorders, and IBD, effector T cells are highly effective at promoting tissue damage.
Role of IL-15 in the licensing of cytotoxic T cells to kill epithelial cells and induce active celiac disease
The induction of non-classical MHC class I molecules and the upregulation of IL-15 on IECs, as well as the dysregulated activation of IELs that acquire cytotoxic properties, are a hallmark of CD and were shown to be critically involved in the development of villous atrophy. Cytotoxic IELs are not gluten-specific but rather kill epithelial cells based on the recognition of stress signals (4, 6, 166). In healthy individuals, IELs mainly express the inhibitory CD94/NKG2A receptor (5, 131) and only low levels of the activating NKG2D receptor (6). In contrast, IELs from CD patients lack the inhibitory CD94/NKG2A receptor (185) and express high levels of the activating NKG2D and CD94/NKG2C receptors (96, 185). Concomitantly, IECs in the inflamed mucosa of CD patients express high levels of the stress-inducible MIC molecules (96, 186), and non-classical MHC class I molecule HLA-E (185), which are the main ligands for NKG2D and CD94/NKG2C, respectively. IL-15 was shown to upregulate the activating NKG2D receptor, endowing cytotoxic IELs with the ability to kill IECs expressing the stress-induced MIC molecules (6, 96, 187). IL-15 stimulation induces CD94 expression in IELs by increasing CD94 transcription and its expression on the cell surface (5). However, it does not affect the expression of NKG2A, NKG2C, or DAP12 (5, 185). Moreover, IL-15 induces the expression of NKG2D (6, 96) by increasing NKG2D and DAP10 transcription (96). Furthermore, IL-15 acts as a costimulatory molecule for the NKG2D cytolytic pathway (96, 187), hence endowing IELs with lymphokine-activated killer (LAK) activity (6, 96), i.e. with the capacity to kill in a T-cell receptor (TCR)-independent manner. In addition to its ability to endow IELs with LAK activity, IL-15 lowers the overall activation threshold of IELs (6, 94, 186). This could result in the recognition of low affinity epithelial self-antigens and the ability to kill epithelial cells in absence of cognate antigens but in a TCR-dependent manner. This scenario is supported by studies in mice showing that CD8+ T cells can reject solid tumors expressing IL-15 in a TCR-dependent manner despite the fact that they do not express the cognate antigen for the CD8+ T cells mediating their rejection (188).
In our view, it is very likely that the destruction of epithelial cells by IELs involves both the TCR and NK receptors. Importantly, only epithelial cells expressing IL-15 and ligands for activating NK receptors will be destroyed. Hence, despite the fact that IELs in the presence of IL-15 act as innate-like lymphocytes that kill epithelial cells based on stress signals, this destruction is highly specific. This led us to propose that IELs in CD are autoreactive and that CD is a model for organ specific autoimmunity (189). This concept is further supported by the presence of autoantibodies specific for TG2, which are required for the diagnosis of CD.
Role of IL-15 in promoting survival of abnormal intraepithelial lymphocytes and promoting refractory celiac disease
In the case of RCD, sustained expression of high levels of IL-15 in the epithelium (150, 190) leads to the expansion of a subset of CD3− cytotoxic IELs that have undergone profound genetic reprogramming of their NK functions, ultimately acquiring an aberrant and highly activated NK cell-like phenotype (148). IL-15 is thought to contribute to the expansion and survival of these IELs with an aberrant phenotype by exerting anti-apoptotic action on IELs (150, 190, 191). Indeed, IL-15 is able to activate an anti-apoptotic cascade, involving phosphorylation of Jak3 and STAT5 and the increased expression of the anti-apoptotic B cell lymphoma-extra large (Bcl-xL) protein (190). In addition to playing a critical role in the sustained survival of these abnormal IELs, IL-15 also increases their cytolytic capacities (186). Refractory sprue is mimicked in an IL-15 transgenic mouse model where human IL-15 is expressed in intestinal epithelial cells. This upregulation of IL-15 is associated with the expansion of activated NK-like cytotoxic IELs and villous atrophy (190, 192). The link between IL-15, the increase in NK-like cytotoxic IELs and villous atrophy, is supported by the finding that blocking IL-15 signaling prevents villous atrophy (192), suggesting that neutralizing IL-15 or blocking its signaling may be a treatment for RCD.
IL-15 and genetic risk factors of celiac disease
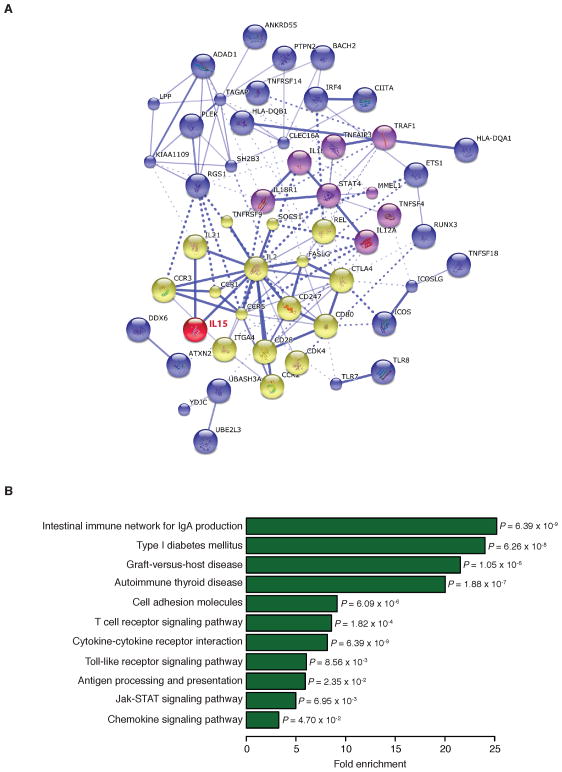
Because of its large contribution to CD immunopathogenesis, one would have expected the identification of il15 as a CD susceptibility gene by genome wide association studies. However, no genetic association has yet been found for the gene encoding IL-15 (193). This lack of association suggests that the increased levels of IL-15 in patients might be the consequence of the deregulation of genes capable to modulate the levels of the cytokine; that is, trans effects. By analyzing the functional interactions among CD susceptibility genes and IL-15, we found that several of the genes were associated with IL-15, suggesting that the increased levels of IL-15 observed in CD patients probably results from functional variation in this CD-susceptibility network (Fig. 3). Interestingly, among the genes that have direct associations with IL-15 in this network are the γc cytokines IL-21 and IL-2, which share many common structural and functional properties with IL-15. More generally, the genes associated with IL-15 are strongly enriched for key pathways that are central for the pathogenesis of CD such as IgA production, T-cell receptor signaling, or antigen processing and presentation. We also observed a significant enrichment for genes belonging to the autoimmune thyroiditis and Type 1 Diabetes pathways, an observation in accordance with several reports showing that IL-15 upregulation is associated with increased risk for such diseases (124, 125, 194, 195).
Fig. 3. Interactions between IL-15 and celiac disease susceptibility genes.
(A). Network of known interactions between celiac disease (CD)-associated genes and IL-15. We used the STRING database to look for known functional interactions among CD susceptibility genes, as well as functional interactions between CD susceptibility genes and IL-15 (red). The figure only shows CD-associated genes that are directly or indirectly connected with IL-15. STRING database assembles information about both known and predicted protein-protein interactions on the basis of numerous sources, including experimental repositories, computational prediction method, and public text collections. We grouped genes based on the distance matrix obtained from the String global scores. We used the KMEANS algorithm setting the number of groups to three. Proteins pairs with a higher global core (i.e. stronger evidence that they interact together) are grouped together on the same cluster. The three clusters are represented by different colors (yellow, purple, and blue). IL-15 is directly connected with the ‘yellow cluster’. The thickness of the lines connecting the genes is proportional to String global scores supporting the evidence of an interaction. Solid and dashed lines represent intra- and inter-cluster connections, respectively. (B). KEGG pathway enrichment analysis for the CD-associated genes shown in panel A. The y-axis reports the fold enrichments observed for genes in a particular pathway (named in y-axis) using all human genes as our background expectation.
Functional redundancy between IL-21, type-1 IFN, and IL-15 in celiac disease
Due to shared receptor components and signaling pathways, γc cytokines theoretically present a high degree of redundancy. In fact, IL-15 and IL-21 exert many overlapping activities in regards to CD immunopathogenesis (Fig. 4) including the ability to render effector CD4+ T cells resistant to the suppressive effects of regulatory T cells (196), the ability to drive the production of IFN-γ (160), and the ability to upregulate cytotoxic activity in IELs (197). Although in vitro studies have demonstrated that IL-21 by itself has very little effect, if any, on the proliferation of CD8+ T cells, IL-21 synergizes with IL-15 to promote CD8+ T-cell activation and expansion, production of IFN-γ, and upregulation of granzyme B and perforin (198). The fact that IL-15 enhances the production of IL-21 suggests the establishment of an amplification loop that could foster the ongoing inflammatory response (199). In agreement with the hypothesis that IL-21 could contribute to the induction of inflammation and tissue damage in CD is the finding that potential CD patients not only lack IL-15 overexpression in the LP, but also fail to express IL-21 (200). Although they do not share structural components and signaling pathways, Type I interferon (IFN) is another cytokine that could exert redundant effects with IL-15 (Fig. 4). IFN-α expression is dysregulated in the small intestine mucosa of CD patients (201–203). In addition, clinical observations of the development of CD in hepatitis C patients treated with IFN-α (204) as well as the induction of inflammatory anti-gluten responses and the generation of TG2 antibodies following rotavirus infections (205) suggest that IFN-α likely plays a critical role in the induction of inflammatory T-cell responses against gluten. However, whether and how Type I IFN contributes to CD pathogenesis remains to be determined.
Fig. 4. Overlapping functions of IL-15, IL-21, and Type I interferon.
Both IL-15 and IL-21 render effector CD4+ T cells resistant to the suppressive functions of regulatory T cells. Both IL-15 and Type I interferon endow dendritic cells with inflammatory properties and have been shown to mediate loss of oral tolerance and to promote Th1 immunity. All three cytokines have the ability to confer cytotoxic properties to CD8+ T cells.
Conclusions and future directions
Overall, the involvement of IL-15 in multiple steps of the NKG2D cytolytic pathway, together with its confirmed roles in abrogating oral tolerance to dietary gluten and interfering with the suppressive activity of intestinal regulatory T cells, makes this cytokine a key player involved in the dysregulation of immune responses in CD. Future studies will also better delineate the role of IL-15 in other organ-specific autoimmune disorders. In particular, it will be important to determine whether IL-15 is critical in the development of autoreactive T-cell responses and the destruction of the tissues targeted by the autoimmune process. In this regard, it is interesting to note that LADA (latent autoimmune diabetes in adults) patients, unlike type-1 diabetes patients, lack IL-15 overexpression in islet β-cells (124), suggesting that, as observed in potential CD, upregulation of IL-15 in tissue cells is critical to license cytotoxic T cells to kill the tissue targeted by the autoimmune process.
Although the exact factors and mechanisms responsible for triggering IL-15 upregulation have yet to be defined, it has been suggested that gliadin peptides could promote IL-15 expression by IECs (186, 206). However, this effect is likely indirect, due to the upregulation of multiple inflammatory mediators as a consequence of T-cell activation. Because IL-15 can be induced by many inflammatory stimuli, including cytokines and TLR ligands (207, 208), other factors, notably microbial components that are enriched in the intestinal compartment, could promote sustained IL-15 expression. However, it is important to acknowledge that we know very little about the mechanisms underlying IL-15 dysregulation in CD and when this dysregulation occurs.
Due to the central role of IL-15 in the immunopathogenesis of CD, there is a growing interest in developing novel therapies able to dampen the actions of IL-15. To inhibit IL-15 activity and to prevent its deleterious effect on oral tolerance and IELs activation, several agents have been developed, including antibodies specific for IL-15 or IL-2/15Rβ, and Jak inhibitors. The humanized antibody (Hu-Mik-β-1) directed towards IL-2/15Rβ prevents the transpresentation of IL-15 by antigen-presenting cells to neighboring NK cells and CD8+ T cells (9, 209). The administration of TMβ-1 (the anti-mouse equivalent of Hu-Mik-β-1) to IL-15 transgenic mice results in the abrogation of inflammatory cytokine production and in the reversal of intestinal damages (165, 192). The Food and Drug Administration has authorized the usage of Hu-Mik-β-1 to treat CD patients with the type II form of RCD (210), who develop enteropathy-associated T-cell lymphoma with a two year survival of less than 30% (146, 211). Another approach consists of interfering with the IL-15 signaling pathway. It involves the administration of the Jak2/3 inhibitor tofacitinib that has been shown to completely reverse the intestinal pathological changes observed in the T3b-hIL-15 transgenic mouse model (212). Finally, ex vivo experiments have demonstrated the capacity of the humanized anti-IL-15 monoclonal antibody AMG714 to inhibit the activation of Jak3 and STAT5 in the mucosa of type II RCD patients. In addition, the administration of AMG714 to IL-15 transgenic mice restores IELs apoptosis and consequently inhibits their accumulation (190). Thus, these anti-IL-15 therapies represent promising therapeutic approaches, especially for patients with refractory disease. However, because of the potential redundancy with IL-21, type-1 IFN and yet undefined cytokines, we cannot exclude that combination therapies or therapies targeting common signaling pathways may be necessary to achieve a therapeutic effect.
Acknowledgments
We thank celiac disease patients and their family members as well as the University Of Chicago Celiac Disease Center for supporting our research. We thank Luis B. Barreiro for fruitful discussions and for help preparing Figure 3. We thank Valentina Discepolo for the immunohistochemistry. We thank M. Zurenski and S. Kim for critical reading of the manuscript. This work was supported by grants from the Digestive Diseases Research Core Center at the University of Chicago and from the US National Institutes of Health (RO1DK67180) to B.J. and by grants from the Canadian Celiac Association and from the Canadian Institutes of Health Research to V.A.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Bamford RN, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4940–4. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton JD, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4935–9. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabstein KH, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 5.Jabri B, et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology. 2000;118:867–79. doi: 10.1016/S0016-5085(00)70173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts AI, et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–30. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 7.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 10.Sugamura K, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Giri JG, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–30. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston JA, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci U S A. 1995;92:8705–9. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JX, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–9. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki T, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–7. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–31. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Suen KL, Barbacid M, Bolen JB, Fargnoli J. Interleukin-2-induced tyrosine phosphorylation of Shc proteins correlates with factor-dependent T cell proliferation. J Biol Chem. 1994;269:5518–22. [PubMed] [Google Scholar]
- 17.Hatakeyama M, et al. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991;252:1523–8. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 18.Gu H, et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol. 2000;20:7109–20. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan P, Babbage JW, Thomas G, Cantrell D. p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol Cell Biol. 1999;19:4729–38. doi: 10.1128/mcb.19.7.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedmann MC, Migone TS, Russell SM, Leonard WJ. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc Natl Acad Sci U S A. 1996;93:2077–82. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravichandran KS, Igras V, Shoelson SE, Fesik SW, Burakoff SJ. Evidence for a role for the phosphotyrosine-binding domain of Shc in interleukin 2 signaling. Proc Natl Acad Sci U S A. 1996;93:5275–80. doi: 10.1073/pnas.93.11.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravichandran KS, Burakoff SJ. The adapter protein Shc interacts with the interleukin-2 (IL-2) receptor upon IL-2 stimulation. J Biol Chem. 1994;269:1599–602. [PubMed] [Google Scholar]
- 23.Evans GA, et al. Analysis of interleukin-2-dependent signal transduction through the Shc/Grb2 adapter pathway. Interleukin-2-dependent mitogenesis does not require Shc phosphorylation or receptor association. J Biol Chem. 1995;270:28858–63. doi: 10.1074/jbc.270.48.28858. [DOI] [PubMed] [Google Scholar]
- 24.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–80. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Dubois S, Shou W, Haneline LS, Fleischer S, Waldmann TA, Muller JR. Distinct pathways involving the FK506-binding proteins 12 and 12.6 underlie IL-2-versus IL-15-mediated proliferation of T cells. Proc Natl Acad Sci U S A. 2003;100:14169–74. doi: 10.1073/pnas.2335979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulfone-Paus S, et al. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 1999;13:1575–85. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 27.Ratthe C, Girard D. Interleukin-15 enhances human neutrophil phagocytosis by a Syk-dependent mechanism: importance of the IL-15Ralpha chain. J Leukoc Biol. 2004;76:162–8. doi: 10.1189/jlb.0605298. [DOI] [PubMed] [Google Scholar]
- 28.Bergamaschi C, et al. Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol. 2009;183:3064–72. doi: 10.4049/jimmunol.0900693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergamaschi C, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–99. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 30.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 31.Ring AM, et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol. 2012;13:1187–95. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowley J, Monie A, Hung CF, Wu TC. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur J Immunol. 2009;39:491–506. doi: 10.1002/eji.200838594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–9. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota N, Takase M, Uchiyama H, Olsen SK, Kanagawa O. No requirement of trans presentations of IL-15 for human CD8 T cell proliferation. J Immunol. 2010;185:6041–8. doi: 10.4049/jimmunol.0901834. [DOI] [PubMed] [Google Scholar]
- 35.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–25. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergamaschi C, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120:e1–8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouchaud G, et al. Interleukin-15 and its soluble receptor mediate the response to infliximab in patients with Crohn’s disease. Gastroenterology. 2010;138:2378–87. doi: 10.1053/j.gastro.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 38.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59:479–90. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418–26. [PubMed] [Google Scholar]
- 40.Bamford RN, Battiata AP, Burton JD, Sharma H, Waldmann TA. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc Natl Acad Sci U S A. 1996;93:2897–902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura H, Washizu J, Nakamura N, Enomoto A, Yoshikai Y. Translational efficiency is upregulated by alternative exon in murine IL-15 mRNA. J Immunol. 1998;160:936–42. [PubMed] [Google Scholar]
- 42.Tagaya Y, et al. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci U S A. 1997;94:14444–9. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onu A, Pohl T, Krause H, Bulfone-Paus S. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J Immunol. 1997;158:255–62. [PubMed] [Google Scholar]
- 44.Gaggero A, et al. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur J Immunol. 1999;29:1265–74. doi: 10.1002/(SICI)1521-4141(199904)29:04<1265::AID-IMMU1265>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 45.Tan X, Lefrancois L. Novel IL-15 isoforms generated by alternative splicing are expressed in the intestinal epithelium. Genes Immun. 2006;7:407–16. doi: 10.1038/sj.gene.6364314. [DOI] [PubMed] [Google Scholar]
- 46.Muller JR, Waldmann TA, Kruhlak MJ, Dubois S. Paracrine and transpresentation functions of IL-15 are mediated by diverse splice versions of IL-15Ralpha in human monocytes and dendritic cells. J Biol Chem. 2012;287:40328–38. doi: 10.1074/jbc.M112.378612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 48.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 51.Waldmann TA. Targeting the interleukin-15/interleukin-15 receptor system in inflammatory autoimmune diseases. Arthritis Res Ther. 2004;6:174–7. doi: 10.1186/ar1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winau F, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–29. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Chang CL, Lai YG, Hou MS, Huang PL, Liao NS. IL-15Ralpha of radiation-resistant cells is necessary and sufficient for thymic invariant NKT cell survival and functional maturation. J Immunol. 2011;187:1235–42. doi: 10.4049/jimmunol.1100270. [DOI] [PubMed] [Google Scholar]
- 54.Burkly L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–6. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 55.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–21. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elewaut D, et al. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med. 2003;197:1623–33. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40. [PubMed] [Google Scholar]
- 58.Cui G, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci U S A. 2014;111:1915–20. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castillo EF, Acero LF, Stonier SW, Zhou D, Schluns KS. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood. 2010;116:2494–503. doi: 10.1182/blood-2010-03-277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183:4948–56. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colpitts SL, Stoklasek TA, Plumlee CR, Obar JJ, Guo C, Lefrancois L. Cutting edge: the role of IFN-alpha receptor and MyD88 signaling in induction of IL-15 expression in vivo. J Immunol. 2012;188:2483–7. doi: 10.4049/jimmunol.1103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golden-Mason L, et al. Hepatic interleuklin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clin Exp Immunol. 2004;138:94–101. doi: 10.1111/j.1365-2249.2004.02586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortier E, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–22. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–21. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–54. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol. 2006;176:6180–5. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- 67.Kiang W, et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med. 2013;210:2465–76. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Zhang H, Ma H, Lu B, Li Y, Li J. Inhibitory effect of dietary n-3 polyunsaturated fatty acids to intestinal IL-15 expression is associated with reduction of TCRalphabeta+CD8alpha+CD8beta-intestinal intraepithelial lymphocytes. J Nut Brioche. 2008;19:475–81. doi: 10.1016/j.jnutbio.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Becker TC, et al. Interleukin 15 is required for proliferate renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ku CC, Murakami M, Sakamoto A, Kipper J, Mar rack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 72.Iodole JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 73.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–50. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schluns KS, Klonowski KD, Lefrancois L. Tran regulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103:988–94. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 75.Schluns KS, Williams K, Ma A, Zen XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Sun S, Hwang I, Tough DF, Sprint J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 77.Carson WE, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Distant JP, Muller W, Guy-Grand D, Fischer A, Rajas K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A. 1995;92:377–81. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu TS, et al. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor alpha-chain. J Immunol. 2002;168:705–12. doi: 10.4049/jimmunol.168.2.705. [DOI] [PubMed] [Google Scholar]
- 80.Matsuda JL, Zhang Q, Nonie R, Richardson SK, Howell AR, Gaping L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsuda JL, et al. Homeostasis of V alpha 14i NKT cells. Nat Immunol. 2002;3:966–74. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 82.Gory LE, et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187:6335–45. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koki R, Burkett P, Chine M, Chain S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–8. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 84.Lucas M, Chatterley W, Oberlin K, Michele P, Dieffenbachia A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C, Zhang J, Nil J, Tina Z. Interleukin-15 improves cytotoxicity of natural killer cells via up regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42:128–36. doi: 10.1016/j.cyto.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 86.Labadie V, Discepolo V, Jabri B. Intraepithelial lymphocytes in celiac disease immunopathology. Semen Immunopathol. 2012;34:551–66. doi: 10.1007/s00281-012-0316-x. [DOI] [PubMed] [Google Scholar]
- 87.Lai YG, et al. IL-15 does not affect IEL development in the thymus but regulates homeostasis of putative precursors and mature CD8 alpha alpha+ IELs in the intestine. J Immunol. 2008;180:3757–65. doi: 10.4049/jimmunol.180.6.3757. [DOI] [PubMed] [Google Scholar]
- 88.Ma LJ, Acero LF, Zeal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. 2009;183:1044–54. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reinbeck HC, McDermott RP, Mira S, Dimness A, Padlock DK. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–13. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 90.Inagaki-O’Hara K, Nishimura H, Miami A, Yoshikai Y. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing gamma delta T cell receptor in mice. Eur J Immunol. 1997;27:2885–91. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 91.Lai YG, et al. IL-15 modulates the balance between Bcl-2 and Bam via a Jak3/1-PI3K-Akt-ERK pathway to promote CD8alphaalpha+ intestinal intraepithelial lymphocyte survival. Eur J Immunol. 2013;43:2305–16. doi: 10.1002/eji.201243026. [DOI] [PubMed] [Google Scholar]
- 92.Navasota K, Yamada H, Yajima T, Kakemono Y, Kiwanis H, Yoshikai Y. Enforced expression of Bcl-2 partially restores cell numbers but not functions of TCRgammadelta intestinal intraepithelial T lymphocytes in IL-15-deficient mice. J Immunol. 2007;178:757–64. doi: 10.4049/jimmunol.178.2.757. [DOI] [PubMed] [Google Scholar]
- 93.Zhao H, Nguyen H, Kang J. Interleukin 15 controls the generation of the restricted T cell receptor repertoire of gamma delta intestinal intraepithelial lymphocytes. Nat Immunol. 2005;6:1263–71. doi: 10.1038/ni1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ebert EC. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439–45. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 95.Ebert EC. IL-15 converts human intestinal intraepithelial lymphocytes to CD94 producers of IFN-gamma and IL-10, the latter promoting Fas ligand-mediated cytotoxicity. Immunology. 2005;115:118–26. doi: 10.1111/j.1365-2567.2005.02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 97.Maiuri L, et al. IL-15 drives the specific migration of CD94+ and TCR-gammadelta+ intraepithelial lymphocytes in organ cultures of treated celiac patients. Am J Gastroenterol. 2001;96:150–6. doi: 10.1111/j.1572-0241.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 98.Fuchs A, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–81. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Sabatino A, Calarota SA, Vidali F, Macdonald TT, Corazza GR. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011;22:19–33. doi: 10.1016/j.cytogfr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Perera PY, Lichy JH, Waldmann TA, Perera LP. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect. 2012;14:247–61. doi: 10.1016/j.micinf.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gill N, Paltser G, Ashkar AA. Interleukin-15 expression affects homeostasis and function of B cells through NK cell-derived interferon-gamma. Cell Immunol. 2009;258:59–64. doi: 10.1016/j.cellimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 102.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–90. [PubMed] [Google Scholar]
- 103.Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J Immunol. 2004;173:6676–83. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 104.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci U S A. 2005;102:8662–7. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cassatella MA, McDonald PP. Interleukin-15 and its impact on neutrophil function. Curr Opin Hematol. 2000;7:174–7. doi: 10.1097/00062752-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 106.Hoontrakoon R, et al. Interleukin-15 inhibits spontaneous apoptosis in human eosinophils via autocrine production of granulocyte macrophage-colony stimulating factor and nuclear factor-kappaB activation. Am J Respir Cell Mol Biol. 2002;26:404–12. doi: 10.1165/ajrcmb.26.4.4517. [DOI] [PubMed] [Google Scholar]
- 107.Masuda A, Matsuguchi T, Yamaki K, Hayakawa T, Yoshikai Y. Interleukin-15 prevents mouse mast cell apoptosis through STAT6-mediated Bcl-xL expression. J Biol Chem. 2001;276:26107–13. doi: 10.1074/jbc.M011475200. [DOI] [PubMed] [Google Scholar]
- 108.Colpitts SL, et al. Transcriptional regulation of IL-15 expression during hematopoiesis. J Immunol. 2013;191:3017–24. doi: 10.4049/jimmunol.1301389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asquith DL, McInnes IB. Emerging cytokine targets in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:246–51. doi: 10.1097/BOR.0b013e3280eec78c. [DOI] [PubMed] [Google Scholar]
- 110.McInnes IB, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 111.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–95. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 112.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–76. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 113.Harada S, et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1508–16. doi: 10.1002/1529-0131(199907)42:7<1508::AID-ANR26>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 114.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells In vitro and in the SCID mouse-human rheumatoid arthritis model In vivo. J Clin Invest. 1998;101:1261–72. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rentzos M, Rombos A. The role of IL-15 in central nervous system disorders. Acta Neurol Scand. 2012;125:77–82. doi: 10.1111/j.1600-0404.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- 116.Vaknin-Dembinsky A, Brass SD, Gandhi R, Weiner HL. Membrane bound IL-15 is increased on CD14 monocytes in early stages of MS. J Neuroimmunol. 2008;195:135–9. doi: 10.1016/j.jneuroim.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22:160–8. doi: 10.1684/ecn.2011.0294. [DOI] [PubMed] [Google Scholar]
- 118.Tang F, et al. Interleukin 15 primes natural killer cells to kill via NKG2D and cPLA2 and this pathway is active in psoriatic arthritis. PLoS One. 2013;8:e76292. doi: 10.1371/journal.pone.0076292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Villadsen LS, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112:1571–80. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Losy J, Niezgoda A, Zaremba J. IL-15 is elevated in sera of patients with relapsing-remitting multiple sclerosis. Folia Neuropathol. 2002;40:151–3. [PubMed] [Google Scholar]
- 121.Aringer M, et al. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:876–81. doi: 10.1093/rheumatology/40.8.876. [DOI] [PubMed] [Google Scholar]
- 122.Bo H, et al. Elevated expression of transmembrane IL-15 in immune cells correlates with the development of murine lupus: a potential target for immunotherapy against SLE. Scand J Immunol. 2009;69:119–29. doi: 10.1111/j.1365-3083.2008.02197.x. [DOI] [PubMed] [Google Scholar]
- 123.Robak E, Robak T, Wozniacka A, Zak-Prelich M, Sysa-Jedrzejowska A, Stepien H. Proinflammatory interferon-gamma--inducing monokines (interleukin-12, interleukin-18, interleukin-15)--serum profile in patients with systemic lupus erythematosus. Eur Cytokine Netw. 2002;13:364–8. [PubMed] [Google Scholar]
- 124.Chen J, et al. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Ralpha. Proc Natl Acad Sci U S A. 2013;110:13534–9. doi: 10.1073/pnas.1312911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuczynski S, Winiarska H, Abramczyk M, Szczawinska K, Wierusz-Wysocka B, Dworacka M. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:231–6. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Kirman I, Nielsen OH. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am J Gastroenterol. 1996;91:1789–94. [PubMed] [Google Scholar]
- 127.Leon AJ, et al. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. doi: 10.1155/2009/580450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Z, Geboes K, Colpaert S, D’Haens GR, Rutgeerts P, Ceuppens JL. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164:3608–15. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 129.Nishiwaki T, et al. Possible involvement of the interleukin-15 and interleukin-15 receptor system in a heightened state of lamina propria B cell activation and differentiation in patients with inflammatory bowel disease. J Gastroenterol. 2005;40:128–36. doi: 10.1007/s00535-004-1510-y. [DOI] [PubMed] [Google Scholar]
- 130.Vainer B, Nielsen OH, Hendel J, Horn T, Kirman I. Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine. 2000;12:1531–6. doi: 10.1006/cyto.2000.0744. [DOI] [PubMed] [Google Scholar]
- 131.Jabri B, et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–99. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 132.Allez M, et al. CD4+NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132:2346–58. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 133.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–7. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 135.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–25. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 136.Husby S, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 137.Lohi S, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 138.MacDonald TT, Bajaj-Elliott M, Pender SL. T cells orchestrate intestinal mucosal shape and integrity. Immunol Today. 1999;20:505–10. doi: 10.1016/s0167-5699(99)01536-4. [DOI] [PubMed] [Google Scholar]
- 139.Dieterich W, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–21. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 140.Holmes GK, Asquith P, Stokes PL, Cooke WT. Cellular infiltrate of jejunal biopsies in adult coeliac disease (ACD) in relation to gluten withdrawal. Gut. 1973;14:429. [PubMed] [Google Scholar]
- 141.Kilander AF, Nilsson LA, Gillberg R. Serum antibodies to gliadin in coeliac disease after gluten withdrawal. Scand J Gastroenterol. 1987;22:29–34. doi: 10.3109/00365528708991852. [DOI] [PubMed] [Google Scholar]
- 142.Savilahti E, Viander M, Perkkio M, Vainio E, Kalimo K, Reunala T. IgA antigliadin antibodies: a marker of mucosal damage in childhood coeliac disease. Lancet. 1983;1:320–2. doi: 10.1016/s0140-6736(83)91627-6. [DOI] [PubMed] [Google Scholar]
- 143.Sulkanen S, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–8. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 144.Bardella MT, et al. Coeliac disease: a histological follow-up study. Histopathology. 2007;50:465–71. doi: 10.1111/j.1365-2559.2007.02621.x. [DOI] [PubMed] [Google Scholar]
- 145.Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–8. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cellier C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 148.Cellier C, et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–81. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 149.Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:413–24. doi: 10.1016/j.bpg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 150.Mention JJ, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–45. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 151.Maiuri L, Ciacci C, Auricchio S, Brown V, Quaratino S, Londei M. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 152.Di Sabatino A, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–77. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shan L, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 154.Vader LW, et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med. 2002;195:643–9. doi: 10.1084/jem.20012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim CY, Quarsten H, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci U S A. 2004;101:4175–9. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Henderson KN, et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity. 2007;27:23–34. doi: 10.1016/j.immuni.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 157.Siegel M, et al. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 159.Bodd M, et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2012;3:594–601. doi: 10.1038/mi.2010.36. [DOI] [PubMed] [Google Scholar]
- 160.Fina D, et al. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut. 2008;57:887–92. doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 161.Molberg O, Kett K, Scott H, Thorsby E, Sollid LM, Lundin KE. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol. 1997;46:103–9. doi: 10.1046/j.1365-3083.1997.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 162.Nilsen EM, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 163.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–40. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 164.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.DePaolo RW, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–4. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9:858–70. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 167.Korneychuk N, et al. Interleukin 15 and CD4 T Cells Cooperate to Promote Small Intestinal Enteropathy in Response to Dietary Antigen. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 168.de Kleer IM, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 169.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 171.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 173.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Makita S, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–30. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 175.Maul J, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 176.Saruta M, et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281–90. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 177.Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–9. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]
- 178.Holmen N, et al. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12:447–56. doi: 10.1097/00054725-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 179.Holtta V, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175–84. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- 180.Ruprecht CR, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Benahmed M, et al. Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology. 2007;132:994–1008. doi: 10.1053/j.gastro.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 182.Ben Ahmed M, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:6763–70. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 183.Zanzi D, et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol. 2011;106:1308–17. doi: 10.1038/ajg.2011.80. [DOI] [PubMed] [Google Scholar]
- 184.Borrelli M, et al. Immunoregulatory pathways are active in the small intestinal mucosa of patients with potential celiac disease. Am J Gastroenterol. 2013;108:1775–84. doi: 10.1038/ajg.2013.303. [DOI] [PubMed] [Google Scholar]
- 185.Meresse B, et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J Exp Med. 2006;203:1343–55. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hue S, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 187.Tang F, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J Exp Med. 2009;206:707–19. doi: 10.1084/jem.20071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu RB, et al. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc Natl Acad Sci U S A. 2013;110:8158–63. doi: 10.1073/pnas.1301022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol. 2013;13:294–302. doi: 10.1038/nri3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Malamut G, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120:2131–43. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schmitz F, et al. Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut. 2013;62:509–19. doi: 10.1136/gutjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 192.Yokoyama S, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009;106:15849–54. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Abadie V, Sollid LM, Barreiro LB, Jabri B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol. 2011;29:493–525. doi: 10.1146/annurev-immunol-040210-092915. [DOI] [PubMed] [Google Scholar]
- 194.Ajjan RA, Watson PF, Weetman AP. Detection of IL-12, IL-13, and IL-15 messenger ribonucleic acid in the thyroid of patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1997;82:666–9. doi: 10.1210/jcem.82.2.3760. [DOI] [PubMed] [Google Scholar]
- 195.Kaiser P, Rothwell L, Vasicek D, Hala K. A role for IL-15 in driving the onset of spontaneous autoimmune thyroiditis? J Immunol. 2002;168:4216–20. doi: 10.4049/jimmunol.168.8.4216. [DOI] [PubMed] [Google Scholar]
- 196.Peluso I, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–9. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 197.Ebert EC. Interleukin 21 upregulates perforin-mediated cytotoxic activity of human intra-epithelial lymphocytes. Immunology. 2009;127:206–15. doi: 10.1111/j.1365-2567.2008.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sarra M, et al. IL-15 positively regulates IL-21 production in celiac disease mucosa. Mucosal Immunol. 2012;6:244–55. doi: 10.1038/mi.2012.65. [DOI] [PubMed] [Google Scholar]
- 200.Sperandeo MP, et al. Potential celiac patients: a model of celiac disease pathogenesis. PLoS One. 2011;6:e21281. doi: 10.1371/journal.pone.0021281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Di Sabatino A, et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology. 2007;133:1175–87. doi: 10.1053/j.gastro.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 202.Monteleone G, et al. Role of interferon alpha in promoting T helper cell type 1 responses in the small intestine in coeliac disease. Gut. 2001;48:425–9. doi: 10.1136/gut.48.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Raki M, Beitnes AC, Lundin KE, Jahnsen J, Jahnsen FL, Sollid LM. Plasmacytoid dendritic cells are scarcely represented in the human gut mucosa and are not recruited to the celiac lesion. Mucosal Immunol. 2013 doi: 10.1038/mi.2012.136. [DOI] [PubMed] [Google Scholar]
- 204.Cammarota G, Cuoco L, Cianci R, Pandolfi F, Gasbarrini G. Onset of coeliac disease during treatment with interferon for chronic hepatitis C. Lancet. 2000;356:1494–5. doi: 10.1016/S0140-6736(00)02880-4. [DOI] [PubMed] [Google Scholar]
- 205.Troncone R, Auricchio S. Rotavirus and celiac disease: clues to the pathogenesis and perspectives on prevention. J Pediatr Gastroenterol Nutr. 2007;44:527–8. doi: 10.1097/MPG.0b013e31804ca0ec. [DOI] [PubMed] [Google Scholar]
- 206.Maiuri L, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–7. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 207.Dafik L, Albertelli M, Stamnaes J, Sollid LM, Khosla C. Activation and inhibition of transglutaminase 2 in mice. PLoS One. 2012;7:e30642. doi: 10.1371/journal.pone.0030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–87. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 209.Morris JC, et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2006;103:401–6. doi: 10.1073/pnas.0509575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J Investig Dermatol Symp Proc. 2013;16:S28–30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
- 211.Delabie J, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148–55. doi: 10.1182/blood-2011-02-335216. [DOI] [PubMed] [Google Scholar]
- 212.Yokoyama S, Perera PY, Waldmann TA, Hiroi T, Perera LP. Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL-15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J Clin Immunol. 2013;33:586–94. doi: 10.1007/s10875-012-9849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]