Abstract
Early work on platelet and erythrocyte vesicles interpreted the phenomena as a discard of material from cells. Subsequently, vesicles were studied as possible vaccines and, most recently, there has been a focus on the effects of vesicles on cell fate. Recent studies have indicated that extracellular vesicles, previously referred to as microvesicles or exosomes, have the capacity to change the phenotype of neighboring cells. Extensive work has shown that vesicles derived from either the lung or liver can enter bone marrow cells (this is a prerequisite) and alter their fate toward that of the originating liver and lung tissue. Lung vesicles interacted with bone marrow cells result in the bone marrow cells expressing surfactants A–D, Clara cell protein, and aquaporin-5 mRNA. In a similar vein, liver-derived vesicles induce albumin mRNA in target marrow cells. The vesicles contain protein, mRNA, microRNA, and noncoding RNA and variably some DNA. This genetic package is delivered to cells and alters the phenotype. Further studies have shown that initially the altered phenotype is due to the transfer of mRNA and a transcriptional modulator, but long-term epigenetic changes are induced through transfer of a transcriptional factor, and the mRNA is rapidly degraded in the cell. Studies on the capacity of vesicles to restore injured tissue have been quite informative. Mesenchymal stem cell-derived vesicles are able to reverse the injury to the damaged liver and kidney. Other studies have shown that mesenchymal stem cell-derived vesicles can reverse radiation toxicity of bone marrow stem cells. Extracellular vesicles offer an intriguing strategy for treating a number of diseases characterized by tissue injury.
Extracellular Vesicles: Basic Considerations
Early reports of membrane enclosed vesicles originating from platelets and red blood cells [1,2] were initially interpreted as these cells emitting their cellular debris. Subsequently, there has been extensive work in characterizing cells from a wide variety of tissues/cells and clinical interest in utilizing vesicles as vaccines for cancer therapy [3]. Studies on the cellular origins of vesicles have made great progress resulting in the awarding of Nobel prizes to Drs. James E. Rothman, Randy W. Schekman, and Thomas C. Sudhof “for their discoveries of machinery regulating vesicle traffic, a major transport system in our cells.” Other work has proceeded on the interactions of vesicles with different, normal, or neoplastic tissues and cells. The nature of such extracellular vesicles has been hotly debated. Various terms have been used to describe them, but most commonly, exosomes and microvesicles. It has been argued that exosomes derive from multivesicular bodies (MVBs), an endosomal intermediate compartment directing endocytosed cargo for lysosomal degradation [4]. The MVBs are also capable of fusing to the plasma membrane and releasing vesicles. These exosomes were defined as lipid bilayer enclosed vesicles with a diameter of ∼50–100 nm. Microvesicles, on the other hand, were felt to be formed directly by blebbing off the plasma membrane; these ranged in size from 100 to 1,000 nm [5]. There was, however, a good deal of overlap between these entities and extensive recent work had indicated that the nature of vesicles varies with the cell of origin and the condition of the cell of origin. Other factors such as gender, age, circadian rhythms, fasting state, medication exposure, and physical activity also may influence the number and nature of vesicles under different conditions [6–11]. Vesicles have been studied most extensively in plasma and serum [12], but have also been isolated from pleural effusions [13], ocular effluent and aqueous humor [14], breast milk [15], ascites [16], amniotic fluid [17], semen [18], saliva [19], cerebrospinal fluid [20], and urine [21]. In addition, vesicles derive from virtually all tissues in culture. Exosomes have been characterized by the presence of membrane proteins such as tetraspanins (CD63, CD81, CD9, and CD82), MHC molecules, cytosolic proteins such as stress proteins, TSG101, Alix, cytoskeletal proteins (actin, tubulin), and milk-fat globule (MFG)-E8 (or lactadherin). However, these proteins are not present on all exosomes, their presence varies with source and condition of exosomes and they may be expressed on vesicular entities in the microvesicle size range. At the initial meeting of The International Society of Extracellular Vesicles in Gothenburg, Sweden (April, 2012), there was much discussion about the naming of vesicles and the distinction between exosomes and microvesicles (MV). Given the overlapping characteristics and the wide differences in characteristics depending upon the source of vesicles along with many other variables, it was finally decided that, not quite by consensus, we should encompass exosomes and microvesicles under the inclusive term, extracellular vesicles. A critical consideration was the appropriate approach to isolate such vesicles for study. A number of methods have been used, but most are using variants of differential ultracentrifugation with or without density gradient separations [22]. Other approaches have included size exclusion, immunoaffinity, and microfluidics [23–25]. Our approaches have focused on the biologic impact of vesicle exposure, and thus, we considered it important to use an inclusive approach and selected differential ultracentrifugation as described by Thery et al. [22] The other approaches have their advocates and may be particularly suited for specific experimental approaches, but are selective in nature each excluding different populations of vesicles. Having selected differential centrifugation where specimens are depleted of cells by a low-speed centrifugation (300 g) and then sequentially subjected to a 10,000 g spin and the supernatant from that spin to a 100,000 g spin, with the final pellet representing the exosome population, we belatedly realized that we were excluding the larger microvesicle population present in the 10,000 g pellet and are now repeating studies looking at the whole population (330–100,000 g), the exosome population (300–10,000–100,000 g) and the larger microvesicle population (300–10,000 g).
These extracellular vesicle populations have been characterized as to protein content, RNA (microRNA, mRNA, noncoding RNA), DNA, mitochondrial DNA, and glycolipids with varying results dependent upon source of vesicles, nature of separative approach, and many other variables.
Vesicle Transfer of RNA and Target Cell Phenotype Change
Our focus has been on the capacity of extracellular vesicles to alter the phenotype of marrow target cells by transferring different nucleotide or protein populations. Seminal work by Ratajczak et al. [26,27] indicated the potential of protein and RNA vesicle transfer to specific target cells. These investigators isolated vesicles from embryonic stem (ES) cells and incubated them with lineage-negative Sca-1+ progenitor stem cells in a murine system. They demonstrated significant (i) enhanced survival and improved expansion of murine hematopoietic progenitors, (ii) upregulation of the expression of early pluripotent (Oct-4, Nanog, and Rex-1) and early hematopoietic stem cell (Scl, HoxB4, and GATA2) markers in these cells, and (iii) induced phosphorylation of MAPK p42/44 and serine–threonine kinase AKT. Furthermore, molecular analysis revealed that ES vesicles are selectively highly enriched in mRNA for several pluripotent transcription factors as compared with parental ES cells. This mRNA could be delivered by ES vesicles to target cells and translated into the corresponding proteins. The biological effects of ES vesicles were inhibited after heat inactivation or pretreatment with RNase, indicating a major involvement of protein and mRNA components of ES-MV in the observed phenomena.
In a similar vein, by studying murine lung originator cells and murine target marrow cells, Aliotta et al. [28–30] showed that lung RNA was transferred to marrow cells inducing the expression of lung-specific mRNAs (surfactants A–D, Clara cell-specific protein, and aquaporin-5) and proteins and altering the function of marrow cells toward that of pulmonary cells. This later was shown by the increase in prosurfactant-positive pulmonary cells after transplant with vesicle exposed marrow.
Valadi et al. [31] also demonstrated that exosomes from a mouse and a human mast cell line (MC/9 and HMC-1, respectively), as well as primary bone marrow-derived mouse mast cells contain RNA from ∼1,300 genes. In vitro translation proved that the exosome mRNAs were functional. Quality control RNA analysis of total RNA derived from exosomes also revealed the presence of small RNAs, including microRNAs. The RNA from mast cell exosomes was transferable to other mouse and human mast cells. After transfer of mouse exosomal RNA to human mast cells, new mouse proteins were found in the recipient cells, indicating that transferred exosomal mRNA can be translated after entering another cell.
In addition, Deregibus et al. [32] showed that endothelial progenitor cell-derived microvesicles could activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. They also showed that transferred mRNA can be translated into proteins in the recipient cells using microvesicles containing the green fluorescence protein mRNA. The group from Torino has since shown the capacity of vesicles from mesenchymal stem cells to transfer RNA, to reverse injury to renal and liver tissue, and to reverse the cancer phenotype of cell line hepatoma, sarcoma, and ovarian cancer cells (see Vesicle Alteration of Cell Fate and Tissue Repair).
Providence Experience
We entered the vesicle field by a lateral movement from the stem cell field. We had been studying the capacity of marrow stem cells to assume the markings of nonhematopoietic cells, skeletal muscle, skin, or lung cells [33–35], predominantly lung cells. These phenomena were termed stem cell plasticity, an area that generated a great deal of needless and destructive controversy [36]. We had demonstrated that engraftment of the green fluorescent protein (GFP)-expressing marrow cells into lethally irradiated mice resulted in the appearance of GFP-positive pulmonary epithelial cells in the lungs of lethally irradiated mice, which had been transplanted with GFP+ marrow cells. Work by others had shown that engrafted marrow cells could give rise to nonhematopoietic cells in a number of different organs. This led to the less then incisive proposal that plasticity could only be demonstrated if the results were robust, from single cells, and functional [37]. This was the impetus for the perspective in nature, ignoratio elenchi, or red herrings [36]. These meaningless criteria nevertheless hurt the field and impaired progress. In our own studies, we had demonstrated that the engraftment of GFP+ marrow cells into lethally irradiated mice resulted in the appearance of GFP+ pulmonary epithelial cells in the lungs of transplanted mice [28]. Subsequent studies showed that when lung tissue was cultured opposite murine marrow cells, but separated from them by a cell impermeable 0.4 μm membrane, the marrow cells expressed the pulmonary-specific mRNAs, surfactants A–D, aquaporin-5, and Clara cell-specific protein [29]. If the lungs were from mice exposed to 500 or 1,000 cGy whole body irradiation 5 days before cell harvest, the expression levels were significantly higher than if the lungs were from unirradiated mice. Conditioned media from the lungs reproduced these mRNA inducing effects, and it was then demonstrated that the active inducer of lung-specific mRNA was in the final pellet of the differential centrifugation (300, 10,000, 100,000 g). In the pellet, after the 100,000 g spin, were extracellular vesicles that turned out to be the active principle in the observed genetic change. We demonstrated that the surfactant protein was expressed in vesicle exposed marrow cells and the marrow cells, which expressed lung-specific mRNA, showed an increased efficiency in forming pulmonary epithelial cells after infusion into lethally irradiated mice. Using PKH26 (red fluorescence)- and CFSE (green fluorescence)-stained vesicles, incubating them with murine marrow, and then separating the marrow cells into cells that expressed fluorescence (had taken up vesicles) or those that did not (had not taken up vesicles), we established that vesicle uptake was necessary for pulmonary mRNA induction in marrow cells [30]. The vesicles were found to be replete with mRNA, microRNA, protein, and noncoding long RNA. There was also some DNA in some vesicle preparations. Adhesion proteins were expressed on their surface [38]. Organ cocultures of murine liver, heart, and brain across from murine marrow cells, but separated from them by a 0.4 μm membrane showed tissue-specific expression of mRNA [30]. These results are summarized in Table 1.
Table 1.
Expression of mRNA in Bone Marrow Cells Following Extracellular Vesicle Exposure
| Originator tissue | mRNA expressed in target marrow tissue |
| Lung | Surfactants A–D, aquaporin-5, Clara cell-specific protein |
| Liver | Albumin, serum amyloid A1 |
| Brain | Glial fibrillary protein, β-3 tubulin, neurofilament heavy chain |
| Heart | Troponin 1, troponin 2, myosin light chain 2 |
In general, the marrow target cells only expressed the tissue-specific mRNA from the originator tissue.
We have found that RNase treatment of vesicles appears to suppress the mRNA induction mediated by the larger vesicles found in conditioned media or in the supernatant after the 10,000 g spin, but not in the exosome supernatant of a 300, 10,000, and 100,000 g differential centrifugation.
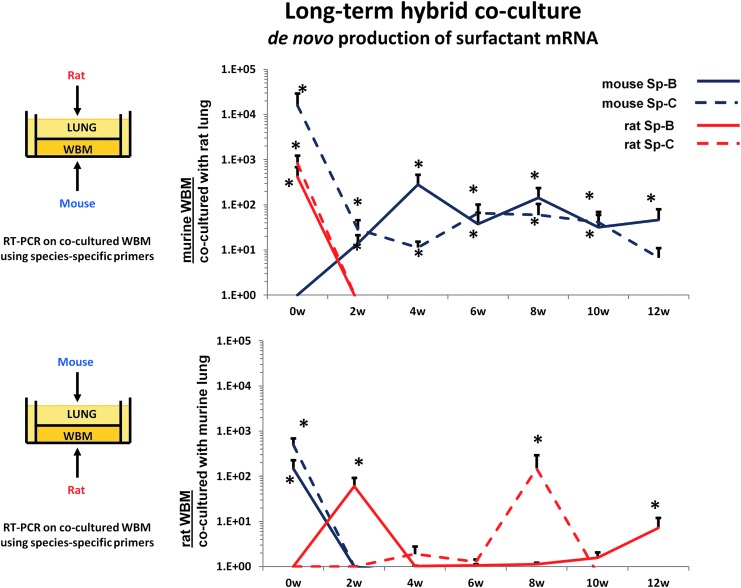
In further experiments, we evaluated the mechanism behind the mRNA induction in marrow cells exposed to murine lung vesicles. We evaluated whether the induced lung mRNA in marrow cells derived from transferred mRNA, from the originator tissue, or whether it was induced from the target tissue. We utilized cocultures of rat lung across from mouse marrow or mouse lung across from rat marrow and then analyzed the tissue for surfactant B and C mRNA using species-specific primers for surfactant B and C. We determined whether the observed mRNA was of rat or mouse origin [39]. Immediately after coculture, surfactant B and C mRNAs were markedly elevated and the mRNAs were of both rat and mouse origin. These cells were then maintained in cytokine supported liquid culture. When rat lung was incubated across from mouse marrow cells, the mouse marrow cells in culture rapidly ceased to express the rat surfactant mRNA, but continued to express mouse surfactant mRNA out to 12 weeks of culture (Fig. 1). Primers specific for rat and mouse surfactants (Sp) B and C were utilized. Cultures were supported by 50 ng steel factor per mL. The fold differences in mRNA levels are shown.
FIG. 1.
Cross-species co-culture of lung cells and marrow cells showing de novo production of surfactant mRNA in target tissue.
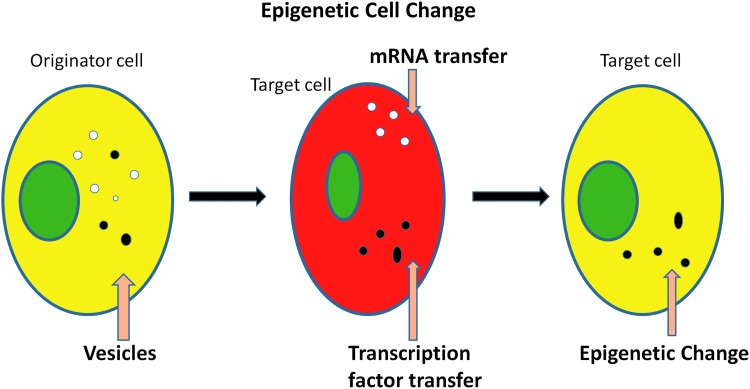
These experiments indicated that rat mRNA and a transcription factor inducing mouse mRNA were transferred to cocultured marrow cells; presumably, the rat mRNA was rapidly degraded, while the murine cells continued to produce surfactant B and C mRNA. Thus, a stable epigenetic change appears to have been induced. Given the RNase sensitivity of the phenomena, we interpret these results as indicating that a RNA species, but not mRNA, induced transcription of pulmonary-specific mRNA in marrow cells. This would indicate action by either microRNA or a long noncoding RNA. These phenotypic changes are illustrated in Figure 2.
FIG. 2.
A model of cellular phenotype modulation by extracellular vesicles.
Lethally irradiated mice transplanted with marrow that had been cocultured with murine lung expressed surfactant C in the marrow, spleen, and liver, while engraftment and expression of lung-specific mRNA in the lungs of transplanted mice were demonstrated utilizing male female transplants to identify cells deriving from marrow.
Vesicle Alteration of Cell Fate and Tissue Repair
We have touched on the work by the Camussi group above. This group showed that mesenchymal stem cells could reverse nonlethal toxic and ischemic renal injury [40,41]. These studies indicated that vesicles released from marrow-derived mesenchymal stem cells were the operative paracrine agent, stimulating proliferation and decreasing apoptosis. Human mesenchymal stem cell-specific mRNAs were shown to be transferred and translated into proteins both in vitro and in vivo in the renal tubular epithelial cells of mice with acute kidney injury. Subsequently, they investigated the effects of mesenchymal stem cell-derived vesicles on severe combined immunodeficiency (SCID) mouse survival in lethal cisplatin-induced acute kidney injury [42]. Two different regimens of MV injection were used. A single administration of vesicles ameliorated renal function and morphology, and improved survival, but did not prevent chronic tubular injury and persistent increase in BUN and creatinine. Multiple injections of vesicles further decreased mortality and at day 21, surviving mice showed normal histology and renal function. The mechanism of protection was mainly ascribed to an antiapoptotic effect of vesicles. In vitro studies demonstrated that vesicles upregulated, in cisplatin-treated human tubular epithelial cells, anti-apoptotic genes such as Bcl-xL, Bcl2, and BIRC8, and downregulated genes that have a central role in the execution phase of cell apoptosis such as Casp1, Casp8, and LTA.
Further work from Torino indicates that vesicles from different sources may have a role in tendon repair [43], improving neovascularization in murine hindlimb ischemia [44] and modulating repair of the damaged murine liver [45].
Our own group in Providence has focused on irradiated or monocrotaline-treated marrow and lung tissue, respectively [46,47]. We showed previously that infusion of GFP+ murine marrow cells into lethally irradiated mice resulted in the formation of GFP+ pulmonary epithelial cells. More recently, we have investigated the impact of vesicles on murine pulmonary hypertension. Monocrotaline administration to mice results in pulmonary hypertension and infusion of vesicles from monocrotaline-treated mice could induce pulmonary hypertension in normal mice infused with these vesicles. Preliminary studies indicate that vesicles from mesenchymal stem cells could reverse established pulmonary hypertension in monocrotaline-treated mice.
In a similar vein, we have evaluated the capacity of mesenchymal stem cell-derived vesicles to reverse marrow stem cell irradiation toxicity. Exposure of irradiated murine marrow (100–500 cGy) to either human or murine mesenchymal stem cell-derived vesicles resulted in a partial reversal of radiation toxicity in an in vitro culture system and in vivo [48]. Additional work on the impact of acetaminophen on liver vesicles has indicated that acetaminophen injury induces increased vesicle release.
Vesicles and Cancer
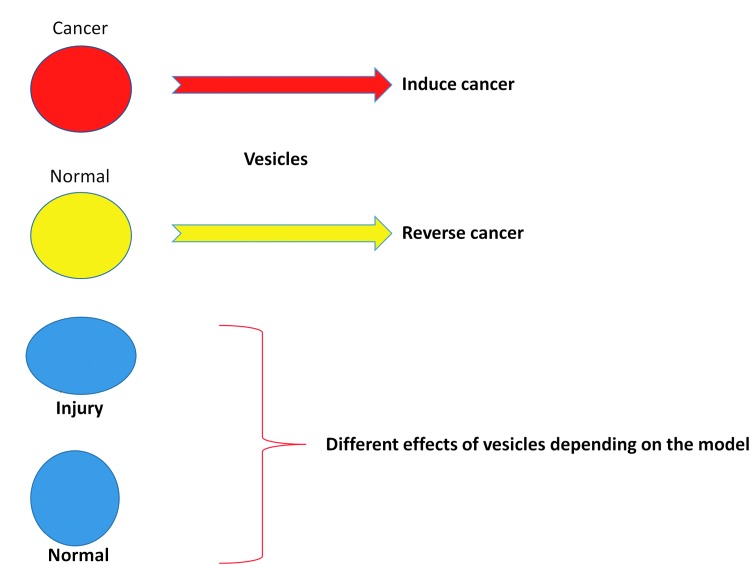
Vesicles can affect the phenotype of cancer cells and normal cells depending upon the originator source. When explant prostate or lung cancer cells from human patients were cocultured across from fresh human marrow cells (normal volunteers), the human marrow cells variably expressed prostate- or lung-specific mRNA [49,50]. This was due to cancer vesicle interactions with marrow cells. In cell line experiments, malignant prostate or breast cancer-derived vesicles induced chemoresistance and anchorage-independent growth, neoplastic characteristics in the normal cell line cells. Conversely, if normal breast or prostate cell vesicles were interacted with malignant cells, there was a reversal of anchorage-independent growth and chemoresistance in the malignant cells [51]. The Torino group has developed intriguing in vitro and in vivo data on reversibility of the cancer phenotype of sarcoma, ovarian, and hepatoma cancer cells [52]. Vesicles derived from human mesenchymal stem cells inhibited cell cycle progression in all these tumors and induced apoptosis in hepatoma and Kaposi's cells and necrosis in ovarian cancer cells [52]. In vivo intratumor administration of vesicles in established tumors generated by subcutaneous injection of these cell lines in SCID mice significantly inhibited tumor growth. However, the timing of vesicle administration is critical as mesenchymal stem cell-derived vesicles promote neoangiogenesis and enhance tumor engraftment when administered at the time of tumor implantation. Vesicles may induce regression of an established tumor by favoring tumor cell apoptosis [52]. Vesicles released from human liver stem cells were also found to inhibit hepatoma growth by delivering antitumor miRNAs, which promote tumor regression [53]. These miRNAs that are lacking in tumor cells may reprogram cells to a more benign phenotype. In normal hepatocytes, the same vesicles exhibited opposite effects because they already contain these microRNAs. Therefore, it seems that the biological effects of vesicles not only depend on the vesicle-carried molecules but also on the functional and metabolic state of recipient cells.
Importance of Treatment of Originator Cell and Cell Cycle State of Target Marrow Cell
The importance of the injury state of an originator tissue was demonstrated in studies showing that the genetic alteration of murine marrow cells varied with the cell cycle status of the marrow cells and whether the originator lung was subjected to irradiation or not [38]. In these studies, murine lineage-depleted Sca-1+ (Lin−/Sca-1+) marrow cells were cultured with interleukin-3 (IL-3), IL-6, IL-11, and steel factor, removed at 0 h (cell cycle phase G0/G1), 24 h (late G1/early S), and 48 h (late S/early G2M) and cocultured with lung tissue, lung-conditioned media (LCM), or lung-derived vesicles from mice exposed to irradiation (500 cGy 5 days previously) or not exposed. Alternatively, Lin−Sca-1+ cells were separated into G0/G1 and S/G2/M cell cycle phase populations by fluorescence-activated cell sorting (FACS) and used in coculture. Separately, lung-derived vesicles from irradiated and nonirradiated mice were analyzed for the presence of adhesion proteins. Peak pulmonary epithelial cell-specific mRNA expression was seen in G0/G1 cytokine-cultured cells cocultured with irradiated lung and in late G1/early S cells cocultured with nonirradiated lung. A similar pattern was seen in cytokine-cultured Lin−Sca-1+ cells cocultured with LCM or lung-derived vesicles and when FACS-separated Lin−Sca-1+ cells were used in coculture. Cells and lung-derived vesicles expressed adhesion proteins, which differed with irradiation exposure and cell cycle phase. This indicated a possible mechanism for differential vesicle cell entry. These data indicate that microvesicle modification of progenitor/stem cells is influenced by cell cycle and the treatment of the originator lung tissue. The concept of different vesicle effects depending on the nature of the originator tissue is presented in Figure 3. The stability of the functional effects of lung-derived vesicles was found to be preserved at 4°C or −20°C with 1% DMSO/PBS out to at least 7 days.
FIG. 3.
The effects of extracellular vesicles on target tissue depend on the nature of the originator tissue.
Translational Potential of Vesicles
The above suggests that extracellular vesicles might have significant therapeutic potential in cancer or in the context of various tissue injuries. Reversal of irradiation injury to marrow stem cells by mesenchymal stem cell-derived vesicles could have a prominent role in therapeutics of bioterrorism victims or in the setting of chemoradiotherapy, while various renal or liver injuries could be approached in a similar fashion. Vesicles could be characterized for their effects and then stored for use in various therapeutic settings. Phase 1 clinical trials are envisioned in the near future.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ. and Sixma JJ. (1999). Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94:3791–3799 [PubMed] [Google Scholar]
- 2.Johnstone RM, Adam M, Hammond JR, Orr L. and Turbide C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420 [PubMed] [Google Scholar]
- 3.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G. and Amigorena S. (1998). Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600 [DOI] [PubMed] [Google Scholar]
- 4.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W. and Geuze HJ. (2000). Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 19:3365–3374 [DOI] [PubMed] [Google Scholar]
- 5.Théry C, Zitvogel L. and Amigorena S. (2002). Exosomes: composition, biogenesis and function. Nat Rev Immunol 8:569–579 [DOI] [PubMed] [Google Scholar]
- 6.Aharon A. and Brenner B. (2011). Microparticles and pregnancy complications. Hromb Res; 127(Suppl 3):S67–S71 [DOI] [PubMed] [Google Scholar]
- 7.Witer KW, Sarbanes SL, Liu J. and Clemens JE. (2011). A Plasma microRNA signature of acute leniviral infection: biomarkers of CAN disease. AIDS 204:1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mobius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, et al. (2009). Time dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol 107:1943–1950 [DOI] [PubMed] [Google Scholar]
- 9.Sossdorf M, Otto GP, Claus RA, Gabriel HH. and Loesche W. (2011). Cell-derived microparticles promote coagulation after moderate exercise. Med Sci Sports Exerc 43:1169–1176 [DOI] [PubMed] [Google Scholar]
- 10.Chaar V, Romana M, Tripette J, Broquere C, Huisse MG, Hue O, et al. (2011). Effect of strenuous physical exercise on circulating cell-derived microparticles. Clin Hemorheol Microcirc 47:15–25 [DOI] [PubMed] [Google Scholar]
- 11.Strohacker K, Breslin WL, Carpenter KC, Davidson TR, Agha NH. and McFarlin BK. (2012). Moderate-intensity, premeal cycling blunts postprandial increases in monocyte cell surface CD18 and CD11a and endothelial microparticles following a high-fat meal in young adults. Appl Physiol Nutr Metab 37:530–539 [DOI] [PubMed] [Google Scholar]
- 12.George JN, Thoi LL, McManus LM. and Reimann TA. (1982). Isolation of human platelet membrane microparticles from plasma and serum. Blood 60:834–840 [PubMed] [Google Scholar]
- 13.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, et al. (2004). Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Repair Cell Mol Biol 31:114–121 [DOI] [PubMed] [Google Scholar]
- 14.Perkumas KM, Hoffman EA, McKay BS, allingham RR. and Stamer WD. (2007). Myocilin-associated exosomes in human ocular samples. Exp eye Res 84:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Admyre C, Johansson SM, Qazi KR, Filen JJ, lahesmaa R, Norman M, et al. (2007). Exosomes with immune modulatory features are present in human breast milk. J Immunol 179:1969–1978 [DOI] [PubMed] [Google Scholar]
- 16.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. (2008). Phase 1 clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 16:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, et al. (2008). Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol 79:12–17 [DOI] [PubMed] [Google Scholar]
- 18.Poliakov A, Spilman M, Dokland T, Amling CL. and Mobley JA. (2009). Structural heterogeneity and protein composition of exosome-like vesicles (prostaomes) in human semen. Prostate 69:159–167 [DOI] [PubMed] [Google Scholar]
- 19.Keller S, Ridinger J, Rupp AK, Janssen JW. and Altevogt P. (2011). Body fluid derived vesicles as a novel template for clinical diagnostics. J Transl Med 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, et al. (2012). Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj DA, Fiume I, Capasso G. and Pocsfalvi G. (2012). A multiplex quantitative proteomics strategy for protein biomarker studies in urinary exosomes. Kidney Int 81:1263–1272 [DOI] [PubMed] [Google Scholar]
- 22.Thery C, amigorena S, Raposo G. and Clayton A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biologic fluids. Curr Protoc Cell Biol DOI: 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- 23.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. (2002). Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 270:211–226 [DOI] [PubMed] [Google Scholar]
- 24.Kadio I, Narayanasamy P, Dash PK, Zhang W. and Gendelman HE. (2012). Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol 189:744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, et al. (2012). Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 6:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 5:847–856 [DOI] [PubMed] [Google Scholar]
- 27.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. (2006). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 9:1487–1495 [DOI] [PubMed] [Google Scholar]
- 28.Aliotta JM, Keaney P, Passero M, Dooner MS, Pimentel J, Greer D, Demers D, Foster B, Peterson A, et al. (2006). Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol 34:230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, et al. (2007). Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 25:2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D, et al. (2010). Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol 38:233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ. and Lötvall JO. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659 [DOI] [PubMed] [Google Scholar]
- 32.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B. and Camussi G. (2007). Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110:2440–2448 [DOI] [PubMed] [Google Scholar]
- 33.Abedi M, Greer DA, Colvin GA, Demers DA, Dooner MS, Harpel JA, Weier HU, Lambert JF. and Quesenberry PJ. (2004). Robust conversion of marrow cells to skeletal muscle with formation of marrow-derived muscle cell colonies: a multifactorial process. Exp Hematol 32:426–434 [DOI] [PubMed] [Google Scholar]
- 34.Dooner M, Cerny J, Colvin G, Demers D, Pimentel J, Greer D, Abedi M, McAuliffe C. and Quesenberry P. (2004). Homing and conversion of murine hematopoietic stem cells to lung. Blood Cells Mol Dis 32:47–51 [DOI] [PubMed] [Google Scholar]
- 35.Badiavas EV, Abedi M, Butmarc J, Falanga V. and Quesenberry P. (2003). Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 196:245–250 [DOI] [PubMed] [Google Scholar]
- 36.Quesenberry PJ, Dooner G, Dooner M, Abedi M. (2005). Developmental biology: Ignoratio elenchi: red herrings in stem cell research. Science 308:1121–1122 [DOI] [PubMed] [Google Scholar]
- 37.Anderson DJ, Gage FH. and Weissman IL. (2001). Can stem cells cross lineage boundaries?. Nat Med 7:393–395 [DOI] [PubMed] [Google Scholar]
- 38.Aliotta JM, Lee D, Puente N, Faradyan S, Sears EH, Amaral A, Goldberg L, Dooner MS, Pereira M. and Quesenberry PJ. (2012). Progenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesicles. Stem Cells Dev 2:1627–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliotta JM, Pereira M, Li M, Amaral A, Sorokina A, Dooner MS, Sears EH, Brilliant K, Ramratnam B, Hixson DC. and Quesenberry PJ. (2012). Stable cell fate changes in marrow cells induced by lung-derived microvesicles. J Extracell Vesicles 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C. and Camussi G. (2011). Microvesicles derived from human adult mesenchymal stem cells protect against ischemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 5:1474–1483 [DOI] [PubMed] [Google Scholar]
- 41.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 5:1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C. and Camussi G. (2012). Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tetta C, Consiglio AL, Bruno S, Tetta E, Gatti E, Dobreva M, Cremonesi F. and Camussi G. (2012). The role of microvesicles derived from mesenchymal stem cells in tissue regeneration; a dream for tendon repair?. Muscles Ligaments Tendons J 16:212–221 [PMC free article] [PubMed] [Google Scholar]
- 44.Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni GP. and Camussi G. (2012). Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol 1:75–85 [DOI] [PubMed] [Google Scholar]
- 45.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C. and Camussi G. (2009). Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 6B:1605–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aliotta JM, Keaney PJ, Warburton RR, DelTatto M, Dooner MS, Passero MA, Quesenberry PJ. and Klinger JR. (2009). Marrow cell infusion attenuates vascular remodeling in a murine model of monocrotaline-induced pulmonary hypertension. Stem Cells Dev 5:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aliotta JM, Pereira M, Amaral A, Sorokina A, Igbinoba Z, Hasslinger A, El-Bizri R, Rounds SI, Quesenberry PJ. and Klinger JR. (2013). Induction of pulmonary hypertensive changes by extracellular vesicles from monocrotaline-treated mice. Cardiovasc Res 3:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen S. (2013). Mesenchymal Stem Cell-Derived Vesicles Reverse Hematopoietic Radiation Damage. Abstract accepted to ASH Annual Meeting, New Orleans, LA [Google Scholar]
- 49.Renzulli JF, 2nd, Del Tatto M, Dooner G, Aliotta J, Goldstein L, Dooner M, Colvin G, Chatterjee D. and Quesenberry P. (2010). Microvesicle induction of prostate specific gene expression in normal human bone marrow cells. J Urol 5:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Tatto M, Ng T, Aliotta JM, Colvin GA, Dooner MS, Berz D, Dooner GJ, Papa EF, Hixson DC, et al. (2011). Marrow cell genetic phenotype change induced by human lung cancer cells. Exp Hematol 11:1072–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panagopoulos K, Cross-Knorr S, Dillard C, Pantazatos D, Del Tatto M, Mills D, Goldstein L, Renzulli J, Quesenberry P. and Chatterjee D. (2013). Reversal of chemosensitivity and induction of cell malignancy of a non-malignant prostate cancer cell line upon extracellular vesicle exposure. Mol Cancer 1:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruno S, Collino F, Deregibus MC, Grange C, Tetta C. and Camussi G. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 5:758–771 [DOI] [PubMed] [Google Scholar]
- 53.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R, Salizzoni M, Tetta C. and Camussi G. (2012). Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells 30:1985–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]