Abstract
Purpose: The objective of this study was to develop a clear, aqueous nanomicellar formulation and evaluate its in vitro ocular biocompatibility as a novel carrier for topical ocular delivery of biotinylated lipid prodrug for the treatment of herpetic keratitis.
Methods: Micellar formulation of Biotin-12Hydroxystearic acid-acyclovir (B-12HS-ACV) was prepared by solvent evaporation/film hydration method with two nonionic surfactants, vitamin E TPGS and octoxynol-40. The optimized formulation was characterized for various parameters including micelle size, polydispersity index (PDI), and zeta-potential and in vitro prodrug release. Human corneal epithelial cells (HCECs) were employed for studying the cytotoxicity of the formulation. Further, mRNA expression levels of various cytokines were also studied with quantitative real-time PCR (qPCR).
Results: Average size was 10.46±0.05 nm with a PDI of 0.086 for blank nanomicelles, and 10.78±0.09 nm with a PDI of 0.075 for prodrug-loaded nanomicelles. Both unloaded and prodrug-loaded nanomicelles had low negative zeta potential. Prodrug encapsulation efficiency of mixed nanomicelles was calculated to be ∼90%. Transmission electron microscopy analysis revealed that nanomicelles were spherical, homogenous, and devoid of aggregates. B-12HS-ACV release from nanomicelles was slow with no significant burst effect. Results show a sustained release of the prodrug from nanomicelles over a period of 4 days. Neither the blank formulation nor the prodrug-loaded micellar formulation demonstrated any cytotoxic effects. Further, incubation of HCECs with blank and prodrug-loaded nanomicellar groups did not significantly alter the expression levels of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ.
Conclusions: In summary, a topical clear, aqueous nanomicellar formulation comprised of vitamin E TPGS and octoxynol-40 loaded with 0.1% B-12HS-ACV was successfully developed. B-12HS-ACV-loaded nanomicelles are small in size, spherical, and homogenous, without any aggregates. The micellar formulations were perfectly transparent similar to pure water. Ocular biocompatibility studies indicated that mixed nanomicelles were nontoxic and noninflammatory to corneal epithelial cells. Therefore, nanomicellar technology represents a promising strategy for the delivery of biotinylated lipid prodrugs of ACV.
Introduction
Topical ocular drug delivery has always been a challenging task for pharmaceutical scientists. Current efforts in ophthalmic drug delivery are directed toward sustained/controlled drug release, prolonging the residence time or contact time of drug delivery system, and enabling improved corneal absorption. A variety of ocular drug delivery systems, such as in situ gelling systems,1–3 mucoadhesives,4–5 nanoparticles,6–8 inserts,9,10 and soft contact lenses,11–13 have been investigated. Although appeared to be promising, these systems are commonly associated with lack of patient compliance (vision interference, irritation, and discomfort), high manufacturing cost (lack of ability to scale up the production), and ultimately approval by regulatory authorities.14
These problems render topical ocular formulations in the form of aqueous solutions as suitable and alternative drug delivery systems. However, a major issue in the field of ocular drug absorption is poor drug bioavailability from topical instillation that arises from rapid elimination owing to precorneal factors, such as tear turnover, lachrymation, nasolacrimal drainage, metabolic degradation, nonproductive adsorption/absorption, and, most importantly, poor corneal permeation. The residence time of topically applied drugs being very short (<5 min), only 1%–5% of the applied drug permeates the cornea and reaches intraocular tissues. Thus, commercially available eye drops are often ineffective and require repeated instillations.15 Despite these disadvantages, eye drops are most compliant dosage forms due to their ease of application, minimal risk of infection compared to implantation or injection-based drug delivery systems, ease of dose adjustment, low interference with vision, and moreover, highly patient compliant.14,16 In addition, a homogenous aqueous solution may offer several advantages including the simplicity of large-scale manufacturing process.
The objective of this study was to formulate a clear, aqueous nanomicellar formulation and evaluate in vitro ocular biocompatibility as a novel carrier for topical delivery of biotinylated lipid prodrug for the treatment of herpetic keratitis. To meet the requirements of ocular delivery, a combination of two nonionic surfactants, D-alpha-tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS) costabilized with octyl phenol ethoxylate (octoxynol-40), were selected as material components.17 These nonionic surfactants were chosen because of their biocompatibility, stability, and minimal toxicity compared with cationic, anionic, or amphoteric polymeric surfactants.16,18,19 Moreover, these surfactants have been reported to possess very minimal hemolytic activity, toxicity, irritation, and inflammation to the ocular structures. Vitamin E TPGS is a component of FDA-approved product Agenerse® (Amprenavir, an antiviral HIV protease inhibitor) marketed by GlaxoSmithKline Pharmaceuticals. Octoxynol-40 is currently used in a marketed formulation (Acular® and Acular LS®) of Allergan, Inc. Also, the presence of vitamin E TPGS can act as efflux transporter inhibitor that facilitates drug absorption and higher bioavailability.20,21
Recently we have developed a novel targeted lipid prodrug strategy to improve cellular absorption of acyclovir (ACV). This approach combines both the lipid and transporter targeted delivery to generate synergistic effect.22–23 Targeted lipid prodrugs of ACV exhibited higher affinity for sodium-dependent multivitamin transporter (SMVT). Biotinylated lipid prodrugs of ACV demonstrated synergistic improvement in cellular uptake presumably due to recognition of the prodrugs by SMVT on the cornea and lipid mediated transcellular diffusion. Biotin-12Hydroxystearicacid-Acyclovir (B-12HS-ACV) (Fig. 1) was selected as a model prodrug because of its enhanced binding affinity toward SMVT, higher cellular uptake, lack of cytotoxicity, and excellent in vitro antiviral activity against HSV-1 and −2. As evident by the EC50 values, B-12HS-ACV displayed 34-fold and 60-fold increase in antiviral efficacy against HSV-1 and HSV-2, respectively.22,23
FIG. 1.
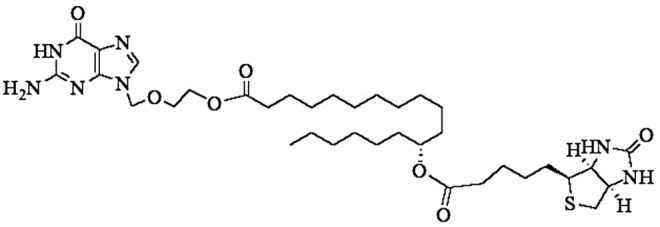
Structure of Biotin-12Hydroxystearicacid-Acyclovir (B-12HS-ACV).
An important aspect of ocular drug delivery is to evaluate drug carriers not only in terms of efficacy but also in terms of biocompatibility. Formulation components are expected to be safe and reliable, effective for its intended use, and biocompatible. Biocompatibility evaluation serves as an indicator of the interaction between cells and surfactants/biopolymers. It confirms that solution/device may not produce direct/indirect injury to corneal tissues; inflammatory, immunologic, or systemic effects; and does not induce delayed adverse effects.24 In vitro biocompatibility studies are often conducted in cells. Therefore, selection of a cell line is extremely important because it must reproduce a toxic or an inflammatory response that is similar to what occurs in vivo.25,26 Therefore, we chose human corneal epithelial cells (HCECs) as an in vitro cell culture model to study the expression profiles of inflammatory cytokines and chemokines. It will also be the medium to evaluate cytotoxic effects of the nanomicellar formulation. Hence, the objective of this study was to develop a clear, aqueous nanomicellar formulation and to evaluate in vitro ocular biocompatibility as a novel carrier for topical delivery of biotinylated lipid prodrug of ACV for the treatment of herpetic keratitis.
Methods
Materials
B-12HS-ACV was synthesized according to a previously published method.23 D-alpha-tocopheryl polyethylene glycol 1000 succinate (vitamin E-TPGS; NF grade) was procured from Eastman Chemical Co. (Kingsport, TN). Igepal (octoxynol-40) was obtained from Rhodio, Inc. (New Jersey, NJ). Escherichia coli–derived lipopolysaccharide (LPS) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12) was obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was procured from Atlanta Biologicals (Lawrenceville, GA). Culture flasks (75 cm2 and 25 cm2 growth area) were purchased from Corning Costar Corp. (Cambridge, MA). The buffers for cDNA synthesis and amplification (oligodT, dNTP, magnesium chloride, and M-MLV reverse transcriptase) were purchased from Promega Corporation (Madison, WI). Light Cycler 480® SYBR I green master mix was obtained from Roche Applied Science (Indianapolis, IN). Quantitative primers used in the study were custom designed and were obtained from Invitrogen Life Technologies (Carlsbad, CA). All the buffer components, solvents, and other chemicals were obtained from Fisher Scientific Co. (Fair Lawn, NJ) and were utilized without further purification.
Preparation of micelles
Micellar formulation of B-12HS-ACV was prepared by solvent evaporation method. Briefly, 1 mg of prodrug, 45 mg of vitamin E TPGS, and 20 mg of octoxynol-40 were each dissolved separately in 1 mL of 95% ethanol. All the three solutions were mixed together to form a homogenous solution. This solution was then evaporated under vacuum to form a solid thin film. Subsequently, this film was rehydrated with 500 μL of distilled deionized (DDI) water and sonicated for 20 min in bath sonicator (50/60 Hz; 125 W) to form mixed micelles. Further, this homogenous solution was diluted with 500 μL of tear buffer and a clear aqueous solution was obtained. Subsequently, pH of the aqueous formulation was adjusted to 6.8 with Oakton pH meter (Model: pH 510 series; Oakton Instruments, Vernon Hills, IL) and filtered through 0.22-μm nylon filter to remove unentrapped prodrug aggregates and other foreign particulates. Also, unloaded micelles were prepared by a similar method as described earlier.
Critical micelle concentration determination
Critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles are spontaneously formed. To determine the CMC of binary mixture of vitamin E TPGS and octoxynol-40 in DDI water, an ultraviolet-visible (UV-Vis) spectroscopy method was employed, using iodine as a hydrophobic probe following previously published reports.27,28 The KI/I2 standard solution was prepared by dissolving 0.5 g of iodine and 1 g of potassium iodide in 50 mL of DDI water. Samples of polymeric surfactant solution with different concentrations were prepared. To each of the vitamin E TPGS/octoxynol-40 binary mixture (ratio 4.5:2) solutions, 25 μL of KI/I2 solution was added. The mixtures were then incubated for 12 h at room temperature preventing from light. The UV absorbance values of varying polymeric surfactant concentrations at 366 nm were measured with a UV-Vis spectrometer. The absorption intensity was plotted against logarithm of polymeric surfactant concentration. The CMC values correspond to the concentration of the polymeric surfactant at which a sharp increase in absorbance is noted.
Characterization of micelles
Micelle size and size distribution
The mean hydrodynamic diameter and polydispersity of micelles were determined by dynamic laser scattering (DLS) using a Zetasizer HS 3000 (Malvern Instruments, UK) with a detection angle of 90° at 25°C. The average values of 3 measurements of 12 runs were calculated for all samples.
Drug encapsulation efficiency
The amount of prodrug encapsulated in the micelles was measured by LC-MS/MS. One milliliter of micelle solution was freeze-dried and dissolved in 1 mL dichloromethane (DCM) to reverse the micelle structure. Subsequently, DCM was evaporated and 1 mL mobile phase (70:30 v/v acetonitrile/water) was added to dissolve the prodrug. The solution was then filtered by 0.22-μm nylon syringe filter and analyzed for prodrug content. Encapsulation efficiency (EE) was calculated according to Equation 1.
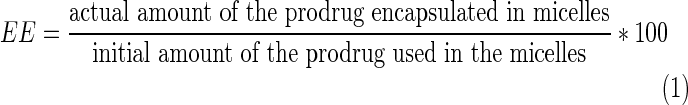 |
Transmission electron microscopy analysis
Morphology of nanomicelles was determined by transmission electron microscopy (TEM; Philips CM12 STEM, Hillsboro, OR).29–32 Briefly, a drop of aqueous formulation containing micelles was placed on a carbon-coated copper grid and excess fluid was removed with a piece of filter paper. Further, the sample was stained with 1.5% phosphotungstic acid solution and excess solution was removed with a filter paper. TEM image was then taken after the sample was completely dried.
In vitro prodrug release
A fixed volume (1 mL) of prodrug-loaded micellar solution was suspended in dialysis bag (molecular mass cut-off 1 kDa), and subsequently placed into vials containing 5 mL DPBS (pH 7.4) buffer solution. All the samples were then placed in a shaking water bath at 60 rpm maintained at a constant temperature of 37°C. The entire buffer medium was replaced at every predetermined time point. The collected incubation medium containing the released prodrug was immediately stored at −80°C until further analysis. As a control, an equal amount (1 mg) of prodrug was dissolved in 1 mL of ethanol and suspended into a separate dialysis bag. Prior to analysis, samples were thawed and the supernatant was extracted by a well-established procedure and analyzed by LC-MS/MS.22,23
LC-MS/MS analysis
A fast and sensitive LC-MS/MS method has been recently developed in multiple reaction monitoring (MRM) with electrospray (ES) positive ionization mode for the detection of targeted lipid prodrug, B-12HS-ACV.22,23 QTRAP® LC-MS/MS mass spectrometer (API 3200; Applied Biosystems/MDS Sciex, Foster City, CA) was employed to analyze prodrug samples obtained from in vitro drug release and entrapment efficiency studies. XTerra® RP8 Column (5 μm, 4.6×50 mm; Waters Corporation, Milford, MA) was applied for chromatographic separation with an isocratic mobile phase. The mobile phase consisted of 70% acetonitrile, 30% water, and 0.1% formic acid that were pumped at a flow rate of 0.2 mL/min. Precursor ions of the analytes as well as the internal standard were determined from spectra obtained during the infusion of standard B-12HS-ACV solutions with an infusion pump connected directly to the electrospray ionization source. Precursor ions were subjected to collision-induced dissociation to determine the product ions. MRM transitions at m/z [M+H]+ generated were 735.6/257.3 for B-12HS-ACV and 256/152 for ganciclovir (GCV; internal standard). Peak areas for all components were automatically integrated by Analyst™ software and peak-area ratios (area of analytes to area of internal standard) were plotted against concentration by weighted linear regression (1/concentration). The analytical data resulted from B-12HS-ACV with MRM method exhibited linearity. This method generated rapid and reproducible results.
Stocks and stock dilutions of B-12HS-ACV were prepared according to a recently published procedure.23 All the release samples were subjected to liquid–liquid extraction method. GCV was selected as internal standard to ensure reproducibility and reliability of this analytical method. Prior to analysis, samples were thawed at room temperature. Two hundred microliters of samples along with 20 μL of GCV (5 μg/mL) were extracted with 1 mL of organic solvent containing isopropanol (IPA) and DCM (2:3 ratio). The samples were vortexed for ∼2 min and centrifuged at 12,000 g for 15 min at 4°C. Organic layer (850 μL) was transferred into eppendorf tubes and evaporated to dryness under speed vacuum with a Speedvac (SAVANT Instruments, Inc., Holbrook, NY). The residue was then reconstituted in 200 μL of mobile phase, vortexed for 1 min, and transferred into prelabeled vials with silanized inserts. Subsequently, 15 μL of the reconstituted solution was injected onto LC-MS/MS. Appropriate calibration standards of B-12HS-ACV were prepared and a calibration curve was generated using calibration standards.
Cell culture
HCECs were utilized in this study and cultured following a previously published protocol.33 HCECs were a generous gift from Dr. Araki-Sasaki (Kinki Central Hospital, Japan). Briefly, HCECs were grown at 37°C in a cell culture incubator with 95% air and 5% CO2. Culture medium consisted of DMEM/F-12 supplemented with 15% (v/v) FBS (heat inactivated), 15 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) 22 mM sodium bicarbonate, 100 mg each of penicillin and streptomycin, 5 μg/mL insulin, and 10 ng/mL of human epidermal growth factor. Cells of passage numbers between 27 and 30 were utilized for all the experiments in this study.
Cytotoxicity assay
In vitro cytotoxicity of unloaded and prodrug-loaded nanomicelles was carried out with Cell Titer 96® Aqueous Non-Radioactive Cell Proliferation Assay Kit (Promega, Madison, WI). HCECs were grown on 96-well plates at a density of 10,000 cells per well. Clear transparent aqueous micellar solution was made in culture media and sterile filtered with 0.22-μm nylon membrane filters under laminar flow to obtain sterile formulations. Aliquots of blank and prodrug-loaded nanomicelles (100 μL) in culture medium were added to each well and cells were incubated for 1 h with the formulations. Cell proliferation in the presence of unloaded and loaded nanomicelles was compared with a positive control (medium without drug) and a negative control (10% Triton-X 100). After 1 h of incubation, 20 μL of freshly prepared dye solution (following manufacturer's protocol) was added to each well and incubated for 2½ hours enabling the dye to react. The amount of formazan formed was measured using a 96-well microtiter plate reader (SpectraFluor Plus; Tecan, Maennedorf, Switzerland), absorbance set at 490 nm wavelength. Cytotoxicity measurements can be estimated by the amount of formazan formed that is directly dependent on the cell viability.
Quantitative gene expression analysis
Expression levels of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ on HCECs were determined at the molecular level with quantitative real-time PCR (qPCR). Cells were exposed to unloaded and prodrug-loaded micellar formulation as well as LPS (100 ng/mL) for 1 h. Cells were lysed with TRIzol® reagent. Chloroform was added to the cell lysate for phase separation. Subsequently, the aqueous phase (containing RNA) was separated and IPA was added to allow RNA precipitation. The generated RNA was washed twice with 75% ethanol and resuspended in RNase-DNase free water. The concentration and purity of RNA was determined with Nanodrop (Thermo Fisher Scientific, Wilmington, DE). cDNA was obtained by reverse transcription of RNA using oligodT as a template and M-MLV reverse transcriptase. The reverse transcription conditions were as follows: denaturation of the template RNA for 5 min at 70°C; reverse transcription for 60 min at 42°C followed by final extension at 72°C for 5 min. Following reverse transcription, qPCR was performed with LightCycler® SYBR green technology (Roche). cDNA (80 ng) was added to each well and subjected to amplification using specific primers listed in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was employed as an internal standard to normalize the amount of cDNA in each well. The specificity of these primers was also confirmed with melting-curve analysis. The comparative threshold method was used to calculate the relative amount of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ in different samples according to previously published methods.33,34
Table 1.
Quantitative Real-Time Polymerase Chain Reaction Primers for Glyceraldehyde-3-Phosphate Dehydrogenase, Interleukin 1- Beta, Interleukin 6, Interleukin 8, Interleukin 17, Tumor Necrosis Factor Alpha, and Interferon Gamma
| Gene | Sequence 5′→3′ |
|---|---|
| GAPDH | |
| Forward | ATCCCTCCAAAATCAAGTGG |
| Reverse | GTTGTCATGGATGACCTTGG |
| IL-1β | |
| Forward | CACTACAGCAAGGGCTTCAGG |
| Reverse | GTCCATGGCCACAACAACTG |
| IL-6 | |
| Forward | CCACTCACCTCTTCAGAACGAA |
| Reverse | GGCAAGTCTCCTCATTGAATCC |
| IL-8 | |
| Forward | CTTGGCAGCCTTCCTGATTT |
| Reverse | CAGCCCTCTTCAAAAACTTC |
| IL-17 | |
| Forward | GCCATAGTGAAGGCAGGAAT |
| Reverse | CAGGTTGACCATCACAGTCC |
| TNF-α | |
| Forward | CCTGCCCCAATCCCTTTATT |
| Reverse | CCCTAAGCCCCCAATTCTCT |
| IFN-γ | |
| Forward | GGCTTTTCAGCTCTGCATCG |
| Reverse | TCCTGGCAGTAACAGCCAAGA |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
All the experiments were conducted at least in quadruplicate (n=4) and the results were expressed as mean±standard deviation (SD). Statistical comparison of mean values was performed with Student's t-test and one-way analysis of Variance using GraphPad Prism (La Jolla, CA). A P-value of <0.05 was considered to be statistically significant.
Results
Preparation and characterization of micelles
CMC determination
CMC is a characteristic of in vitro and in vivo stability of micelles. Low CMC values of vitamin E TPGS/octoxynol-40 binary mixture bring about high stability of mixed nanomicelles in solutions upon dilution. In the current study, the micelle formation was monitored by using iodine as a hydrophobic probe. Solubilized I2 prefers to partition in the hydrophobic microenvironment of copolymer, causing the conversion of I3− to I2 from the excess KI in the solution. CMC of vitamin E TPGS and octoxynol-40 was calculated to be 0.025 and 0.107 wt%, respectively. Interestingly, CMC of vitamin E TPGS/octoxynol-40 was found to be 0.012 wt%. Low CMC of the micelles suggests high stability and ability to maintain integrity even upon dilution.
Micelle size and size distribution
The solvent evaporation method was employed for the preparation of B-12HS-ACV-encapsulated micelles. Micellar systems were characterized relative to micelle size, polydispersity index (PDI), and zeta-potential. Average micelle diameter was 10.46±0.05 nm with a PDI of 0.086 for unloaded micelles, and 10.78±0.09 nm with a PDI of 0.075 for prodrug-loaded micelles (Fig. 2). PDI values suggest that the nanomicelles exhibit narrow particle size distribution. Both unloaded and prodrug-encapsulated nanomicelles carry negative zeta potential (−2.26 mV for unloaded and −1.59 mV for prodrug-loaded nanomicelles) (Table 2).
FIG. 2.
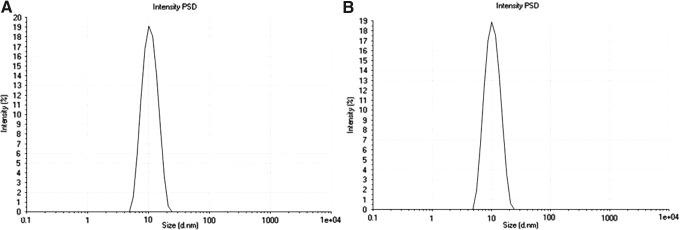
Size distribution of nanomicelles: (A) nanomicelles with no drug inside, and (B) prodrug-loaded nanomicelles. PSD, particle size distribution.
Table 2.
Characteristics of the Mixed Nanomicelle Systems: Particle Size, Size Distribution, Zeta Potential, and Entrapment Efficiency
| Sample | Nanomicelle effective diameter (nm) | Polydispersity index | Zeta potential | Entrapment efficiency |
|---|---|---|---|---|
| Vitamin E TPGS (4.5%)+octoxynol-40 (2.0%) | 10.46 | 0.086 | −2.26 | – |
| Prodrug/vitamin E TPGS (4.5%)+octoxynol-40 (2.0%) | 10.78 | 0.075 | −1.59 | ∼90% |
Vitamin E TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate.
Drug EE
Drug EE is an important factor for drug delivery carriers. The prodrug EE of mixed nanomicelles was calculated to be ∼90%. These results demonstrate that mixed nanomicelles achieve high prodrug EE (Table 2).
TEM analysis
The morphology of the nanomicelles was investigated by TEM. TEM analysis showed that the micelles were spherical and homogenous, and devoid of aggregates (Fig. 3A). Unloaded (blank) and prodrug-encapsulated nanomicelles did not differ in terms of morphology. The particle sizes visualized by TEM were very similar and in agreement with the size obtained by DLS.
FIG. 3.
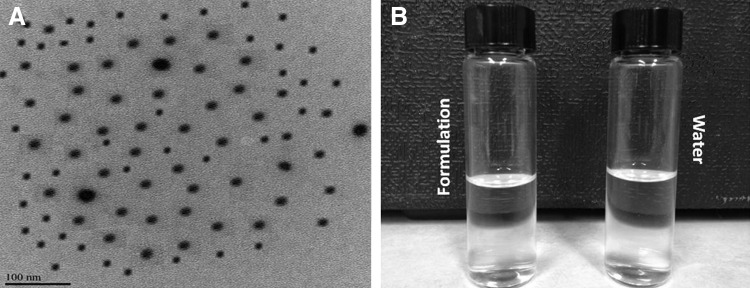
(A) TEM picture of prodrug-encapsulated nanomicelles, and (B) 0.1% prodrug nanomicellar formulation on the left compared with distilled deionized water on the right side. TEM, transmission electron microscopy.
Figure 3B compares 0.1% prodrug encapsulated micellar formulation on the left with DDI water on the right side. As the figures illustrate, the micellar formulations are perfectly transparent comparable to DDI water.
In vitro prodrug release
In vitro–release kinetics of biotinylated lipid prodrug B-12HS-ACV from mixed nanomicelles was investigated at a physiological pH of 7.4 at 37°C. An equal quantity of prodrug dissolved in 1 mL of ethanol served as a control. B-12HS-ACV release from ethanolic solution was much faster in comparison to prodrug in micelles. Almost 100% prodrug release occurs in ∼6 h. Figure 4 represents the release kinetic profile of encapsulated prodrug from the nanomicelles. Release half-lives of B-12HS-ACV from nanomicelles were slow and not associated with any significant burst effect. The results suggest a sustained release of the prodrug from the nanomicelles over a period of 4 days.
FIG. 4.
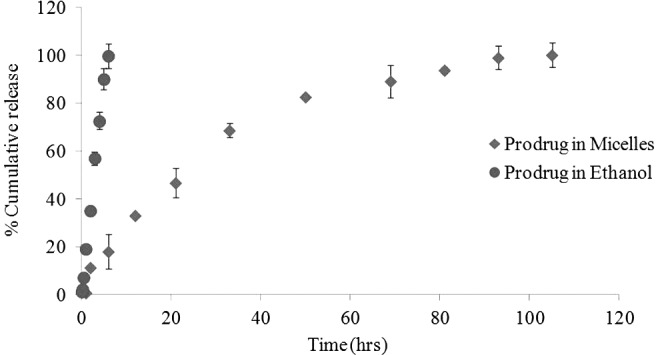
Release profile of prodrug B-12HS-ACV from the mixed nanomicelle systems under sink conditions at 37°C. Values represent mean±standard deviation (n=4).
Cytotoxicity assay
Cytotoxicity assay was performed on HCECs for a period of 1 h to evaluate the cytotoxic effects of unloaded and prodrug-encapsulated nanomicelles. Since eye drops are rapidly cleared from the surface of the eye,35,36 it was assumed that a 1-h incubation period would be sufficient to evaluate any toxic effects. Triton X-100 caused significant toxicity because it reduced the percentage of cell viability to ∼6% of the whole cell number. Neither blank formulation nor prodrug-encapsulated micellar formulation demonstrated any cytotoxic effects as evident by the cell viability. Results from this assay clearly suggest that our mixed nanomicellar formulation was safe for topical ocular application (Fig. 5).
FIG. 5.
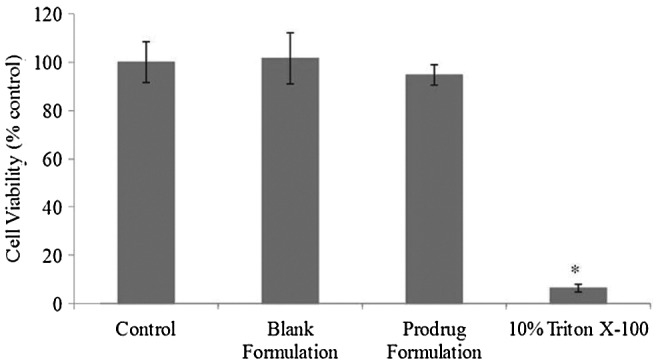
Cytotoxicity assay in the presence of blank and prodrug-loaded formulation on human corneal epithelial cells (HCECs) for 1 h. Data represent mean percentage of viable cells±standard deviation (n=4). A P-value of <0.05 is considered to be statistically significant and denoted by *.
Quantitative gene expression analysis
Expression levels of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ on HCECs were determined at the molecular level with qPCR. The quality of RNA extracted from HCECs was assessed by measuring A260/A280 ratios. The ratio was found to be 1.83 suggesting that the RNA was pure. LPS (100 ng/mL) was selected as a positive control in all the experiments.
Relative fold expression of IL-6 and IL-1β
Figure 6 shows the mRNA expression of IL-6 and IL-1β in HCECs exposed to blank and prodrug-encapsulated nanomicelles for 1 h. There was no statistically significant difference in the IL-6 and IL-1β gene expression among the control, blank, and prodrug-loaded nanomicelle groups. LPS induced the expression of IL-6 and IL-1β by about 8.5- and 3.5-fold times, respectively.
FIG. 6.
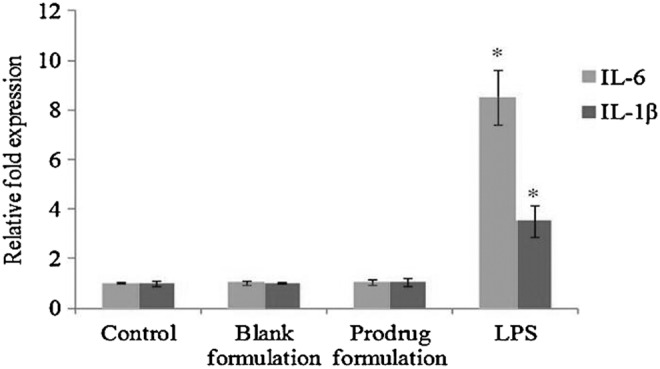
Relative fold induction of IL-6 and IL-1β in HCECs treated with blank and prodrug-loaded formulation and lipopolysaccharide (100 ng/mL) for 1 h. Values represent mean±standard deviation (n=4). A P-value of <0.05 is considered to be statistically significant and denoted by *.
Relative fold expression of IL-8 and IL-17
mRNA expression levels of IL-8 (3-fold) and IL-17 (5-fold) were significantly elevated in HCECs following LPS exposure relative to control. However, incubation of HCECs with blank and prodrug-loaded micelles groups did not significantly alter the expression levels of IL-8 and IL-17 (Fig. 7).
FIG. 7.
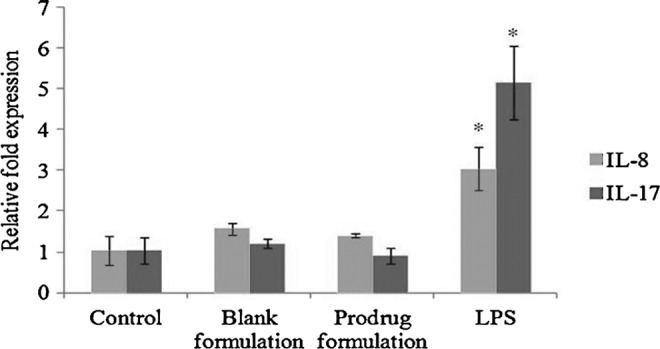
Relative fold induction of IL-8 and IL-17 in HCECs treated with blank and prodrug-loaded formulation and lipopolysaccharide (100 ng/mL) for 1 h. Values represent mean±standard deviation (n=4). A P-value of <0.05 is considered to be statistically significant and denoted by *.
Relative fold expression of TNF-α and IFN-γ
A significant upregulation of TNF-α (9-fold) and IFN-γ (4-fold) expression was observed in LPS-treated cells compared with control. Similar to earlier results, there was no statistically significant difference in the TNF-α and IFN-γ gene expression among the control, blank, and prodrug-encapsulated nanomicelles groups (Fig. 8).
FIG. 8.
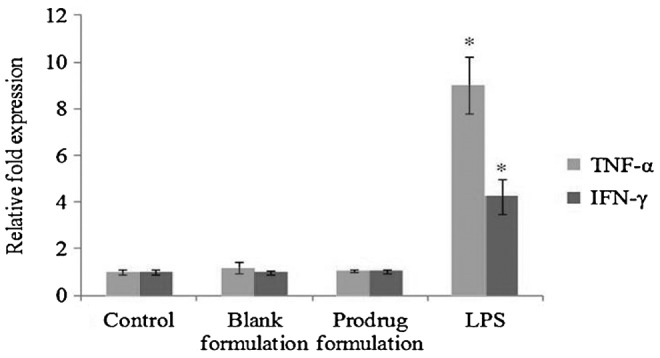
Relative fold induction of TNF-α and IFN-γ in HCECs treated with blank and prodrug-loaded formulation and lipopolysaccharide (100 ng/mL) for 1 h. Values represent mean±standard deviation (n=4). A P-value of <0.05 is considered to be statistically significant and denoted by *.
Discussion
Topical administration by commercial eye drops is often ineffective in achieving therapeutic drug levels due to the presence of static and dynamic barriers and rapid elimination by precorneal factors, i.e., blinking and nasolacrimal drainage.15,37 Therefore, many ophthalmic disorders require a systemic administration, which requires high systemic doses to achieve therapeutic levels, with the potential of systemic side effects. Alternatively, a topical aqueous ophthalmic formulation that can overcome ocular barriers and deliver the drug directly to the target tissue would enhance treatment efficacy with lower doses and reduced/minimal side effects. To improve ocular bioavailability, utilization of a carrier system that can protect the drug, improve uptake by corneal epithelia, and enable a sustained drug/prodrug release after administration would be more attractive. To this extent, nanosized colloidal carriers offer many advantages. Indeed, the particle size is one key factor for transport through biological membranes and nanosized particles permeate the corneal epithelial layers through both transcellular and paracellular pathways. In the last decade, many colloidal carrier systems including nanoparticles, nanomicelles, microparticles, and liposomes have been developed for topical ophthalmic application.38,39 Among these systems, micelles appear to be promising carriers for ophthalmic applications because of their simple preparation method, nanosize, optical transparency, stability, high EE, and localized drug release. Moreover, the ability of nanomicelles to prevent/minimize drug degradation reduces adverse effects and enhances drug permeation through ocular epithelia with minimal/no irritation. Act of these properties ultimately leads to enhanced ocular bioavailability.40–43
In this study, mixed nanomicelles prepared from vitamin E TPGS and octoxynol-40 were used as colloidal carriers for ocular delivery of biotinylated lipid prodrug B-12HS-ACV. The mixed micelles have a low CMC suggesting high stability and ability to maintain integrity even upon dilution. Mixed micelles were characterized for their size, PDI, and entrapment efficiency. The micellar size is an actual representation of the hydrodynamic diameter of the particles in Brownian motion. Dynamic light scattering demonstrated that blank micelles are about 10.46 nm, while mean size of prodrug-encapsulated micelles is slightly bigger, about 10.78 nm. Also, these micelles display a narrow and unimodal particle size distribution. However, no significant changes in micelles size or size distribution were recorded. Since these nanomicelles are in the same size range as membrane receptors, proteins, and other biomolecules, these carriers may have the ability to bind with cellular barriers.44 Drug EE is an important factor for any nanotechnology-based drug delivery carriers. As evident by the EE of ∼90%, it can be seen that mixed nanomicelles can achieve a high degree of drug/prodrug encapsulation. This is mainly because of the presence of hydrophobic core of mixed micelles that allows lipophilic drugs to strongly interact with this hydrophobic core facilitating larger amounts of drug to entrapment. TEM analysis further confirmed that the nanomicelles were spherical and homogenous, and devoid of aggregation. Further, the mixed nanomicellar formulations were perfectly transparent comparable to DDI water. Such optically clear ophthalmic solutions may be advantageous over suspensions because the ingredients are completely dissolved and usually do not impair or interfere with the vision of the patient. However, the major drawback associated with ophthalmic suspensions is the presence of particles large enough to produce eye irritation and may cause blurred vision.
B-12HS-ACV release from ethanolic solution was much faster in comparison to prodrug encapsulated in nanomicelles. Almost 100% prodrug release occurs in ∼6 h. However, in vitro prodrug release profile from nanomicelles demonstrates slower release of B-12HS-ACV without a significant burst effect. The results clearly suggest a sustained release of the prodrug from nanomicelles over a period of 4 days. These results further indicate that the addition of octoxynol-40 to vitamin E TPGS might assist in stabilizing the total micellar composite structure and had a significant effect in extending prodrug release. The first nonionic surfactant vitamin E TPGS acts as the main spherical structure and the second nonionic surfactant octoxynol-40 adds strength to the nanomicellar structure by inserting itself between two polymeric chains of the first nonionic surfactant. Such stabilization is believed to result in the formation of aqueous solutions of extremely hydrophobic drugs that have optical clarity.45,46 Further, mixed nanomicelles are more stable than that of pure polymeric micelles due to the presence of vitamin E TPGS and octoxynol-40 composite polymers, which might possibly enhance hydrophobic interactions between the polymeric chains in the micellar core, thus enhancing colloidal stability of the system.47,48
Nanosized materials can be efficiently taken up and internalized by cells. Such accumulated components could possibly interact with cells and tissues and, consequently, cause adverse effects.49 Any alteration in corneal epithelial integrity or tissue disruption due to possible toxicity of the formulations or drug delivery systems would provide inaccurate results regarding drug entry into cornea. Therefore, mixed nanomicelles were evaluated for their ocular biocompatibility. The outer surface of the corneal epithelium, being relatively impermeable, serves as a major barrier to foreign substances. Moreover, these cells are susceptible to trauma caused by xenobiotics. Hence, HCECs were chosen as an in vitro cell culture model to evaluate the cytotoxic effects of the nanomicellar formulation on cell viability and to determine the gene expression profiles of inflammatory cytokines and chemokines. HCECs are one of the most frequently investigated corneal cell culture model.50–52 Moreover, functional aspects and molecular expression of SMVT were studied with HCECs. These cells have been employed for studying the cellular accumulation of biotin-conjugated antiviral drugs.33
Cytotoxic effects of unloaded and prodrug-encapsulated nanomicelles were evaluated by (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Since eye drops are rapidly cleared from the surface of the eye,35,36,53 it was assumed that a 1-h incubation time would be sufficient to examine any toxic effects. All the micelle formulations did not affect cell viability after 1 h of incubation. Results from this assay clearly suggest that mixed nanomicellar formulation is safe and suitable for topical ocular application. A variety of inflammatory cytokines and chemokines are involved in ocular surface inflammation. Following exposure to a medication, proinflammatory cytokines and chemokines may be produced by corneal epithelial cells, which could promote a pathological inflammatory response, including induction of cellular infiltration and cytotoxicity in the cornea epithelium.54–58 Therefore, we studied mRNA expression levels of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ at the molecular level with qPCR. In this study, LPS was selected as an inflammation inducer because of its potential ability to induce a large number of inflammatory mediators. LPS is a major component of the outer membranes of gram-negative bacteria, and induces inflammation by stimulating host innate immune system responses. Induction of multiple proinflammatory cytokines by LPS is due to its ability to bind to intramembranal complex of CD-14 and Toll-like receptors.54,59–62 LPS caused significant upregulation of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ expression in HCECs. However, incubation of HCECs with blank and prodrug-encapsulated nanomicelles groups did not significantly alter the expression levels of IL-1β, IL-6, IL-8, IL-17, TNF-α, and IFN-γ. All the in vitro ocular biocompatibility tests revealed that nanomicelle formulations are biocompatible and suitable for topical ocular application.
Conclusion
In summary, a topical aqueous optically clear mixed nanomicellar formulation comprised of vitamin E TPGS and octoxynol-40 loaded with 0.1% biotinylated lipid prodrug of ACV was successfully developed for the treatment of herpetic keratitis. B-12HS-ACV-loaded nanomicelles are relatively small in size, spherical and homogenous, and devoid of aggregates. The nanomicelle formulations were perfectly transparent and comparable to water. Ocular biocompatibility studies indicated that mixed nanomicelles were nontoxic and noninflammatory to corneal epithelial cells. Therefore, nanomicellar technology represents a promising novel strategy for delivery of biotinylated lipid prodrugs of ACV to treat herpetic keratitis.
Acknowledgments
This study has been supported by NIH grants R01EY09171-16 and R01EY010659-14. We would like to thank Dr. Vladimir Dusevich, UMKC School of Dentistry, for helping with the operation of TEM. Also, the authors are thankful to Dr. Kun Cheng from Division of Pharmaceutical Sciences, UMKC School of Pharmacy for allowing us to use real-time PCR machine for our studies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mandal S., Thimmasetty M.K., Prabhushankar G., and Geetha M.Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int. J. Pharm. Investig. 2:78–82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anumolu S.S., Singh Y., Gao D., Stein S., and Sinko P.J.Design and evaluation of novel fast forming pilocarpine-loaded ocular hydrogels for sustained pharmacological response. J. Control. Release. 137:152–159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanjawade B.K., Manvi F.V., and Manjappa A.S.In situ-forming hydrogels for sustained ophthalmic drug delivery. J. Control. Release. 122:119–134, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Shen J., Wang Y., Ping Q., Xiao Y., and Huang X.Mucoadhesive effect of thiolated PEG stearate and its modified NLC for ocular drug delivery. J. Control. Release. 137:217–223, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Ludwig A.The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug. Deliv. Rev. 57:1595–1639, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Musumeci T., Bucolo C., Carbone C., Pignatello R., Drago F., and Puglisi G.Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int. J. Pharm. 440:135–140, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Lu Y., Zhong Y., Wang Q., Wu W., and Gao S.Ocular delivery of cyclosporine A based on glyceryl monooleate/poloxamer 407 liquid crystalline nanoparticles: preparation, characterization, in vitro corneal penetration and ocular irritation. J. Drug. Target. 20:856–863, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Diebold Y., Jarrin M., Saez V., Carvalho E.L., Orea M., Calonge M., Seijo B., and Alonso M.J.Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials. 28:1553–1564, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Khurana G., Arora S., and Pawar P.K.Ocular insert for sustained delivery of gatifloxacin sesquihydrate: preparation and evaluations. Int. J. Pharm. Investig. 2:70–77, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawar P.K., Katara R., and Majumdar D.K.Design and evaluation of moxifloxacin hydrochloride ocular inserts. Acta. Pharm. 62:93–104, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Peng C.C., Ben-Shlomo A., Mackay E.O., Plummer C.E., and Chauhan A.Drug delivery by contact lens in spontaneously glaucomatous dogs. Curr. Eye Res. 37:204–211, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Braga M.E., Yanez F., Alvarez-Lorenzo C., Concheiro A., Duarte C.M., Gil M.H., and de Sousa H.C.Improved drug loading/release capacities of commercial contact lenses obtained by supercritical fluid assisted molecular imprinting methods. J. Control. Release. 148:e102–e104, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Kapoor Y., Thomas J.C., Tan G., John V.T., and Chauhan A.Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials. 30:867–878, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Ali Y., and Lehmussaari K.Industrial perspective in ocular drug delivery. Adv. Drug. Deliv. Rev. 58:1258–1268, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gaudana R., Jwala J., Boddu S.H., and Mitra A.K.Recent perspectives in ocular drug delivery. Pharm. Res. 26:1197–1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao J.Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug. Deliv. Rev. 60:1663–1673, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Velagaleti P.R., Anglade E., Khan I.J., Gilger B.C., and Mitra A.K.Topical delivery of hydrophobic drugs using a novel mixed nanomicellar technology to treat diseases of the anterior & posterior segments of the eye. Drug. Deliv. Technol. 10:42–47, 2010 [Google Scholar]
- 18.Zografi G., ed. Interfacial phenomena. In: Gennaro A.R., ed. Remington: The Science and Practice Pharmacy. Easton, PA: Mark Publishing; 1995 [Google Scholar]
- 19.Hall D.G.Thermodynamics of micelle formation. In: Schick M.J., ed. Nonionic Surfactants: Physical Chemistry, Surfactant Science Series. New York, NY: Marcel Dekker; 1987; p. 233–296 [Google Scholar]
- 20.Zhang Z., Tan S., and Feng S.S.Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 33:4889–4906, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Wempe M.F., Wright C., Little J.L., Lightner J.W., Large S.E., Caflisch G.B., Buchanan C.M., Rice P.J., Wacher V.J., Ruble K.M., and Edgar K.J.Inhibiting efflux with novel non-ionic surfactants: Rational design based on vitamin E TPGS. Int. J. Pharm. 370:93–102, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Vadlapudi A.D., Vadlapatla R.K., Earla R., Sirimulla S., Bailey J.B., Pal D., and Mitra A.K.Novel biotinylated lipid prodrugs of acyclovir for the treatment of Herpetic Keratitis (HK): transporter recognition, tissue stability and antiviral activity. Pharm. Res. 30:2063–2076, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadlapudi A.D., Vadlapatla R.K., Kwatra D., Earla R., Samanta S.K., Pal D., and Mitra A.K.Targeted lipid based drug conjugates: a novel strategy for drug delivery. Int. J. Pharm. 434:315–324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai J.Y.Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 21:1899–1911, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Jones C.F., and Grainger D.W.In vitro assessments of nanomaterial toxicity. Adv. Drug. Deliv. Rev. 61:438–456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai J-Y, Li Y-T, and Wang T-P.In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different cross-linkers. Int. J. Mol. Sci. 11:5256–5272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena V., and Hussain M.D.Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int. J. Nanomedicine. 7:713–721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Z., Hao J., Yuan S., Li Y., Juan W., Sha X., and Fang X.Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int. J. Pharm. 376:176–185, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Xiao K., Luo J., Lee J., Pan S., and Lam K.S.A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release. 144:314–323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei L., Gohy J-F, Willet N., Zhang J-X, Varshney S., and Jérôme R.Morphology of core-shell-corona aqueous micelles: II. Addition of core-forming homopolymer. Polymer. 45:4375–4381, 2004 [Google Scholar]
- 31.Chen W., Chen H., Hu J., Yang W., and Wang C.Synthesis and characterization of polyion complex micelles between poly(ethylene glycol)-grafted poly(aspartic acid) and cetyltrimethyl ammonium bromide. Colloids. Surf A. 278:60–66, 2006 [Google Scholar]
- 32.Jiang Z., Zhu Z., Liu C., Hu Y., Wu W., and Jiang X.Non-enzymatic and enzymatic degradation of poly(ethylene glycol)-b-poly(ɛ-caprolactone) diblock copolymer micelles in aqueous solution. Polymer. 49:5513–5519, 2008 [Google Scholar]
- 33.Vadlapudi A.D., Vadlapatla R.K., Pal D., and Mitra A.K.Functional and Molecular Aspects of Biotin Uptake via SMVT in Human Corneal Epithelial (HCEC) and Retinal Pigment Epithelial (D407) Cells. AAPS. J. 14:832–842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vadlapatla R.K., Vadlapudi A.D., Kwatra D., Pal D., and Mitra A.K.Differential effect of P-gp and MRP2 on cellular translocation of gemifloxacin. Int. J. Pharm. 420:26–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang J.C.Ocular drug delivery conventional ocular formulations. Adv. Drug. Deliv. Rev. 16:39–43, 1995 [Google Scholar]
- 36.Järvinen K., Järvinen T., and Urtti A.Ocular absorption following topical delivery. Adv. Drug. Deliv. Rev. 16:3–19, 1995 [Google Scholar]
- 37.Gaudana R., Ananthula H.K., Parenky A., and Mitra A.K.Ocular drug delivery. AAPS. J. 12:348–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultana Y., Maurya D.P., Iqbal Z., and Aqil M.Nanotechnology in ocular delivery: current and future directions. Drugs Today (Barc). 47:441–455, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Sahoo S.K., Dilnawaz F., and Krishnakumar S.Nanotechnology in ocular drug delivery. Drug. Discov. Today. 13:144–151, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Trivedi R., and Kompella U.B.Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond). 5:485–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholkar K., Patel A., Vadlapudi A.D., and Mitra A.K.Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent. Patents. Nanomed. 2:82–95, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vadlapudi A.D., and Mitra A.K.Nanomicelles: an emerging platform for drug delivery to the eye. Ther. Deliv. 4:1–3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cholkar K., Patel S.P., Vadlapudi A.D., and Mitra A.K.Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharmacol. Ther. 29:106–123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan A.C., and Ying J.Y.Nanomaterials for in situ cell delivery and tissue regeneration. Adv. Drug. Deliv. Rev. 62:731–740, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Mitra A.K., Natesan S., and Velagaleti P.R.Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors. US20110300195 A1 2011 [Google Scholar]
- 46.Mitra A.K., Velagaleti P. R., and Grau U.M.Topical drug delivery systems for ophthalmic use. US20100310642 A1 2010 [Google Scholar]
- 47.Gao Y., Li L.B., and Zhai G.Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids. Surf. B. Biointerfaces. 64:194–199, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Butt A.M., Amin MCIM, Katas H., Sarisuta N., Witoonsaridsilp W., and Benjakul R.In vitro characterization of pluronic F127 and D—tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. J. Nanomater. 2012:11, 2012 [Google Scholar]
- 49.Lewinski N., Colvin V., and Drezek R.Cytotoxicity of nanoparticles. Small. 4:26–49, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Vellonen K.S., Mannermaa E., Turner H., Hakli M., Wolosin J.M., Tervo T., Honkakoski P., and Urtti A.Effluxing ABC transporters in human corneal epithelium. J. Pharm. Sci. 99:1087–1098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karla P.K., Quinn T.L., Herndon B.L., Thomas P., Pal D., and Mitra A.Expression of multidrug resistance associated protein 5 (MRP5) on cornea and its role in drug efflux. J. Ocul. Pharmacol. Ther. 25:121–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Araki-Sasaki K., Ohashi Y., Sasabe T., Hayashi K., Watanabe H., Tano Y., and Handa H.An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 36:614–621, 1995 [PubMed] [Google Scholar]
- 53.Di Tommaso C., Torriglia A., Furrer P., Behar-Cohen F., Gurny R., and Moller M.Ocular biocompatibility of novel Cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int. J. Pharm. 416:515–524, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Erdinest N., Shmueli O., Grossman Y., Ovadia H., and Solomon A.Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 53:4396–4406, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Wang C., Shi X., Chen X., Wu H., Zhang H., Xie J., Yang X., Gou Z., and Ye J.17-beta-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cells. Mol. Vis. 18:1115–1122, 2012 [PMC free article] [PubMed] [Google Scholar]
- 56.Gorbet M.B., Tanti N.C., Crockett B., Mansour L., and Jones L.Effect of contact lens material on cytotoxicity potential of multipurpose solutions using human corneal epithelial cells. Mol. Vis. 17:3458–3467, 2011 [PMC free article] [PubMed] [Google Scholar]
- 57.Offord E.A., Sharif N.A., Mace K., Tromvoukis Y., Spillare E.A., Avanti O., Howe W.E., and Pfeifer A.M.Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest. Ophthalmol. Vis. Sci. 40:1091–1101, 1999 [PubMed] [Google Scholar]
- 58.Sharif N.A., Wiernas T.K., Howe W.E., Griffin B.W., Offord E.A., and Pfeifer A.M.Human corneal epithelial cell functional responses to inflammatory agents and their antagonists. Invest. Ophthalmol. Vis. Sci. 39:2562–2571, 1998 [PubMed] [Google Scholar]
- 59.Fukuda K., Kumagai N., Yamamoto K., Fujitsu Y., Chikamoto N., and Nishida T.Potentiation of lipopolysaccharide-induced chemokine and adhesion molecule expression in corneal fibroblasts by soluble CD14 or LPS-binding protein. Invest. Ophthalmol. Vis. Sci. 46:3095–3101, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Kumagai N., Fukuda K., Fujitsu Y., Lu Y., Chikamoto N., and Nishida T.Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 46:114–120, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y., Joshi-Barve S., Barve S., and Chen L.H.Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J. Am. Coll. Nutr. 23:71–78, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Chow J.C., Young D.W., Golenbock D.T., Christ W.J., and Gusovsky F.Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689–10692, 1999 [DOI] [PubMed] [Google Scholar]


