Abstract
The incidence of premature ovarian failure (POF), also known as ovarian insufficiency, has been increasing in recent years. Although some treatments are currently available, improved treatment strategies are urgently required. Many researchers have reported that human endometrial stem cells (HuMenSCs), which exhibit stem/progenitor cell properties in vitro repaired damaged cells in vivo. Thus, we aimed to determine whether HuMenSCs can serve as cell therapy tools and be used for the treatment of POF. After treating with cyclophosphamide, on the first estrus period (we predicted mouse estrus cycle was generally 5 days), HuMenSCs were injected into a cyclophosphamide-induced mouse model of POF. The results revealed that the HuMenSCs could survive within POF mouse ovaries for at least 14 days in vivo; further, ovaries of the HuMenSCs-transplanted group expressed higher levels of ovarian markers [AMH, inhibin α/β, and follicle-stimulating hormone receptor (FSHR)], and the proliferative marker Ki67. In addition, the ovarian weight, plasma E2 level, and the number of normal follicles increased over time in the HuMenSC group compared with the control group. Further, microarray analysis of cDNA expression patterns revealed that, after HuMenSC transplantation, the gene mRNA expression patterns in the ovarian cells following stimulation of the host ovarian niche became increasingly similar to those observed in human ovarian tissue compared with the pretransplantation mRNA expression pattern in HuMenSCs. Hence, we can safely conclude that the mesenchymal stem cell properties and in vivo survival of HuMenSCs make them ideal seed cells for stem cell transplantation in the treatment of POF.
Introduction
Premature ovarian failure (POF) is a condition that causes amenorrhea and hypergonadotropic hypoestrogenism before the age of 40, and it affects 1% of women in the general population [1–5]. Patients with POF exhibit several typical characteristics [2,6–8]: (i) primary or secondary amenorrhea; (ii) at least intermittent hypoestrogenism; (iii) hypergonadotropinism; and (iv) age at the time of onset is below 40 years. In some patients with POF, laparoscopy reveals a lack of developing follicles and ovarian biopsy shows a network of connective tissue interspersed with fibroblasts. Previous studies have reported that the uterus and vaginal mucosa in patients with POF undergo atrophy due to lack of estrogen stimulation from inactive ovaries [4,9]. The incidence of POF has increased in recent years. Currently, POF cannot be reversed and although treatments are available, there is an urgent need for improved treatment strategies. Regenerative medicine research suggests that due to the self-renewal capacity and multiplex differentiation potential of stem cells, they could be used to treat various human diseases. Currently, Lee et al. have reported the impact of bone marrow transplantation on the generation of immature oocytes and were able to rescue long-term fertility in a preclinical mouse model of chemotherapy-induced POF [10]. At the same time, Ghadami et al. treated POF by using intraovarian injection of an adenoviral vector expressing human follicle-stimulating hormone receptor (FSHR) to restore folliculogenesis in FSHR(−/−) FSHR knockout (FORKO) mice [11]. Moreover, our previous study showed that after the CD44+/CD105+ human amniotic fluid cells (HuAFCs) were transplanted into the ovarian tissue of POF mice, these stem cells exhibited natural cell cycles and self-renewal in the ovarian tissues in the long term. Therefore, due to the mesenchymal stem cell properties and long-term survival conferred by CD44+/CD105+ HuAFCs, we found a novel way of treating POF by using CD44+/CD105+ HuAFCs as seed cells in vivo [12]. Furthermore, our results indicated that it is possible to use stem cells for the treatment of POF.
Human endometrial stem cells (HuMenSCs), which were isolated from menstrual blood, possess the adult stem cell-like characteristics of self-renewal, high proliferative potential in vitro, and the ability to differentiate toward diverse cell lineages in induction media [13]. These cells were directly harvested from the endometrium and first described by Gargett [14]. Thereafter, several research groups have expanded on the knowledge of HuMenSCs, which exhibit stem/progenitor cell properties in vitro and can also repair several types of damaged cells in vivo [13,15–18]. Studies by Meng et al. and Patel et al. revealed that HuMenSCs had high expression levels of mesenchymal stem cell surface markers, including CD29, CD44, CD49f, CD90, CD105, and CD117, and embryonic stem cell markers (Oct4 and SSEA3/4) [13,17]. On the other hand, other researchers have confirmed that HuMenSCs can be induced to differentiate into a variety of somatic cell types under special conditions, including adipocytes, osteoblasts, chondrocytes, neurons, endotheliocytes, pulmonary epithelial cells, hepatocytes, islet cells, cardiac myocytes, and insulin-producing cells [13,16–20]. Thus, a large body of evidence indicates the strong pluripotent characteristic of HuMenSCs [13,17,18]. HuMenSCs are more easily accessible than other adult stem cells, making them a potential donor source for stem cell therapy. Therefore, in this study we aimed to determine whether HuMenSCs can survive and proliferate in vivo, and investigated whether these cells can differentiate into ovarian-like cells (particularly ovarian granulosa cells) by microenvironment stimulation in POF mouse ovaries, and represent potential seed cells for stem cell transplantation to treat POF.
Materials and Methods
Cells cultured
The HuMenSCs and human fibroblasts (HuFBs) were kindly provided by Prof. Charlie Xiang (S-Evans Biosciences). Briefly, the HuMenSCs were isolated from five women's menstrual blood samples. All human materials were obtained according to consent regulation and approved by the Ethical Review Committee of the World Health Organization of Collaborating Center of Research in Human Production authorized by Hangzhou Municipal Government. The menstrual blood samples were layered over Ficoll solution (GE Healthcare Life Sciences) and centrifuged at 400 g for 15 min to pellet erythrocytes. The interface cells were harvested, washed thrice and finally resuspended at a concentration of 1×106 cells/mL in culture medium comprising DMEM: F12 (1:1) medium supplemented with 10% KnockOut™ Serum Replacement, 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, penicillin (25 U/mL)-streptomycin (925 mg/mL), and mixtured when the culture dishes were coated with 10 μg/μL fibronectin (Sigma-Aldrich). These cells were incubated in a humidified tissue culture incubator containing 5% CO2 at 37°C. When all cells were cultured on passage 10th, the ulterior experiments were made.
Flow cytometry analysis
All cells were suspended (1×104 cells/mL) and stained with the primary antibody (CD146, PDGFRβ, CD29, CD44, CD105, CD34, and CD45 FITC conjugate, BD Biosciences) on ice in Dulbecco's phosphate-buffered saline containing 10% BSA. Staining was compared with an isotype control antibody (mouse IgG1-FITC; BD Biosciences) to correct for nonspecific binding. Evaluation of antibody staining by flow cytometry (FCM) was performed using a FACS Aria (Quanta SC; Beckman Coulter, Inc.).
Western blotting analysis
Cells were lysed using a 2× loading lysis buffer (50 mM Tris–HCl, pH 6.8, 2% sodium dodecyl sulfate, 10% β-mercaptoethanol, 10% glycerol, and 0.002% bromophenol blue). The total amount of proteins from the cultured cells was subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Hybrid-polyvinylidene difluoride (PVDF) membranes (Millipore). After blocking with 5% (w/v) nonfat dried milk in Tris-buffered saline containing Tween-20 (TBST; 25 mM Tris/HCl, pH 8.0, 125 mM NaCl, and 0.05% Tween-20), the PVDF membranes were washed four times (15 min each) with TBST at room temperature and incubated with primary antibody (Table 1). Following extensive washing, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:1,000; Santa Cruz Technology) for 1 h. After washing four times (15 min each) with TBST at room temperature, the immunoreactivity was visualized by enhanced chemiluminescence using ECL kit from Perkin-Elmer Life Science.
Table 1.
Primary Antibodies List
| Antibodies | Companies | Applications |
|---|---|---|
| Rabbit anti-human Ki67 | Santa Cruz Technology | IF (1:200) |
| WB (1:1,000) | ||
| Rabbit anti-human AMH | Santa Cruz Technology | IF (1:200) |
| WB (1:1,000) | ||
| Rabbit anti-human FSHR | Santa Cruz Technology | IF (1:200) |
| WB (1:1,000) | ||
| Rabbit anti-human Inhibinα | Santa Cruz Technology | IF (1:200) |
| WB (1:1,000) | ||
| Rabbit anti-human Inhibinβ | Santa Cruz Technology | WB (1:1,000) |
| Rabbit anti-GAPDH | Cell Signaling Technology | WB (1:1,000) |
FSHR, follicle-stimulating hormone receptor; IF, immunofluorescence.
Immunofluorescence staining
Briefly, the cultured cells or tissue sections were washed thrice with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min. All ovary tissues were prepared and embedded in optimal cutting temperature compound (Sakura Finetek), and serial 10 μm sections were cut by cryostat. Moreover, the cells were incubated first with primary antibodies (Table 1) overnight at 4°C, and then with Cy3-conjugated goat anti-rabbit IgG antibody (1:200; Sigma-Aldrich) and 5 μg/mL DAPI (Sigma-Aldrich) at room temperature for 30 min after blocking. Then, the cells were thoroughly washed with TBST and viewed through a fluorescence microscope (DMI3000; Leica).
Hematoxylin-eosin staining
Briefly, all fresh ovary tissues were washed thrice with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min, dehydrated through a graded series of ethanol, vitrified in xylene, and embedded in paraffin. Next, serial 6 μm sections were cut and stained with hematoxylin-eosin.
ELISA assay
The mouse estradiol (E2) and follicle stimulating hormone (FSH) ELISA kit (Westang Bio) was used according to the protocol description to determine the level of E2 or FSH in mouse plasma. Briefly, 100 μL of mouse E2 or FSH at concentrations of 8,000, 4,000, 2,000, 1,000, 500, 250, and 125 pg/mL or 10, 5, 2.5, 1.25, 0.625, 0.312, and 0.156 ng/mL or diluted mouse plasma were added to anti-E2 or FSH antibody precoated microtest wells and incubated for 60 min. After 3 times of washing, the HRP-conjugated detection antibodies were added followed by substrate solution. The absorbance was determined at a wavelength of 450 nm.
The mouse model of POF and in vivo xenograft experiments
Hebetic femal C57BL/6 mice (n=80), between 4 and 5 weeks of age, were obtained from the Shanghai Tongji University with Institutional Animal Care and Use Committee approval in accordance with institutional guidelines. All mice were maintained for 14 days, three to four per cage, in a temperature-controlled colony room under standard light–dark cycle with free access to food and water. The study protocol was in accordance with the article [21]. To build the POF model, mice had single intraperitoneal injection of 70 mg/kg cyclophosphamide (Sigma-Aldrich) at 7 weeks of age. The animals were divided into four groups: a blank control group [20 animals of healthy wild-type (WT) mouse] nontreated cyclophosphamide and nongrafted with any cells in both ovaries, a negative control group (20 animals of POF model) grafted with HuFBs in both ovaries, an experimental group (20 animals of POF model) grafted with HuMenSCs in the both ovaries, a vehicle group (20 animals of POF model) did not graft any cells. One week after POF models were built, each experimental model received an injection of ∼10 μL of single-cell suspension, containing ∼1×104 cell spheres, which was harbored with a green fluorescence dye (DiOC18(3), 3, 3′-dioctadecyloxacarbocyanine perchlorate), or PBS. The above steps were in accordance with the previously described method [21]. The experiments of the animal models in both groups were conducted 21 days after the transplantation.
Isolation cells from the mouse POF ovaries by DiOC18(3)-positive cell sorting and cDNA microarray analysis
After mouse ovaries were collected and dissociated with 0.125% trypsin–EDTA solution, they were incubated at 10°C in PBS for 15 min and then washed twice. The DiOC18(3)-positive cells were isolated by FACS (FACSAria; BD Bioscience). Total RNAs of DiOC18(3)+ cells, HuMenSCs, and primary cells from human normal ovarian tissue (hOvary) were labeled using Agilent's Low RNA Input Fluorescent Linear Amplification kit. Cy3-dCTP or Cy5-dCTP was incorporated during reverse transcription of 5 μg total RNAs into cDNA. Different fluorescently labeled cDNA probes were mixed in 30 μL hybridization buffer (3×saline-sodium citrate (SSC), 0.2% SDS, 5×Denhardt's solution, and 25% formamide) and applied to the microarray (CapitalBio human mRNA microarray V2.0; CapitalBio) following incubation at 42°C for 16 h. After hybridization, the slide was washed with 0.2% SDS/2×SSC at 42°C for 5 min, and then washed with 0.2×SSC at room temperature for 5 min. The fluorescent images of the hybridized microarray were scanned with an Agilent Whole Human Genome 4×44 microarray scanner system. Images and quantitative data of the gene-expression levels were analyzed by Agilent's Feature Extraction software, version 9.5.
Statistical analysis
Each experiment was performed as least thrice, and data were shown as the mean±standard error where applicable; differences were evaluated with Student's t-test. A P value less than 0.05 was considered statistically significant.
Results
HuMenSCs express high levels of mesenchymal stem cell biomarkers
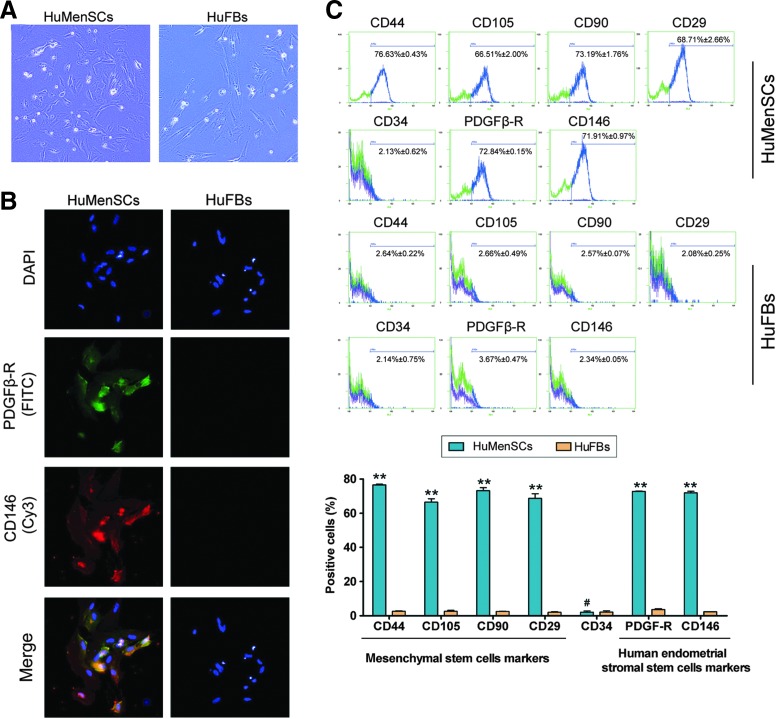
Microscopic analysis revealed no difference in the morphology between HuMenSCs and HuFBs. Then, to evaluate the degree of mesenchymal stem cell “stemness,” we analyzed the expression levels of mesenchymal stem cell biomarkers using FCM (Fig. 1). Expression levels of the “stemness” markers CD29, CD44, CD90, and CD105 were ∼30-fold higher in the HuMenSCs than in the HuFBs. Furthermore, the HuMenSCs showed high expression levels of the endometrial cell markers CD146 and PDGFRβ. In addition, immunofluorescence (IF) staining revealed similar results as the FCM, suggesting that HuMenSCs possess more “stemness” than HuFBs.
FIG. 1.
Morphology and biomarkers determined of HuMenSCs. (A) Primary HuMenSCs and HuFBs have similar phenotypes. Original magnification, 200×. (B) Results of the immunofluorescence staining show that HuMenSCs but not HuFBs expressed CD146 and PDGFβ-R. Original magnification, 200×. (C) Flow cytometric analysis of human mesenchymal stem cell marker expression in HuMenSCs and HuFBs in vitro. Expression of the “stemness” markers was higher in HuMenSCs than in HuFBs. **P<0.01 versus HuFBs; #P>0.05 versus HuFBs; n=3. HuFBs, human fibroblasts; HuMenSCs, human endometrial stem cells. Color images available online at www.liebertpub.com/scd
Characteristics of a mouse model of chemotherapy-induced POF
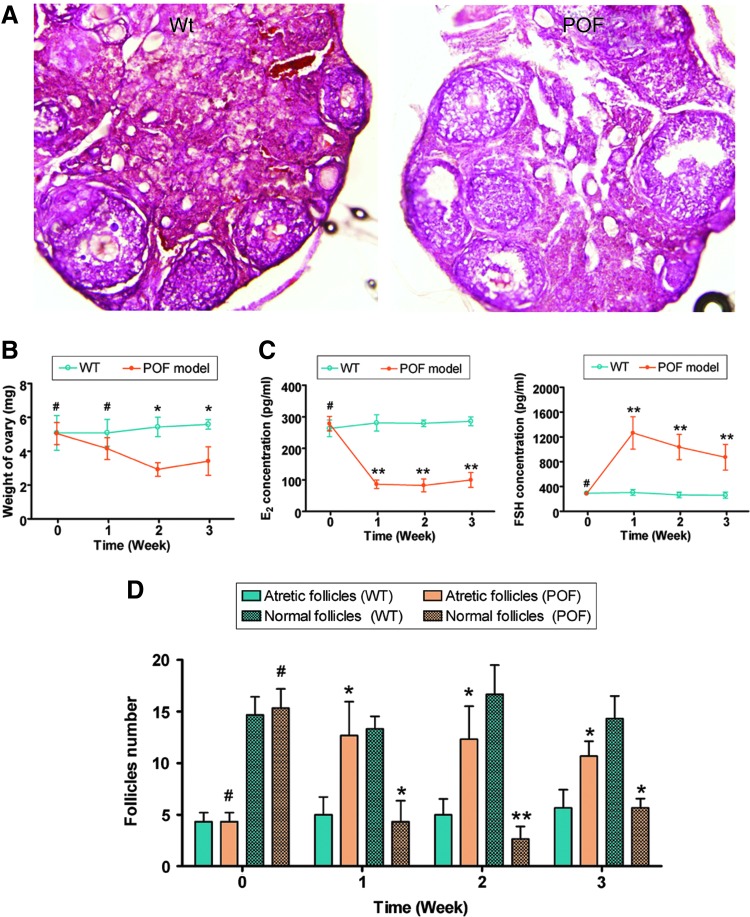
We first established a mouse model of POF. Seven days after the injection of chemotherapy drugs, the ovaries of the POF mice and the normal healthy mice were collected for pathological analysis. Mouse plasma samples were collected to test the E2 and FSH levels in different groups. Pathological results revealed that the ovaries of normal healthy mice contained a large number of follicles in all stages ranging from immature to mature (Fig. 2). In contrast, the atrophied ovaries of the POF model mice primarily consisted of interstitial cells in a fibrous matrix, with a reduced number of follicles of each stage. Additionally, ovaries of the POF mice contained an increased number of collapsed oocytes, and the size of their ovaries was smaller than those of normal healthy mice (Fig. 2). Furthermore, The weight of the ovaries in the POF model mice (1 week: 4.157±0.643 mg; 2 week: 2.923±0.401 mg; 3 week: 3.417±0.834 mg) was significantly lower than that in normal healthy mice (1 week: 5.093±0.799 mg; 2 week: 5.433±0.571 mg; 3 week: 5.587±0.287 mg; P<0.05). (Fig. 2). The plasma E2 and FSH levels and ovarian pathology were investigated. After injection with cyclophosphamide, the plasma E2 levels decreased over time in the POF group (1 week: 86.3967±13.323 pg/mL; 2 week: 82.4967±20.362 pg/mL; 3 week: 99.7668±23.653 pg/mL) but not in the control group (1 week: 280.603±25.983 pg/mL; 2 week: 279.287±10.234 pg/mL; 3 week: 285.940±14.025 pg/mL). Meanwhile, the plasma FSH levels increased over time in the POF group (1 week: 86.397±13.323 pg/mL; 2 week: 1264.557±260.913 pg/mL; 3 week: 1264.557±260.913 pg/mL) but not in the control group (1 week: 304.840±47.981 pg/mL; 2 week: 265.657±44.305 pg/mL; 3 week: 260.500±51.046 pg/mL). Results of the ELISA assays revealed that mice in the POF group lacked hormonal maintenance from the ovaries (Fig. 2). We counted the number of follicles at every stage (atretic or normal follicles) in each group. The results showed a significantly higher number of atretic follicles in the ovaries of the POF group than in the WT group. There were fewer normal follicles in the POF group than in the WT group.
FIG. 2.
Establishment of a mouse model of premature ovarian failure (POF). (A) Ovarian pathology of the wild-type (WT) group and POF group at 2 weeks after injection of cyclophosphamide. The ovaries of the WT group contained a large number of follicles at all developmental stages; whereas the atrophied ovaries of the POF mice were predominantly composed of interstitial cells in a fibrous matrix with a reduced number of follicles at each stage and an increased number of collapsed oocytes. Original magnification, 100×. (B) The weight of the ovaries in the POF model mice was significantly lower than that in normal healthy mice. *P<0.05 versus WT; #P>0.05 versus WT; n=3. (C) Plasma E2 and FSH levels as determined by ELISA at various time points after the injection of cyclophosphamide; **P<0.01 versus WT; #P>0.05 versus WT; n=3. (D) Follicle count revealed that there were significantly more atretic follicles in ovaries of the POF mice than in ovaries of the WT mice, but fewer normal follicles in the POF mice than in the WT mice. **P<0.01 versus WT; *P<0.05 versus WT; #P>0.05 versus WT; n=3. FSH, follicle stimulating hormone. Color images available online at www.liebertpub.com/scd
HuMenSCs survive and expressed ovarian granulosa cell-specific proteins in the ovaries of POF mice
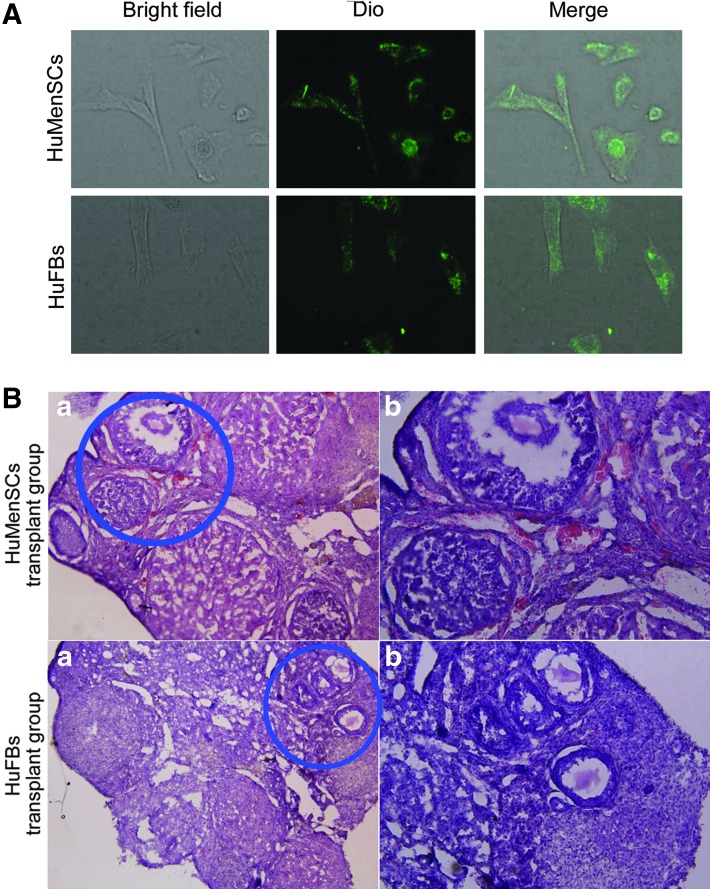
To investigate the effect of transplanting HuMenSCs, DiO (green fluorescence)-labeled HuMenSCs or HuFBs (negative control) were injected into the ovaries of POF model mice (Fig. 3). The mice were then examined after 14 days to confirm the presence of transplanted cells. First, hematoxylin and eosin-stained ovarian tissues showed that the number of atretic follicles in the HuMenSC transplantation group was significantly reduced but the number of mature follicles was increased (Fig. 3). In contrast, in the HuFBs transplanted group, ovarian tissues were found to contain a larger number of atretic follicles. We then determined the survival of transplanted cells in the ovaries of POF mice. The IF assay revealed that the DiO-positive cells were elevated in the ovaries of mice transplanted with HuMenSCs, whereas the HuFB transplant group had very few DiO-positive cells (Fig. 4). As anticipated, DiO- (green fluorescence)-positive HuMenSCs were observed along the injection tract after 14 days in the ovaries of POF mice, indicating that HuMenSCs could survive transplantation within the POF mouse ovaries for at least 14 days in vivo, and morphological changes in the ovaries could be seen thereafter. However, DiO-positive HuFB cells could not be detected along the injection tract in the ovaries of POF mice, confirming that only grafted HuMenSCs were capable of in vivo survival after implantation.
FIG. 3.
Fluorescent dye of labeled cells transplanted into ovaries of POF mice. (A) Each group of cells was labeled with the green fluorescent dye DiO in vitro. (B) Hematoxylin and eosin staining of ovaries in each transplant group in POF mice at 2 weeks after injection of HuMenSCs or HuFBs. Follicular atresia and ovarian fibrosis were significantly improved in the HuMenSC group but not in the HuFB group. (a) Original magnification, 100×. (b) Area encircled in blue is depicted at high magnification. Original magnification, 200×. Color images available online at www.liebertpub.com/scd
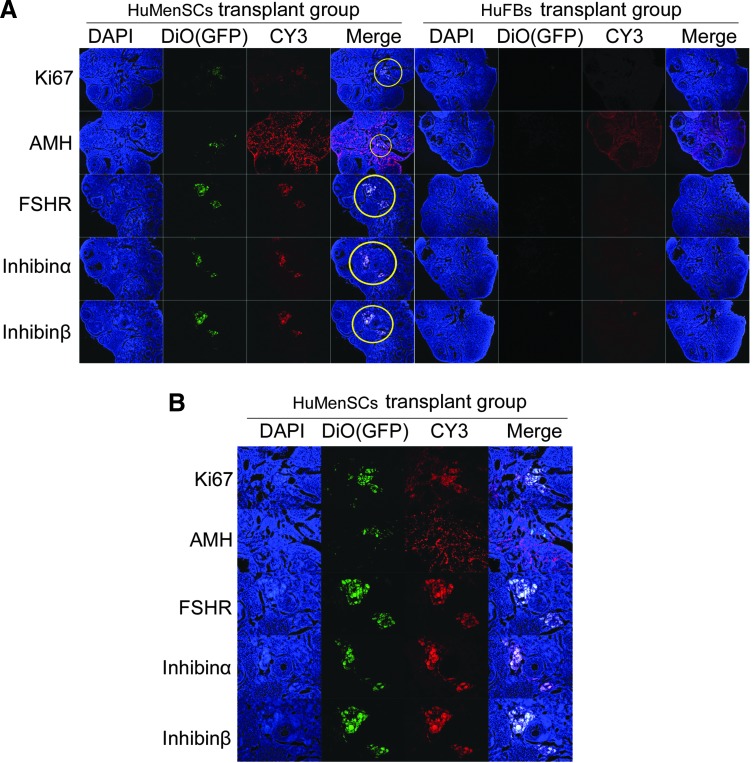
FIG. 4.
Expression of ovarian granulosa cell-specific biomarkers after cell transplantation. (A) Survival of HuMenSCs in the ovary of a POF mouse model and changes in expression of specific ovarian granulosa cell biomarkers. Immunofluorescence staining analysis demonstrated that DiO (green fluorescence), which was used as a marker of transplanted cells, was expressed at a higher level in HuMenSCs transplanted into the mouse ovaries. Ki67 (red fluorescence), a cell proliferation factor, was also expressed at a higher level in the transplanted HuMenSCs. The transplanted cells survived for at least 2 weeks in vivo. However, DiO and Ki67 signals were not found in the HuFB group. Thus, only HuMenSCs could be detected along the injection tract in POF mouse ovaries. In addition, AMH, FSHR, inhibin α, and inhibin β protein levels were elevated in the ovarian tissues of mice transplanted with HuMenSCs compared with those in the ovarian tissues of mice transplanted with HuFBs. Original magnification, 100×. (B) Area encircled in yellow is depicted at a high magnification. Original magnification, 200×. FSHR, follicle-stimulating hormone receptor. Color images available online at www.liebertpub.com/scd
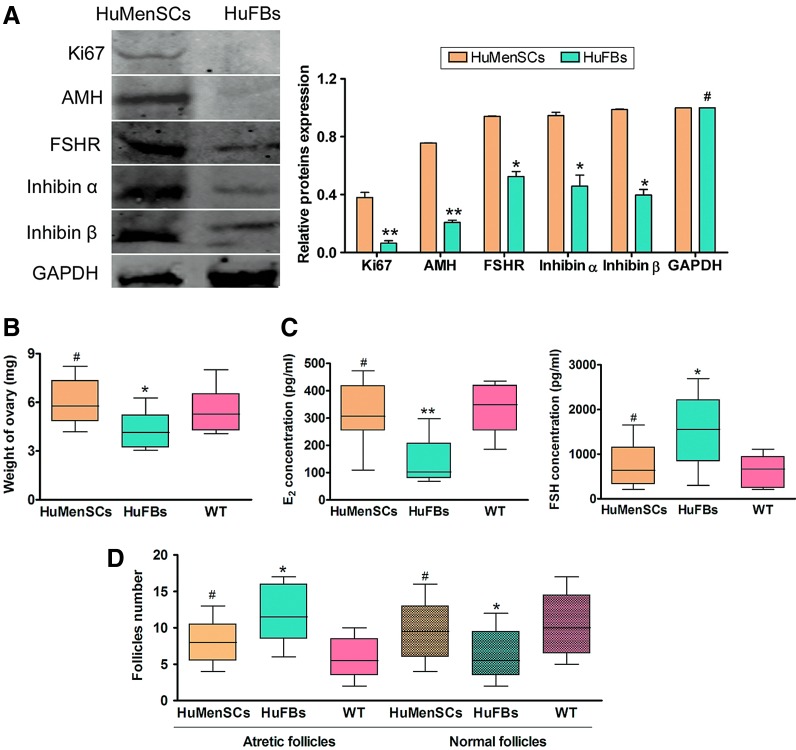
On other hand, western blotting and IF staining were used to investigate the levels of ovarian granulosa cell-specific proteins (inhibin α, inhibin β, AMH, and FSHR) and the cellular proliferation marker Ki67 between the two transplant groups after 14 days of transplantation. IF staining revealed that the inhibin α, inhibin β, AMH, FSHR, and Ki67 expression levels were elevated in the ovarian tissues of the HuMenSC group compared with those of the HuFB group (Fig. 4). Similarly, western blotting revealed that inhibin α, Inhibin β, AMH, FSHR, and Ki67 were significantly higher in the HuMenSC transplant group than in the HuFB group (5). These results indicated that HuMenSCs differentiated into ovarian granulosa-like cells in the ovaries of POF mice following stimulation of the ovarian niche.
Transplanted HuMenSCs improve ovarian weight and hormone secretion in POF mice
At 14 days after transplantation, the weight of ovaries in each group was determined. There was almost no statistically significant difference of the ovarian weight between HuMenSC-transplanted mice (5.968±0.419 mg) and in the WT group (5.507±0.394 mg; P>0.05) at 14 days after transplantation. Then, the ovarian weight in the HuFBs group (4.088±0.328) was obviously decreased compared with that in the WT group (P>0.05) (Fig. 5). Meanwhile, the ELISA revealed there was no statistically significant difference in the plasma E2 and FSH levels between the POF mice transplanted with HuMenSCs (E2: 322.904±33.895 pg/mL; FSH: 732.051±145.482 pg/mL) and the WT group (E2: 333.435±27.729 pg/mL; FSH: 621.836±108.427 pg/mL; P>0.05). However, the plasma E2 level decreased with time in the HuFBs groups (135.666±24.974 pg/mL) compared with the WT groups (P>0.01) (Fig. 5), whereas the plasma FSH level increased with time in the HuFBs group (1544.813±242.789 pg/mL) compared with the WT group (P>0.05) (Fig. 5). We counted the number of follicles in each groups; there was almost no statistically significant difference in both normal follicles and atretic follicles between the HuMenSC-transplanted POF mice and the WT group (Table 2). But, the HuFBs group had a higher number of atretic follicles than the HuFBs group (P>0.05) (Fig. 5).
FIG. 5.
Detection of specific biomarkers and hormone levels as well as ovarian weight change after cell transplantation. (A) Western blotting showed that the Ki67, AMH, FSHR, inhibin α, and inhibin β protein expression levels in the ovarian tissue of the HuMenSCs group were significantly higher than the corresponding levels in the HuFBs group. **P<0.01 versus HuFBs group; *P<0.05 versus HuFBs group; #P>0.05 versus HuFBs group; n=15). (B) Detection of ovarian weight after cell transplantation. There was no statistically significant difference in ovarian weight between the HuMenSCs-transplanted mice and the WT group at 14 days after transplantation. But, the ovarian weight in HuFBs-transplanted mice significantly decreased compared with that in the WT group at 14 days after transplantation. *P<0.05 versus WT; #P>0.05 versus WT group; n=15. (C) Detection of hormone levels after cell transplantation. There was no statistically significant difference in the plasma E2 and FSH levels between the POF mice transplanted with HuMenSCs and the WT group. Then, the plasma E2 level decreased and FSH level increased over time in the POF mice transplanted with HuMenSCs compared with the HuFBs group. **P<0.01 versus WT; *P<0.05 versus WT; #P>0.05 versus WT group; n=15. (D) Detection of follicle numbers after cell transplantation. There was no statistically significant difference in both normal follicles and atretic follicles between the HuMenSCs-transplanted POF mice and the WT group. *P<0.05 versus WT; #P>0.05 versus WT group; n=15. Color images available online at www.liebertpub.com/scd
Table 2.
Detection of Follicle Numbers After Cell Transplantation
| WT group | HuMenSCs transplant | HuFBs transplant | |
|---|---|---|---|
| (n=15) | (n=15) | (n=15) | |
| Atretic follicles | 5±2 | 7±1 | 12±1 |
| Normal follicles | 10±1 | 10±2 | 6±2 |
HuFBs, human fibroblasts; HuMenSCs, human endometrial stem cells; WT, wild-type.
Microarray analysis of cDNA expression patterns in HuMenSCs before transplantation in POF mouse ovaries versus normal human ovarian tissue
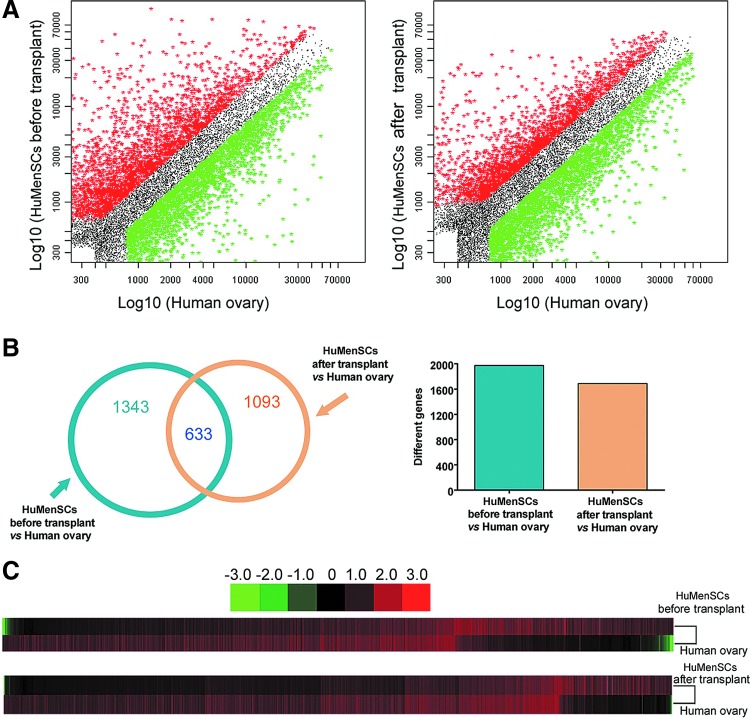
To determine whether HuMenSCs were induced to differentiate into human ovarian cells following stimulation in the ovarian tissue of POF mice after transplantation, we prepared a CapitalBio human mRNA microarray V2.0 containing 35,000 oligonucleotide probes complementary to known mammalian gene cDNAs. A FCM sorting system was used to isolate and enrich a specific subpopulation (DiO+) of the HuMenSCs after transplantation (tHuMenSCs) in the host ovaries of POF mice in 14 days, namely, the tHuMenSCs. Next, the gene mRNA expression pattern of tHuMenSCs, HuMenSCs before transplantation (bHuMenSCs), and human normal ovarian tissue (hOvary) were compared. Significance analysis of microarray data and a fold change criterion (tHuMenSCs/hOvary ratio and bHuMenSCs/hOvary ratio) of >5.0 or <0.5 and a Q value of <0.01 were used to identify significant differences. On the basis of these criteria, we identified 1,343 gene mRNAs that were differentially expressed in the bHuMenSCs and hOvary and 1,093 gene mRNAs that were differentially expressed in the tHuMenSCs and hOvary (Fig. 6). The Venn diagram (Fig. 6C) depicts the correlation between the mRNA microarray results of tHuMenSCs:hOvary and bHuMenSCs:hOvary. The differential expression of 710 gene mRNAs in bHuMenSCs and 460 gene mRNAs in tHuMenSCs were confirmed (indicated by overlaps in Fig. 6C). The two groups (tHuMenSCs: hOvary and bHuMenSCs:hOvary) shared 633 differentially expressed genes (Fig. 6). Interestingly, the scatter plot of microarray results revealed a certain number of mRNA expressions that differed between the tHuMenSCs and hOvary and bHuMenSCs and hOvary groups, and the number of differentially expressed mRNAs was significantly lower in tHuMenSCs and hOvary group than in the bHuMenSCs and hOvary group (Fig. 6). These results indicate that the gene mRNA expression patterns in the tHuMenSCs showed increasing similarities to the expression patterns in human ovarian tissues compared with the bHuMenSCs.
FIG. 6.
Microarray analysis of cDNA expression patterns in HuMenSCs before transplantation in ovaries of POF mice versus normal human ovarian tissue. (A) Scatter plot analysis indicated mRNA expression differed between HuMenSCs after transplant (tHuMenSCs) versus normal human ovarian cells (hOvary) and the HuMenSCs before transplant (bHuMenSCs) versus hOvary groups. There were significantly fewer differentially expressed gene mRNAs in the tHuMenSCs versus hOvary group than in the bHuMenSCs versus hOvary group. (B) A Venn diagram was used to depict the correlation between the different results of the tHuMenSCs:hOvary and bHuMenSCs:hOvary groups tested by gene mRNA microarray. (C) Cluster analysis of differentially expressed gene mRNAs. Color images available online at www.liebertpub.com/scd
Discussion
The ability to isolate human embryonic stem cells (HuESCs) has facilitated the treatment of human genetic and degenerative diseases through the application of pluripotent cell-based tissue engineering and regenerative medicine. However, there are insurmountable difficulties in terms of the ethics and safety of the use of HuESCs, which has rendered its clinical applications limited. To date, human mesenchymal stem cell (HuMSC) transplantation for the treatment of irreversible genetic defects and diseases is widely used in transferred and regenerative medicine. Moreover, HuMSC transplantation into specific tissues and organs has been reported to produce a significant efficacy in the treatment of certain genetic diseases and acute injuries such as muscular dystrophies, diabetes, heart failure, and spinal cord injury. HuMSCs can overcome the ethical problems associated with the use of HuESCs and reduce the possibility of immune rejection; therefore, if solutions become available for the source limitations HuMSCs may become an attractive source for cell therapy. Many studies have indicated that when stem cells were transplanted into a specific microenvironment they are stimulated by the niche, and while the release of cell growth factors stimulate the surrounding tissue regeneration, the cells may also be induced to differentiate into specific tissue or organ-like cells. However, stem cell transplantation in the treatment of preconditions must be safe and effective. Stem cells transplanted into the host could not only induce immune rejection by the host but also result in cancerous tissue or distortions in the host body. Thus far, little research has investigated stem cell transplantation for the repair of damaged ovaries to treat POF. In this study, we transplanted a novel and special source of HuMSCs, HuMenSCs derived from the endometrium, into the ovaries of a POF mice model to evaluate their effect on ovarian repair.
The method described in this study has a number of advantages over current approaches.
First, the method of isolation and enrichment of HuMenSCs is very convenient. Previous studies have demonstrated that female menstrual blood contains a large number of MenSCs [13,16] and that HuMenSCs are excreted in each menstrual cycle along with blood cells [13,16]. Xiang and colleagues and Meng et al. established a method to successfully isolate and enrich a large number of active HuMenSCs from the menstrual blood [13,16]. Furthermore, our findings indicate that HuMenSCs derived from the endometrium express high levels of mesenchymal stem cell biomarkers, suggesting that these cells possess the “stemness” of mesenchymal stem cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). The source and the method of enrichment and isolation of these cells are very simple, and we were able to isolate and enrich a large number of living HuMenSCs with stem cell characteristics by using a simple method. This solves the problem of insufficient seed cells and collection difficulties.
Second, HuMenSCs are similar to HuMSCs derived from the other sources. They possess the basic characteristics of mesenchymal stem cells, which have the ability to differentiate into a variety of somatic cells and possess proliferation capabilities. The only difference is that HuMenSCs are derived from menstrual blood of premenopausal women. This source determines that the transplantation characteristics of these cells are superior to those of other HuMSCs. Although POF patients exhibit loss of ovarian dysfunction and mature oocytes and the ability to conceive, HuMenSCs derived from the endometrium are potentially suitable seed cells of transplantation in the treatment of POF. Many types of stem cells such as HuESCs, iPS cells, and HuMSCs exist and can serve as seed cells for cell transplantation for the treatment of nonreversible disease. However, because of the source of HuESCs, there are potential obstacles such as ethical limitations and tumorigenicity in vivo. Furthermore, as iPS cells contain some factors involved in transgenic mutations and random integration of lentiviruses, many concerns have arisen regarding their safety and therefore, these cells are not currently suitable for use as seed cells in therapy. In addition, although other sources of HuMSCs are relatively safe, they have been shown to exhibit immunogenicity after long-term culture in vitro; therefore, they are associated with potential autoimmune rejection following transplantation. HuMenSCs derived from menstruation are safe and practical for use as transplantation seed cells to treat POF, and they are considerably advantageous compared with other stem cells. On the other hand, it would be collected HuMenSCs through the biopsy of endometrium for women.
Third, the results of our research reveal that the ovarian microenvironment (niche) of POF mice stimulated and induced differentiation in HuMenSCs. Although studies are increasingly concerned with the effect of induction of differentiation in stem cells by niche (in vitro/in vivo), the differentiation of HuMenSCs induced by POF ovaries has not yet been studied. Previous studies revealed that HuMenSCs could be induced to differentiate into multiple cell lineages (such as adipocytes, osteoblasts, chondrocytes, neurons, endotheliocytes, pulmonary epithelial cells, hepatocytes, islet cells, cardiac myocytes, and insulin-producing cells) in a particular niche [13,16–20]. Thus, HuMenSCs had a great potential to become a donor cell source for regenerative medicine. Our study found that even in ovarian tissue of the POF mouse model, HuMenSCs could be induced to differentiate into ovarian tissue-like cells, especially into ovarian granulosa-like cells. The results of the cDNA microarray assay revealed stark differences in the gene expression pattern between HuMenSCs before transplantation and human ovarian tissues. HuMenSCs cells before induction rarely expressed high levels of the genes found in human ovarian tissue; however, HuMenSCs after transplantation survived in the POF ovaries for several days and showed gene expression profiles similar to those observed in human ovarian tissue. These results strongly illustrated that niche-stimulated HuMenSCs in POF mouse ovary were induced to differentiate into ovarian tissue-like cells. Moreover, the study results confirmed that HuMenSCs could be induced to differentiate into ovarian tissue-like cells in vivo; further, our results also suggest that HuMenSCs can potentially repair ovarian failure caused by chemotherapy and delay the progress of POF.
In conclusion, HuMenSCs are advantageous over mesenchymal cells as they are derived from menstrual blood, and we found that they could be stimulated to differentiate into ovarian tissue-like cells in an ovarian microenvironment in POF ovarian tissue. These stimulated cells demonstrated the ability to achieve further restoration of ovarian damage in POF. Therefore, HuMenSCs could be used as seed cells for the treatment of POF.
Supplementary Material
Acknowledgments
This work was supported by grant from National Natural Science Foundation of China (no. 81202811) and Shanghai Municipal Health Bureau Fund (no. 20124320) to Te Liu.
Author Disclosure Statement
We declare no potential conflicts of interest.
References
- 1.Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Goswami SK, Chakravarty BN. and Kabir SN. (2003). Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum Reprod 18:2031–2038 [DOI] [PubMed] [Google Scholar]
- 2.Beck-Peccoz P. and Persani L. (2006). Premature ovarian failure. Orphanet J Rare Dis 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persani L, Rossetti R. and Cacciatore C. (2010). Genes involved in human premature ovarian failure. J Mol Endocrinol 45:257–279 [DOI] [PubMed] [Google Scholar]
- 4.McGuire MM, Bowden W, Engel NJ, Ahn HW, Kovanci E. and Rajkovic A. (2011). Genomic analysis using high-resolution single-nucleotide polymorphism arrays reveals novel microdeletions associated with premature ovarian failure. Fertil Steril 95:1595–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vujovic S, Ivovic M, Tancic-Gajic M, Marina L, Barac M, Arizanovic Z, Nenezic A, Ivanisevic M, Micic J, Sajic S. and Micic D. (2012). Premature ovarian failure. Srp Arh Celok Lek 140:806–811 [PubMed] [Google Scholar]
- 6.Qin Y, Sun M, You L, Wei D, Sun J, Liang X, Zhang B, Jiang H, Xu J. and Chen ZJ. (2012). ESR1, HK3 and BRSK1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebar RW. (2008). Premature ovarian “failure”. in the adolescent. Ann N Y Acad Sci 1135:138–145 [DOI] [PubMed] [Google Scholar]
- 8.Kalantari H, Madani T, Zari Moradi S, Mansouri Z, Almadani N, Gourabi H. and Mohseni Meybodi A. (2013). Cytogenetic analysis of 179 Iranian women with premature ovarian failure. Gynecol Endocrinol 29:588–591 [DOI] [PubMed] [Google Scholar]
- 9.Duncan M, Cummings L. and Chada K. (1993). Germ cell deficient (gcd) mouse as a model of premature ovarian failure. Biol Reprod 49:221–227 [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM. and Tilly JL. (2007). Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol 25:3198–3204 [DOI] [PubMed] [Google Scholar]
- 11.Ghadami M, El-Demerdash E, Salama SA, Binhazim AA, Archibong AE, Chen X, Ballard BR, Sairam MR. and Al-Hendy A. (2010). Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(−/−) FORKO mice. Mol Hum Reprod 16:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Xu F, Du X, Lai D, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W. and Liu Z. (2010). Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem 340:265–273 [DOI] [PubMed] [Google Scholar]
- 13.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, et al. (2007). Endometrial regenerative cells: a novel stem cell population. J Transl Med 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gargett CE. (2004). Stem cells in gynaecology. Aust N Z J Obstet Gynaecol 44:380–386 [DOI] [PubMed] [Google Scholar]
- 15.Santamaria X, Massasa EE, Feng Y, Wolff E. and Taylor HS. (2011). Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther 19:2065–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Xiang D, Zhang JL, Allickson J. and Xiang C. (2011). Plasticity of human menstrual blood stem cells derived from the endometrium. J Zhejiang Univ Sci B 12:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr., Kormos R. and Benetti F. (2005). Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg 130:1631–1638 [DOI] [PubMed] [Google Scholar]
- 18.Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, et al. (2008). Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 26:1695–1704 [DOI] [PubMed] [Google Scholar]
- 19.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ. and Allickson JG. (2008). Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 17:303–311 [DOI] [PubMed] [Google Scholar]
- 20.Li HY, Chen YJ, Chen SJ, Kao CL, Tseng LM, Lo WL, Chang CM, Yang DM, Ku HH, et al. (2010). Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther 335:817–829 [DOI] [PubMed] [Google Scholar]
- 21.Liu T, Huang Y, Guo L, Cheng W. and Zou G. (2012). CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. Int J Med Sci 9:592–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.